Abstract
While it is widely acknowledged that exercise has positive effects on cognitive function, the specific impacts of different types of exercises, particularly open and closed skill exercises, on cognitive impairment continue to be a debated topic. In this study, we used fNIRS and cognitive psychology tasks to investigate the effects of different types of exercises on cognitive function and brain activity in young adults. We conducted an observational study to assess the cognitive function of participants who had engaged in these exercises for a long period. Additionally, we examined the effects of open skill exercise (badminton) and closed skill exercise (calisthenics) on localized blood flow in the prefrontal lobe of the brain using an experimental research method. Specifically, during the Stroop task, the badminton group exhibited significantly higher △HbO2 in channel 18, corresponding to the dorsolateral prefrontal cortex, compared to the calisthenics group (F = 4.485, P < 0.05, η2 = 0.074). In the 2-back task, the calisthenics group showed significantly higher △HbO2 in channel 17, corresponding to the frontopolar area, dorsolateral prefrontal cortex and inferior prefrontal gyrus, than the badminton group (F = 8.842, P < 0.01, η2 = 0.136). Our findings reveal that open skill exercises are more effective in enhancing cognitive inhibition, thereby increasing attention capacity, self-regulation, and flexibility in response to environmental changes. Conversely, closed skill exercises demonstrate greater efficacy in improving working memory within cognitive functions, showcasing an enhanced capacity for information processing and storage. These data indicate that while both open and closed skill exercises are beneficial for cognitive function, they exhibit significant distinctions in some aspects.
Similar content being viewed by others
Introduction
Cognitive functions are crucial for processing, storing, and extracting information within the human brain. These functions encompass memory, executive, language, visuospatiality, and attention. Cognitive functions can be influenced by various factors, with severe cognitive decline potentially leading to cognitive impairment. Dementia, a condition commonly found in the elderly, is marked by significant cognitive deterioration. However, the journal Cell suggests that individuals with dementia may start to develop related brain abnormalities 20–30 years prior to the appearance of cognitive impairment symptoms1. Recent studies have found that cognitive impairment is increasingly being diagnosed at younger ages, with a growing number of cases among middle-aged individuals and even young adults2,3. Dementia is no longer a condition exclusive to the elderly; it now affects individuals across a broader age spectrum. These diseases lead to severe brain damage, and effective drugs or treatments are still lacking. Therefore, early prevention remains the only strategy to enhance cognitive reserve or delay the onset of cognitive decline4,5. These studies emphasize the importance of understanding and addressing cognitive impairment as a condition that affects individuals at various life stages. Some studies suggest that early preventative measures for cognitive function should begin with young adults aged 18–39 years to promote brain health6. Improving cognitive health in young adults is pivotal for preventing cognitive impairment across the lifespan.
Factors contributing to cognitive impairment include modifiable elements, such as exercise, sleep, and diet, as well as non-modifiable factors like age and education7. One study identified regular exercise as a protective factor against mild cognitive impairment8,9, and a randomized controlled trial corroborated the association between cognitive function and regular physical activity10. Therefore, among the modifiable factors, lack of exercise has a greater impact on cognitive function.
Lack of exercise not only negatively impacts cognitive function but also leads to a decrease in its level11. A large body of experimental research in the field of cognitive function in both animals and humans has been conducted. On the one hand, studies have shown that short-term exercise does not significantly affect cognitive function, while on the other hand, acute exercise has been demonstrated to have a beneficial effect12,13. From a neurological perspective, cognitive function progressively declines with age, and the prefrontal cortex utilizes more cognitive resources after exercise to compensate for this decline14. Therefore, the impact of exercise on cognitive function remains a subject of significant controversy. In conclusion, exercise has been proven to be an effective method for early intervention.
Exercise skills are categorized as open or closed skill exercises, depending on the extent to which the external environment is utilized during the exercise15. Various forms of exercise serve distinct roles in influencing cognitive function. For instance, open skill exercises have been shown to enhance cognitive functions such as visuospatial ability, attention, and flexibility16. Closed skill exercises also exert an influence on cognitive functions, notably enhancing aspects like working memory and cognitive inhibition17. Recent research on the effects of different exercise types on cognitive function indicates that open skill exercises are better for improving cognitive function18,19. However, there is also research evidence supporting the benefits of closed skill exercises in slowing cognitive decline20,21. There remains much debate as to which type of skill exercise is more effective in promoting cognitive function.
As research on cognitive function expands, a variety of possible mechanisms have been identified for the effects of physical activity on cognitive function. These mechanisms include the improvement of neuroinflammation and neuroplasticity22, the release of neurotrophic factors23, the enhancement of brain oxygenation function24, and the improvement of brain structure and morphology25. Based on their mechanisms of action, various instruments and devices have been developed to assist in the assessment of cognitive function. These include electroencephalography (EEG), transcranial magnetic stimulation (TMS), functional magnetic resonance imaging (fMRI), functional near-infrared spectroscopy (fNIRS), and other brain imaging technologies26. The advent of fNIRS has become a pivotal research tool in the field of brain science27. This tool evaluates cognitive function by measuring changes in blood flow and oxygenation levels in the brain. It is advantageous due to its wide applicability, ease of operation, high compatibility, low cost, and non-invasive nature. Owing to these benefits, fNIRS is extensively utilized in both clinical settings and scientific research28.
In recent years, the impact of exercise on human cognitive function has garnered increasing attention from scholars worldwide. It is widely acknowledged that exercise has positive effects on cognitive function. However, the specific impacts of different types of exercises, particularly open and closed skill exercises, on cognitive impairment remain a debated topic29,30,31. Designing and implementing diverse exercise programs for scientific studies involving cognitively impaired individuals presents significant challenges. One major challenge is the lack of comprehensive evidence on the brain mechanisms that link exercise to cognitive function. Therefore, it is crucial to thoroughly investigate these brain mechanisms using neuroscientific principles. By doing so, we can better understand how different types of exercise influence cognitive function. Moreover, developing effective exercise interventions for the early prevention of cognitive impairment is an urgent and critical issue. Our study aims to address this by exploring the neural underpinnings of various exercises, ultimately contributing to the creation of targeted exercise programs for cognitive health maintenance.
In summary, this research is designed to explore the impact of open and closed skill exercises on cognitive functions in young adults. In turn, this study will compare and analyze how open and closed skill exercises differently stimulate the brain’s cognitive regions. Ultimately, by synthesizing these findings, the research aims to provide valuable insights into the differential impacts of these exercises on the cognitive function of young adults.
Material and methods
Participants
The observational study selected 97 young adults as participants. These participants were stratified according to their sports specializations, with a focus on individuals regularly engaged in prolonged exercise. 32 individuals were assigned to the open skill exercise group, consisting of 16 males and 16 females. Participants engaged in sports like badminton, volleyball, and soccer (mean ± standard deviation, age: 21.60 ± 1.21 years, BMI: 21.88 ± 2.64 kg/m2, sporting age: 8.63 ± 2.96 years, physical activity: 4976.03 ± 2431.91 MET-min/w). The closed skill exercise group comprised 35 individuals, including 16 males and 19 females, who engaged in sports such as calisthenics, swimming, track and field (mean ± standard deviation, age: 20.84 ± 0.74 years, BMI: 21.48 ± 3.19 kg/m2, sporting age: 7.00 ± 2.94 years, physical activity: 3266.91 ± 1310.72 MET-min/w). The no-exercise group consisted of 30 individuals, with 15 males and 15 females, characterized as young adults who had not participated in regular exercise activities over the past 6 months (mean ± standard deviation, age: 24.59 ± 2.17 years, BMI: 22.01 ± 3.82 kg/m2, physical activity: 1766.52 ± 1318.24 MET-min/w).
The experimental study included 30 young adult females, none of whom had engaged in regular exercise in the past 6 months. The participants were randomly divided into two groups: 15 individuals were placed in the open skill exercise group, engaging in badminton (mean ± standard deviation, age: 24.24 ± 1.43 years, BMI: 20.53 ± 2.98 kg/m2, physical activity: 1535.87 ± 1101.32 MET-min/w), while the other 15 were in the closed skill exercise group, participating in calisthenics (mean ± standard deviation, age: 23.28 ± 2.14 years, BMI: 24.38 ± 4.09 kg/m2, physical activity: 1564.93 ± 1076.79 MET-min/w).
All volunteers met the following inclusion criteria: (1) right-handed; (2) aged between 20 and 25 years; (3) in good health, with no history of hypertension or other serious diseases; (4) abstained from consuming coffee, tea, or other beverages 24 h prior to testing; (5) in good mental health, with no history of drug or alcohol dependence; (6) possessing normal or corrected-to-normal visual acuity, without color blindness or color weakness. The study received approval from the Ethics Committee of Guangxi Normal University. All participants voluntarily joined the study, signing an informed consent form after fully comprehending the experimental content and procedures.
Ethics statement
The studies involving humans were approved by Guangxi Normal University Ethics Committee (No. 20230625002), Guilin, China. All study participants were informed about the purpose of the study and additional information was given as they need. The participants provided their written informed consent to participate in this study. All experiments were performed in accordance with relevant named guidelines and regulations.
Study procedure
In this study, volunteers were recruited via posters and screened based on predefined inclusion and exclusion criteria. Ultimately, 97 young adults were selected as study participants. A questionnaire was utilized to collect basic demographic and athletic information, including height, weight, age, sports specialization, years of sports experience, and competitive level. All participants completed the International Physical Activity Questionnaire (IPAQ) to ensure balanced levels of physical activity across the sample.
In the observational study, participants completed three cognitive tasks and underwent fNIRS assessment. The findings indicated that adult females demonstrated greater focus and were less prone to distraction during fNIRS data collection. Significant physiological differences between males and females, including variations in brain structure and hormone levels, may influence experimental outcomes. Selecting a single gender helps minimize these variables and enhances the accuracy of the results.
To minimize the impact of gender as a confounding variable, 30 adult females were selected as participants for the experimental study and were randomly assigned to either the badminton group or the calisthenics group. Prior to the exercise intervention, participants completed three cognitive tasks and underwent an fNIRS assessment. During the exercise intervention, which included 10 min of preparatory activities, 30 min of moderate-intensity acute exercise, and 10 min of relaxation activities, the same cognitive tasks and fNIRS assessments were performed after the exercise intervention (Fig. 1).
Study design
Our study on cognitive function used both observational and experimental designs. Observational studies identified trends in how open skill exercise and closed skill exercise affect cognitive function in males and females, providing baseline data and potential influencing variables. The experimental phase, focused on females, used fNIRS to compare the effects of these exercises on localized brain blood flow during cognitive tasks, specifically examining prefrontal changes in △HbO2. This method allowed us to assess the interventions’ impact on cognitive function and validate the observational findings.
The observational study utilized a 3 × 3 mixed design, with three types of exercise (open skill, closed skill, no-exercise) and three cognitive tasks (Stroop, 2-back, More-odd shifting). The between-subjects variable was the type of exercise, while the dependent variable was the delta oxy-hemoglobin (△HbO2) levels across 19 channels in the prefrontal cortex (PFC) during the tasks (Fig. 1).
The experimental study utilized a 2 × 2 mixed design, with two groups (badminton, calisthenics) and two time points (pre-intervention, post-intervention). The intervention involved three phases: initial exercise testing, the intervention exercises, and final exercise testing. Independent variables were the types of exercise (badminton and calisthenics groups), while the dependent variable was the delta oxy-hemoglobin (△HbO2) levels in the 19 channels of the PFC during the execution of assigned tasks. Exercise intensity was controlled throughout to ensure experimental rigor (Fig. 1).
fNIRS data acquisition
The fNIRS signals were used a portable functional Near-Infrared brain imaging device (NirSmartII-3000C, Danyang Huichuang Medical Equipment Co., Ltd., China) with wavelengths of 730 nm and 850 nm, and a sampling rate of 11 Hz. Figure 2 shows the distribution of 19 measurement channels, consisting of 7 light source probes and 7 detector probes, placed on the brain. All probes were positioned according to the 10–20 international standard, aiming to capture changes in hemodynamic signals in the prefrontal cortex. 6 regions of interest were divided based on the corresponding positions of the channels in the prefrontal cortex. These channels were located above the right inferior prefrontal gyrus (RIFG), left inferior prefrontal gyrus (LIFG), right dorsolateral prefrontal cortex (RDLPFC), left dorsolateral prefrontal cortex (LDLPFC), right frontopolar area (RFPA), left frontopolar area (LFPA)32 (Table 1).
Experimental paradigm
Various aspects of cognitive function were evaluated using a range of cognitive psychology tasks, with executive function serving as the primary metric for inferring cognitive ability. Executive function refers to the mental process through which an organism consciously controls thoughts and actions, representing a crucial component of cognitive function. Executive function is comprised of three primary subcomponents: inhibitory control, working memory, and cognitive flexibility. Each of these subcomponents is evaluated using a variety of testing methods. This study assessed cognitive function using three task paradigms: Stroop, n-back, and More-odd shifting. (1) In the Stroop task, the formal test consisted of 5 blocks, each with 12 trials and a 30 s interval between blocks. The formal test duration was 5 min33. (2) In the 2-back task, the test was divided into 2 blocks, each containing 18 trials, with a 30 s interval between blocks. The formal test duration was 5 min34. (3) In the More-odd shifting task, the test included 6 blocks of 12 trials each, with the figure presentation time set at 1.5 s, a stimulus interval of 1 s, and a 30 s interval between blocks. The total duration of the formal test was 6 min35.
Exercise program
The acute exercise intervention programs included badminton and calisthenics. Participants began the intervention after a week of acclimatization. A professional coach led the badminton group, focusing on doubles practice sessions. The calisthenics group, under the guidance of a uniformly trained instructor, performed exercises like jumping jacks, side-knee kicks, kicking shuttlecock steps, knee-to-elbow raises, deep squat jumps, and chest expansion with back kicks. Each session of the acute exercise lasted 30 min, with a consistent moderate intensity. Participants’ heart rates were monitored using fitness trackers throughout the intervention to maintain them within 60–70% of their maximum heart rate (HRmax).”
fNIRS data preprocessing
The fNIRS data were processed using NirSpark software. Data preprocessing involved five steps: (1) eliminating artifacts unrelated to the experimental data; (2) converting optical intensity to optical density; (3) applying a band-pass filter (0.01–0.2 Hz) to remove noise and interfering signals36; (4) converting optical density to oxygen concentration following the modified Beer-Lambert law37; and (5) setting the initial time of the hemodynamic response function (HRF) to − 10 s and the end time to 30 s, thus retaining the baseline state as “− 10–0 s” and the time of single block mode as “0–30 s”. Finally, the task-related blood oxygen concentrations were superimposed and averaged to produce a block average result.
Statistical analysis
The sample size was calculated using PASS 15 software (New Canaan, Connecticut, USA), following the guidelines for superiority tests comparing two rates. The calculation determined a sample size of 15, with an alpha value of 0.05 and a power of 0.75. This calculation established that a minimum sample size of 15 was necessary for the experimental study. All data were statistically analyzed using SPSS 26.0 (Chicago, IL, USA) software and expressed as mean ± standard deviation. First, each outcome variable was examined for normality and homoscedasticity. When the data distribution didn’t conform to the normality assumption and the variance violated homogenous, the Kruskal–Wallis H test was applied. When the data exhibited normal distribution and homogeneity of variance, we employed one-way analysis of variance (ANOVA) to evaluate △HbO2 levels in the PFC. In the observational study, and multiple comparisons used the LSD method. In the experimental studies, △HbO2 was analyzed using a paired t-test to determine the differences between pre-exercise and post-exercise measurements. Additionally, △HbO2 was analyzed using two-way ANOVA to evaluate between-group differences and calculate the group × time interaction. Effect sizes were reported as Cohen’d for t-tests and partial eta squared (η2) for ANOVA. Significant differences were considered at P < 0.05.
Results
Results of the observational study
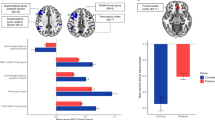
During the Stroop task, males in the open skill exercise group exhibited higher △HbO2 in the LDLPFC (1.14 ± 7.19 (× 10−2 mmol/L)) compared to males in the closed skill exercise group (− 3.84 ± 5.63 (× 10−2 mmol/L)). Additionally, males in the open skill exercise group showed higher △HbO2 in the LFPA (0.76 ± 8.08 (× 10−2 mmol/L)) than males in the closed skill exercise group (− 5.50 ± 7.66(× 10−2 mmol/L)). All these differences were statistically significant (P < 0.05) (Fig. 3A). However, there was no significant difference in △HbO2 levels at the 6 ROIs between the girls in the open practice group and the closed skill practice group in the Stroop task (Fig. 3B). Conversely, in the 2-back task, there was a significant reduction in △HbO2 levels observed in both the open and closed skill exercise groups at the RDLPFC in males (Fig. 3C) and females (Fig. 3D). It is noteworthy that no significant difference in △HbO2 levels was observed between the open and closed skill exercise groups. During the More-odd shifting task, the highest levels of △HbO2 at RFPA, LFPA, and RDLPFC were observed in males belonging to the no-exercise skill group, and the difference was statistically significant (P < 0.05) (Fig. 3E). However, there was no significant difference in △HbO2 levels at the 6 ROIs between the girls in the open practice group and the closed skill practice group in the More-odd shifting task (Fig. 3F).
Changed in △HbO2 concentration across ROIs within the prefrontal cortex (PFC) during the cognitive tasks. (A) Males in Stroop task; (B) Females in Stroop task; (C) Males in 2-back task; (D) Females in 2-back task; (E) Males in More-odd shifting task; (F) Females in More-odd shifting task. RIFG right inferior prefrontal gyrus; LIFG left inferior prefrontal gyrus; RDLPFC right dorsolateral prefrontal cortex; LDLPFC left dorsolateral prefrontal cortex; RFPA right frontopolar area; LFPA left frontopolar area.
Results of experimental study
In the Stroop task, the badminton group showed a significant decrease in △HbO2 levels at channel 4 following acute exercise intervention. Furthermore, a significant interaction effect between skill exercise types and test time was noted at channel 18 during the Stroop task (F = 4.485, P < 0.05, η2 = 0.074). In this channel, the badminton group exhibited a more pronounced change in △HbO2 compared to the calisthenics group. However, no statistically significant differences in △HbO2 levels were observed in the other channels between the two groups (Fig. 4).
Changed in △HbO2 during the Stroop task in the experimental groups before and after the acute exercise intervention. (A1) Badminton group pre-intervention. (A2) Badminton group post-intervention. (B1) Calisthenics group pre-intervention. (B2) Calisthenics group post-intervention. The software used to create the figures is NirSpark version 1.8.1. The NirSpark package URL is http://www.hcmedx.cn/en/Content/2292015.html.
In the badminton group, a significant reduction in △HbO2 levels was observed in channels 4 (T = 2.492, P < 0.05, d = 0.643), 9 (T = 2.547, P < 0.05, d = 0.658), 16 (T = 2.622, P < 0.05, d = 0.677), and 17 (T = 3.249, P < 0.01, d = 0.839) following the acute exercise intervention during the 2-back (P < 0.05). In the calisthenics group, △HbO2 levels in channel 5 were significantly lower (P < 0.05) post-intervention compared to the pre-intervention. Notably, there was a significant interaction effect between exercise types and testing time for channel 17 during the 2-back task (F = 8.842, P < 0.01, η2 = 0.136). In channel 17, the △HbO2 levels were significantly lower in the badminton group (pre-intervention: 5.03 ± 8.83, post-intervention: − 1.98 ± 5.82 (× 10−2 mmol/L)) compared to the calisthenics group (pre-intervention: − 2.93 ± 7.77, post-intervention: 1.15 ± 6.00 (× 10−2 mmol/L)). However, in the other channels, no statistically significant differences in △HbO2 levels were observed between the two groups (Fig. 5).
Changed in △HbO2 during the 2-back task in the experimental groups before and after the acute exercise intervention. (A1) Badminton group pre-intervention. (A2) Badminton group post-intervention. (B1) Calisthenics group pre-intervention. (B2) Calisthenics group post-intervention. The software used to create the figures is NirSpark version 1.8.1. The NirSpark package URL is http://www.hcmedx.cn/en/Content/2292015.html.
Following the acute exercise intervention, the badminton group showed a significant reduction in △HbO2 levels at channel 9 during the More-odd shifting task (T = 2.096, P < 0.05, d = 0.750). Conversely, in the calisthenics group, there were no statistically significant differences (P > 0.05) in △HbO2 across all channels following the acute exercise intervention. Additionally, no significant differences in △HbO2 levels (P > 0.05) were observed between the badminton and calisthenics groups across all channels during the More-odd shifting task post-intervention (Fig. 6).
Changed in △HbO2 during the More-odd shifting task in the experimental groups before and after the acute exercise intervention. (A1) Badminton group pre-intervention. (A2) Badminton group post-intervention. (B1) Calisthenics group pre-intervention. (B2) Calisthenics group post-intervention. The software used to create the figures is NirSpark version 1.8.1. The NirSpark package URL is http://www.hcmedx.cn/en/Content/2292015.html.
Discussion
This study primarily compared cognitive functioning levels among young adults engaged in prolonged exercise and investigated variations in PFC cerebral blood flow during cognitive tasks across open and closed skill exercises. In addition, the study assessed variations in PFC cerebral blood flow before and after acute interventions, which included exercises of both open and closed skills. The study objective was to uncover changes in △HbO2 levels within the PFC of young adults’ brains in response to different skill exercise types.
The effects of prolonged open and closed skill exercises on cognitive
Changes in HbO2 levels within the bilateral dorsolateral prefrontal cortex and the left frontopolar region were associated with the effects of the Stroop task38. This study observed that during the Stroop cognitive task, changes in blood oxygen concentration were significantly greater in males from the open exercise group than in those from the closed skill exercise group, in the RFPA, LFPA, RDLPFC, and LDLPFC regions. Participants in the open exercise group demonstrated improved performance in certain executive functions, particularly in inhibitory control and cognitive flexibility39,40. This suggests that long-term exercise could play a crucial role in maintaining neurocognitive functions41. Specifically, it indicates improved performance in executive functions, particularly inhibitory control and cognitive flexibility, in the open exercise group.
Execution of the 2-back task was associated with an increase in neural activity within the left dorsolateral prefrontal cortex42. This study found that young adults in the no-exercise skill group had significantly higher HbO2 changes in the LDLPFC than those in both the open and closed skill exercise groups during the 2-back cognitive task. According to cognitive load theory, young adults who engage in less physical activity do not deplete their cognitive resources during the 2-back task, allowing them to fully mobilize these resources and increase cerebral blood flow43. Alternatively, the reduced cognitive benefits in young adults from both open and closed skill exercise groups could result from prolonged high-intensity training, consistent with the inverted “U” theory44. Thus, sustained high-intensity exercise could potentially contribute to a reduction in cognitive performance (Supplementary information).
The More-odd shifting task activates the prefrontal cortex of the brain45. In this study, during the More-odd shifting task, △HbO2 was highest in the no-exercise skill group and lowest in the closed exercise group in terms of RFPA, LFPA, RDLPFC, and LDLPFC. Participants in both the open and closed skill exercise groups were students specializing in athletic training. In China, students specializing in sports predominantly focus on athletic training over cultural studies, which may increase their risk of cognitive decline from prolonged dedication to a single sports discipline. Conversely, adults in the no-exercise skill group require extended periods for reflection and study to successfully complete various cultural theory courses, necessitating enhanced cognitive flexibility. Therefore, it is anticipated that the prefrontal △HbO2 levels will be elevated in the no-exercise skill group during the More-odd shifting task.
The effects of acute open and closed skill exercises on cognitive
In the badminton group, post-acute exercise intervention, blood oxygen levels in the RFPA and RDLPFC were observed to be lower than the pre-intervention levels during the performance of the Stroop task. During moderate-intensity exercise, there may be a significant reallocation of brain resources towards the primary motor cortex46,47. Consequently, after an acute exercise intervention, it was observed that activity potentially increased in the primary motor cortex while simultaneously decreasing in the PFC. The “cognitive stimulus hypothesis” suggests that muscle strength initially decreases following acute exercise, yet shows improvement with sustained exercise over time48. The Strength of Self-Control model proposes that tasks demanding cognitive effort can lead to the depletion of cognitive resources49. In summary, the hypothesis was that acute exercise interventions produced similar effects on PFC blood oxygen levels.
In observational studies, it was found that male adults in the open skill exercise group experienced greater changes in blood oxygen concentration compared to those in the closed skill exercise group. This phenomenon arises from the higher cognitive demands associated with open skill exercises, as hemodynamic activity levels in the brain tend to increase linearly with cognitive load50.
The catecholamine hypothesis proposes that enhanced cognitive performance is attributed to the heightened phasic release of dopamine and norepinephrine during moderate-intensity exercise51. The study provided a compelling explanation for the mechanism through which exercise enhances working memory performance. Recent findings suggest that engaging in a two-week closed skill exercise program, such as running, can lead to an increase in hippocampal volume among young individuals52. Closed skill exercise has been shown to positively impact hippocampal volume, which correlates with improvements in working memory53. Improving working memory performance has been associated with larger hippocampal volumes54.
Additionally, studies have indicated that, in other cognitive tasks, older adults exhibit increased brain activation compared to younger adults, a phenomenon considered as compensatory55. Similarly, closed skill exercises require the memorization of a greater number of movements and utilize more cognitive resources than open skill exercises. After an acute exercise intervention of equal intensity, participants engaging in closed skill exercises required additional cognitive resources to successfully complete the cognitive task. Consequently, △HbO2 levels in the prefrontal cortex were higher in the closed skill exercise group than in the open skill exercise group.
In the More-odd shifting task, participants in the badminton group showed improved cognitive performance after participating in an acute exercise session. However, a significant reduction in blood oxygen levels was observed in both the RFPA and RDLPFC areas. Improved cognitive performance after open skill exercise was associated with an increase in the release of neurotrophic factors in participants following the exercise intervention56. The significant decrease in blood oxygen levels can be explained by the cognitive load theory, which posits that cognitive resources are depleted during exercise, leading to a reduction in the PFC following physical activity. Consequently, blood oxygen content in the PFC is diminished after acute exercise.
Conclusion
This study revealed that there was no significant difference in the overall impact on cognitive function between open and closed skill exercises. However, significant differences in cognitive functions were noted. Open skill exercises improved cognitive inhibition, attention, self-regulation, and adaptability to environmental changes. Closed skill exercises enhanced working memory, improving information processing and storage.
Limitations of the study
This study has several limitations. First, it limited the classification of skill exercise modes to two categories, despite the existence of multiple criteria for differentiating skill exercise types. Second, although positive impacts on cognitive functions were observed in young adults through both open and closed skill exercises, there is a paucity of comprehensive assessments across a broader spectrum of age groups. Future research should more thoroughly investigate the effects of exercise on cognitive function by incorporating a wider variety of exercise types and programs. Additionally, the impact of various exercise modalities on cognitive functions warrants further investigation across different age groups, including children, adolescents, adults, and the elderly.
Data availability
The datasets used in the analyses described in this study are available from the corresponding author on reasonable request.
References
Jucker, M. & Walker, L. C. Alzheimer’s disease: From immunotherapy to immunoprevention. Cell. 186, 4260–4270. https://doi.org/10.1016/j.cell.2023.08.021 (2023).
Agüero, P. et al. De Novo PS1 mutation (Pro436Gln) in a very early-onset posterior variant of Alzheimer’s disease associated with spasticity: A case report. Alzheimers Dis. 83, 1011–1016. https://doi.org/10.3233/JAD-210420 (2021).
Sutphen, C. L. et al. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol. 72, 1029–1042. https://doi.org/10.1001/jamaneurol.2015.1285 (2015).
Demurtas, J. et al. Physical activity and exercise in mild cognitive impairment and dementia: An umbrella review of intervention and observational studies. J. Am. Med. Dir. Assoc. 21, 1415–1422. https://doi.org/10.1016/j.jamda.2020.08.031 (2020).
Dieckelmann, M. et al. Effectiveness of exercise interventions to improve long-term outcomes in people living with mild cognitive impairment: A systematic review and meta-analysis. Sci. Rep. 13, 18074. https://doi.org/10.1038/s41598-023-44771-7 (2023).
Farina, F. R. et al. Young adult brain capital: A new opportunity for dementia prevention. J. Alzheimers Dis. 94, 415–423. https://doi.org/10.3233/JAD-230260 (2023).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 396, 413–446. https://doi.org/10.1016/S0140-6736(20)30367-6 (2020).
Zhang, Y. F. et al. Influencing factors of mild cognitive impairment among the Chinese elderly: A meta-analysis. Natl. Med. J. China. 103, 1340–1348. https://doi.org/10.3760/cma.j.cn112137-20220819-01765 (2023).
Li, W. et al. Cost-effectiveness of physical activity interventions for prevention and management of cognitive decline and dementia-a systematic review. Alzheimers Res. Ther. 15, 159. https://doi.org/10.1186/s13195-023-01286-7 (2023).
Hu, Y., Wang, K., Gu, J., Huang, Z. & Li, M. Effect of combined physical and cognitive intervention on fear of falling in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 117, 105173. https://doi.org/10.1016/j.archger.2023.105173 (2024).
Taddei, F., Bultrini, A., Spinelli, D. & Di Russo, F. Neural correlates of attentional and executive processing in middle-age fencers. Med. Sci. Sports Exerc. 44, 1057–1066. https://doi.org/10.1249/MSS.0b013e31824529c2 (2012).
Chang, Y. K., Labban, J. D., Gapin, J. I. & Etnier, J. L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 1453, 87–101. https://doi.org/10.1016/j.brainres.2012.02.068 (2012).
Mograss, M. et al. The effects of acute exercise and a nap on heart rate variability and memory in young sedentary adults. Psychophysiology. 19, e14454. https://doi.org/10.1111/psyp.14454 (2023).
Vermeij, A., van Beek, A. H., Olde Rikkert, M. G., Claassen, J. A. & Kessels, R. P. Effects of aging on cerebral oxygenation during working-memory performance: a functional near-infrared spectroscopy study. PLoS One. 7, e46210. https://doi.org/10.1371/journal.pone.0046210 (2012).
Gu, Q., Zou, L., Loprinzi, P. D., Quan, M. & Huang, T. Effects of open versus closed skill exercise on cognitive function: A systematic review. Front. Psychol. 10, 1707. https://doi.org/10.3389/fpsyg.2019.01707 (2019).
Guo, W. et al. The relationship between different exercise modes and visuospatial working memory in older adults: A cross-sectional study. PeerJ. 4, e2254. https://doi.org/10.7717/peerj.2254 (2016).
Rehfeld, K. et al. Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PLoS One. 13, e0196636. https://doi.org/10.1371/journal.pone.0196636 (2018).
Westendorp, M. et al. Effect of a ball skill intervention on children’s ball skills and cognitive functions. Med. Sci. Sports Exerc. 46, 414–422. https://doi.org/10.1249/MSS.0b013e3182a532b3 (2014).
Gökçe, E., Güneş, E., Arı, F., Hayme, S. & Nalçacı, E. Comparison of the effects of open- and closed-skill exercise on cognition and peripheral proteins: A cross-sectional study. PLoS One. 16, e0251907. https://doi.org/10.1371/journal.pone.0251907 (2021).
Zhou, F. & Qin, C. Acute moderate-intensity exercise generally nhances attentional resources related to perceptual processing. Front. Psychol. 10, 2547. https://doi.org/10.3389/fpsyg.2019.02547 (2019).
Liao, J., Yang, Y. J. & Xu, D. R. Multiyear square dancing is associated with superior mental processing capacity but not memory in middle-aged and older Chinese women: A cross-sectional propensity score matching analysis. J. Phys. Act Health. 17, 736–743. https://doi.org/10.1123/jpah.2019-0336 (2020).
Swain, R. A. et al. On aerobic exercise and behavioral and neural plasticity. Brain Sci. 2, 709–744. https://doi.org/10.3390/brainsci2040709 (2012).
Valenzuela, P. L. et al. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 62, 101108. https://doi.org/10.1016/j.arr.2020.101108 (2020).
Gary, R. A. & Brunn, K. Aerobic exercise as an adjunct therapy for improving cognitive function in heart failure. Cardiol Res Pract. 2014, 157508. https://doi.org/10.1155/2014/157508 (2014).
Erickson, K. I. et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U S A. 108, 3017–3022. https://doi.org/10.1073/pnas.1015950108 (2011).
Lin, Y. et al. Electroencephalography and functional magnetic resonance imaging-guided simultaneous transcranial direct current stimulation and repetitive transcranial magnetic stimulation in a patient with minimally conscious state. Front. Neurosci. 13, 746. https://doi.org/10.3389/fnins.2019.00746 (2019).
Noah, J. A. et al. fMRI validation of fNIRS measurements during a naturalistic task. J. Vis. Exp. 100, e52116. https://doi.org/10.3791/52116 (2015).
Arenth, P. M., Ricker, J. H. & Schultheis, M. T. Applications of functional near-infrared spectroscopy (fNIRS) to neurorehabilitation of cognitive disabilities. Clin. Neuropsychol. 21, 38–57. https://doi.org/10.1080/13854040600878785 (2007).
Serra, L. et al. Walking, running, swimming: An analysis of the effects of land and water aerobic exercises on cognitive functions and neural substrates. Int. J. Environ. Res. Public Health. 19, 16310. https://doi.org/10.3390/ijerph192316310 (2022).
Qi, L. et al. Positive effects of brisk walking and Tai Chi on cognitive function in older adults: An fNIRS study. Physiol. Behav. 273, 114390. https://doi.org/10.1016/j.physbeh.2023.114390 (2024).
Cantrelle, J., Burnett, G. & Loprinzi, P. D. Acute exercise on memory function: Open vs. closed skilled exercise. Health Promot. Perspect. 10, 123–128. https://doi.org/10.34172/hpp.2020.20 (2020).
Rorden, C. & Brett, M. Stereotaxic display of brain lesions. Behav. Neurol. 12(4), 191–200. https://doi.org/10.1155/2000/421719 (2000).
Parris, B. A., Hasshim, N., Wadsley, M., Augustinova, M. & Ferrand, L. The loci of Stroop effects: A critical review of methods and evidence for levels of processing contributing to color-word Stroop effects and the implications for the loci of attentional selection. Psychol. Res. 86(4), 1029–1053. https://doi.org/10.1007/s00426-021-01554-x (2022).
Zohdi, H., Märki, J., Scholkmann, F. & Wolf, U. Cerebral, systemic physiological and behavioral responses to colored light exposure during a cognitive task: A SPA-fNIRS study. Behav. Brain Res. 462, 114884. https://doi.org/10.1016/j.bbr.2024.114884 (2024).
Dong, M., Li, Y. & Zhang, Y. The effect of mindfulness training on executive function in youth with depression. Acta Psychol. 235, 103888. https://doi.org/10.1016/j.actpsy.2023.103888 (2023).
Li, Q. et al. Effects of the multisensory rehabilitation product for home-based hand training after stroke on cortical activation by using NIRS methods. Neurosci. Lett. 717, 134682. https://doi.org/10.1016/j.neulet.2019.134682 (2020).
Wang, Z. et al. Effects of three different rehabilitation games’ interaction on brain activation using functional near-infrared spectroscopy. Physiol. Meas. 41, 125005. https://doi.org/10.1088/1361-6579/abcd1f (2020).
Xiang, M. Q., Lin, L., Song, Y. T., Hu, M. & Hou, X. H. Reduced left dorsolateral prefrontal activation in problematic smartphone users during the Stroop task: An fNIRS study. Front. Psychiatry. 13, 1097375. https://doi.org/10.3389/fpsyt.2022.1097375 (2023).
Giglia, G. et al. Visuospatial attention lateralization in volleyball players and in rowers. Percept. Mot. Skills. 112, 915–925. https://doi.org/10.2466/05.22.27.PMS.112.3.915-925 (2011).
Dai, C. T., Chang, Y. K., Huang, C. J. & Hung, T. M. Exercise mode and executive function in older adults: An ERP study of task-switching. Brain Cogn. 83, 153–162. https://doi.org/10.1016/j.bandc.2013.07.007 (2013).
Zhao, E., Tranovich, M. J., DeAngelo, R., Kontos, A. P. & Wright, V. J. Chronic exercise preserves brain function in masters athletes when compared to sedentary counterparts. Phys. Sportsmed. 44, 8–13. https://doi.org/10.1080/00913847.2016.1103641 (2016).
Rodriguez-Jimenez, R. et al. Differential dorsolateral prefrontal cortex activation during a verbal n-back task according to sensory modality. Behav. Brain Res. 205, 299–302. https://doi.org/10.1016/j.bbr.2009.08.022 (2009).
Venkat, M. V., O’Sullivan, P. S., Young, J. Q. & Sewell, J. L. Using cognitive load theory to improve teaching in the clinical workplace. MedEdPORTAL. 16, 10983. https://doi.org/10.15766/mep_2374-8265.10983 (2020).
Wang, D., Zhou, C., Zhao, M., Wu, X. & Chang, Y. K. Dose-response relationships between exercise intensity, cravings, and inhibitory control in methamphetamine dependence: An ERPs study. Drug Alcohol Depend. 161, 331–339. https://doi.org/10.1016/j.drugalcdep.2016.02.023 (2016).
Wang, H., Tang, W. & Zhao, Y. Acute effects of different exercise forms on executive function and the mechanism of cerebral hemodynamics in hospitalized T2DM patients: A within-subject study. Front. Public Health. 11, 1165892. https://doi.org/10.3389/fpubh.2023.1165892 (2023).
Zheng, K. et al. Changes in working memory performance and cortical activity during acute aerobic exercise in young adults. Front. Behav. Neurosci. 16, 884490. https://doi.org/10.3389/fnbeh.2022.884490 (2022).
Lambourne, K. & Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 1341, 12–24. https://doi.org/10.1016/j.brainres.2010.03.091 (2010).
Egger, F., Benzing, V., Conzelmann, A. & Schmidt, M. Boost your brain, while having a break! The effects of long-term cognitively engaging physical activity breaks on children’s executive functions and academic achievement. PLoS One. 14, e0212482. https://doi.org/10.1371/journal.pone.0212482 (2019).
Kuhnle, C., Hofer, M. & Kilian, B. Self-control as predictor of school grades, life balance, and flow in adolescents. Br. J. Educ. Psychol. 82, 533–548. https://doi.org/10.1111/j.2044-8279.2011.02042.x (2012).
Liu, Z. et al. Effective connectivity analysis of the brain network in drivers during actual driving using near-infrared spectroscopy. Front. Behav. Neurosci. 11, 211. https://doi.org/10.3389/fnbeh.2017.00211 (2017).
McMorris, T. The acute exercise-cognition interaction: From the catecholamines hypothesis to an interoception model. Int. J. Psychophysiol. 170, 75–88. https://doi.org/10.1016/j.ijpsycho.2021.10.005 (2021).
Fink, A. et al. A two-week running intervention reduces symptoms related to depression and increases hippocampal volume in young adults. Cortex. 144, 70–81. https://doi.org/10.1016/j.cortex.2021.08.010 (2021).
Naef, N., Ciernik, A., Latal, B. & Liamlahi, R. Children’s heart and development research group. Hippocampal volume and cognitive performance in children with congenital heart disease. Pediatr. Res. 94, 99–102. https://doi.org/10.1038/s41390-022-02457-2 (2023).
Nauer, R. K., Dunne, M. F., Stern, C. E., Storer, T. W. & Schon, K. Improving fitness increases dentate gyrus/CA3 volume in the hippocampal head and enhances memory in young adults. Hippocampus. 30, 488–504. https://doi.org/10.1002/hipo.23166 (2020).
Langenecker, S. A. & Nielson, K. A. Frontal recruitment during response inhibition in older adults replicated with fMRI. Neuroimage. 20, 1384–1392. https://doi.org/10.1016/S1053-8119(03)00372-0 (2003).
Ramdeo, K. R. et al. The effects of exercise on synaptic plasticity in individuals with mild cognitive impairment: Protocol for a pilot intervention study. JMIR Res. Protoc. 12, e50030. https://doi.org/10.2196/50030 (2023).
Acknowledgements
We thank the participants in this study for their collaboration. We acknowledge all teachers and members for their support during this study.
Funding
This study was supported by the National Social Science Foundation of China (grant no. 21XTY018).
Author information
Authors and Affiliations
Contributions
Q.L. and Y.Z. contributed equally to the experiment and wrote the manuscript. Y.H. contributed significantly to the meticulous revision of the manuscript, Y.F.W. and X.Y. review and editing of manuscripts, Q.H. contributed in the experiment, H.C. found literature and information, Y.B.W. contributed to the graphics, H.W. contributed significantly to the data analysis. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Q., Zhao, Y., Wang, Y. et al. Comparative effectiveness of open and closed skill exercises on cognitive function in young adults: a fNIRS study. Sci Rep 14, 21007 (2024). https://doi.org/10.1038/s41598-024-70614-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70614-0