Abstract
To evaluate the changes of choroidal thickness (CT) and blood flow related to myopia, and its effects of vascular endothelial growth factor (VEGFA) on choroidal vessels in myopia. Subjects were included and divided into emmetropia (EM), non-high myopia (Non-HM) and high myopia (HM) groups. we measured choroidal thickness (CT), choriocapillaris vessel density (VD), and VEGFA content in tears in humans (137 subjects for CT, VD and 84 for tear) and detected the role of VEGFA in the choroid in form-deprivation myopia (FDM) in guinea pigs. Twenty-four guinea pigs were divided into control and FDM groups, and the expression changes of choroidal vessels and VEGFA were observed and compared using immunohistochemistry and Western blotting. Twenty-one guinea pigs were divided into control, FDM + Vehicle and FDM + Conbercept groups. The changes of diopter, axis length and choroidal vessels after intravitreal injection of Conbercept were observed. There were significant differences in CT and VD among the three groups (p < 0.05). VEGFA levels in tears were significantly lower in the myopic groups, with a decreasing trend from EM to Non-HM to HM. The choroidal vascular area fraction of FDM decreased compared to the control group. FDM guinea pigs exhibited reduced choroidal vasculature and significant downregulation of VEGFA expression. Following intravitreal injection of conbercept, the FDM + Conbercept group showed greater myopia, longer axial length, and lower choroidal vascular area fraction compared to the control group. VEGFA may participate in the regulation of choroidal blood vessels and blood flow in the progression of myopia. The reduction in VEGFA may accelerates the progression of myopia.
Similar content being viewed by others
Introduction
The pathogenesis of myopia is still not fully clear, and as a result, a series of serious problems, such as early age of onset, high prevalence, and complications of myopia, have become increasingly prominent1,2. Myopia has become a global public health problem3,4. Previous studies have found that choroidal thinning is observed in myopic children5,6,7 and adults8,9. Alterations in choroidal thickness have also been observed in form deprivation myopia (FDM) guinea pigs10, suggesting that myopia is closely related to structural changes in the choroid. In recent years, with the continuous development and clinical application of optical coherence tomography angiography (OCTA) technology, our observation and understanding of the choroidal changes in pathological statuses have been greatly facilitated11,12,13,14. Based on the OCTA technique, numerous clinical studies have observed choroidal thinning in myopic patients and found that choroidal thinning can be a predictive marker for early pathological changes in myopia11,15,16. However, what causes choroidal thinning in myopic patients remains an intriguing question, and studies on this issue may contribute to the elucidation of myopia pathogenesis.
As the main vascular tissue in the eye, the choroid not only has the functions of regulating eye temperature, secreting cytokines to regulate the growth of the eye, and regulating intraocular pressure but also provides oxygen and nutrients to the outer layer of the retina17,18. The vascular endothelial growth factor (VEGF) is a highly specific endothelial growth factor, which has been associated with increased vascular permeability, extracellular matrix degeneration, vascular endothelial cell migration, proliferation, and angiogenesis. What are the consequences of changes in choroidal structure during myopia formation, and how do they influence the development of myopia? These questions prompted us to investigate the changes in choroidal thickness, blood flow, and VEGFA expression in the choroid in myopia.
Materials and methods
Study population and experimental design
The current clinical study was approved by the Ethics Committee of Jinshan Hospital of Fudan University (IEC 2020-S41, JIEC 2022-S09) and followed the tenets of the Declaration of Helsinki, the patients and healthy subjects were collected at the Department of Ophthalmology, Jinshan Hospital Affiliated to Fudan University. Informed consent was obtained from all recruited individuals. A total of 137 people with no ocular diseases except myopia aged from 18 to 60 years were included in the study. The subjects were divided into three groups according to their spherical equivalent (SE): (1) emmetropia (EM) group: − 0.50D < SE ≤ + 0.50D; (2) non-high myopia (non-HM) group: − 6.00D < SE ≤ − 0.50D; and (3) high myopia (HM) group: SE ≤ -6.0 D and/or AL ≥ 26.5 mm. Subjects with the following diseases were excluded from this study: (1) previous history of ocular trauma or internal eye surgery; (2) pathological myopia (PM), which was defined as type 2 myopic macular disease, specifically diffuse chorioretinal atrophy, or the presence of posterior scleral staphyloma according to the META-PM study19,20; and (3) systemic diseases, such as diabetes and hypertension.
General eye examination of the subjects
Subjects underwent intraocular pressure (IOP), slit lamp and fundus examinations to exclude ocular diseases, such as glaucoma, uveitis, retinal vein obstruction and other ophthalmological diseases other than myopia.
Subjective refraction was used to detect diopter, and SE in diopters (D) was calculated as sphere plus half cylinder. AL, corneal radius ratio (CR), and anterior chamber depth (ACD) were measured by an IOL-Master 500. Vitreous chamber depth (VCD) was measured by A-scan ultrasound. The measurements of the right eye of all subjects were used for the study. To reduce the potential impact of circadian variation, relevant tests were conducted between 13:30 and 17:0021.
Measurement of choroidal thickness and choriocapillaris vessel density with OCTA
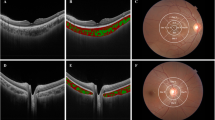
OCTA(Triton, Topcon, Tokyo, Japan) uses an internal fixation point to locate the fovea and papilla and measures the choroidal thickness (CT) and choriocapillaris vessel density. The radial scan mode was selected to measure the subfoveal choroidal thickness (SFCT). Using a 6 mm × 6 mm 3D Disc or Angio mode, the macula and papilla were divided into five subfields by Early Treatment Diabetic Retinopathy Study (ETDRS) grid overlay: central (foveal and papillary) (C), superior (S), inferior (I), temporal (T), and nasal (N). After OCTA selects the corresponding mode, the instrument automatically measures the length of the vertical line located within the choroidal area between two green curves in different regions (from Bruch’s membrane to the choroidal sclera interface), and records it as CT (Fig. S1).
The recorded peripapillary choroidal thicknesses included superior peripapillary choroidal thickness (pCTs), inferior peripapillary choroidal thickness (pCTi), temporal peripapillary choroidal thickness (pCTt) and nasal peripapillary choroidal thickness (pCTn).
The vessel density (VD) measurements included the macular and papillary choriocapillary layers, which were defined as an area extending from the Bruch membrane (BM) to 10.4 μm beneath the BM. The macular choriocapillaris vessel density area was divided into 5 parts: central macular choriocapillaris vessel density (MCVDc), superior macular choriocapillaris vessel density (MCVDs), inferior macular choriocapillaris vessel density (MCVDi), temporal macular choriocapillaris vessel density (MCVDt) and nasal macular choriocapillaris vessel density (MCVDn). The papillary choriocapillaris vessel density area was recorded as central peripapillary choriocapillaris vessel density (PCVDc), superior peripapillary choriocapillaris vessel density (PCVDs), inferior peripapillary choriocapillaris vessel density (PCVDi), temporal peripapillary choriocapillaris vessel density (PCVDt) and nasal peripapillary choriocapillaris vessel density (PCVDn). VD was corrected by the Bennett formula according to AL22. A signal strength intensity (SSI) < 40 and fake images were discarded.
Tear collection and detection of vascular endothelial growth factor A (VEGFA), vascular endothelial growth factor B (VEGFB), vascular endothelial growth factor C (VEGFC) and placental growth factor (PlGF) content
A capillary tear collector (Guangdong Shengze Kanghua Biomedical Co., Ltd., China) was used to collect nonirritating tears on the lateral margin of the lower eyelid. The amount of VEGFA (060752 V, Shanghai Enzyme-linked Biotechnology Co., Ltd., China), VEGFB (060753 V, Shanghai Enzyme-linked Biotechnology Co., Ltd., China), VEGFC (060754 V, Shanghai Enzyme-linked Biotechnology Co., Ltd., China), and PlGF (959663 V, Shanghai Enzyme-linked Biotechnology Co., Ltd., China) in tears was determined by an ELISA kit produced by Shanghai Enzyme Linked Biotechnology.
Animal grouping and experimental design
In this study, forty-five 2-week-old male trichromatic guinea pigs were purchased from Danyang Changyi Experimental Animal Breeding Co., Ltd. The Ethics Committee of the Shanghai Public Health Clinical Center (2022-A009-01) approved the study, which followed the ARVO Statement for the use of animals in ophthalmic and vision research. The experiment was divided into two parts. In the first part, 24 guinea pigs were randomly divided into a control group and a form deprivation myopia (FDM) group. The right eyes of the FDM group were covered with a translucent nontoxic latex for 4 weeks, whereas the control group did not receive any treatment. In the second part, 21 guinea pigs were randomly divided into three groups: the control group, FDM + vehicle group and FDM + conbercept group. The right eyes of the FDM + vehicle and FDM + conbercept groups were covered with a translucent nontoxic latex for 6 weeks, and in the 4th week after the onset of eye blockage, a 10 μl 0.9% sodium chloride or conbercept injection (Chengdu Kanghong Biotechnologies Co. Ltd. China) was administered to the FDM + vehicle group and FDM + conbercept group, respectively.
Measurement of SE and AL in guinea pigs
The eyes of the guinea pigs were dilated using tropicamide eye drops, and SE was assessed by streak retinoscopy (YZ24; 66 Vision Technology Co., Ltd., Suzhou, China). SE was measured 3 times, and the average value was accepted as the end result. AL was measured 10 times using A-scan ultrasound (OD1-AP; Kaixin Electronic Instrument Co., Ltd., Xuzhou, China) after all the guinea pigs were treated with anesthesia (0.5% oxybuprocaine hydrochloride), and the average value of AL was taken as the end result.
Intravitreal injection (IVT)
One day before the intravitreal injection, levofloxacin eye drops were used. Just before IVT injection, the guinea pigs were anesthetized with isoflurane, and the eye surface was disinfected with povidone-iodine. Subsequently, a microsyringe (Hamilton Microlitre™ syringe; Sigma–Aldrich, USA) was used to inject 0.9% sodium chloride or conbercept intravitreally behind the limbus, and levofloxacin eye drops were used for 3 days after injection.
Choroidal flatmounts
To observe the choroidal vessels, the guinea pigs were anesthetized intraperitoneally with an appropriate amount of pentobarbital. After heart perfusion with 4% paraformaldehyde, the eyeballs were dissected and fixed with 4% paraformaldehyde for one hour. Then, the optic nerve, sclera around the optic nerve, anterior segment, retina and residual sclera were removed under a microscope. The choroid was bleached with 3% H2O2, blocked, and incubated with α-SMA (anti-alpha smooth muscle actin) primary antibody (1:400, ab5694, Abcam, USA) at 4 °C overnight, followed by Alexa Fluor 594-labeled secondary antibody (1:200, Life Technologies, USA) before DAPI staining. After DAPI staining, the sample was observed and photographed under a confocal microscope. According to the method of Rust et al.23, the vascular area fraction was quantified using ImageJ.
Detection of VEGFA expression levels in the choroid
The expression of VEGFA in the choroid was detected by immunohistochemistry and Western blotting.
Immunohistochemistry The anesthesia and heart perfusion methods of guinea pigs were performed as described above. The eye was fixed with 4% paraformaldehyde and then embedded in paraffin. The choroid of guinea pigs underwent successive incubation with anti-VEGFA (1:200, PA1-16948, Thermo, USA) and the secondary antibody (1:5000, cat. no. 01334/10146, CoWin Biosciences, China). The sections of the choroid were observed under a light microscope after DAB staining.
Western blotting The animals were euthanized by intraperitoneal injection of sodium pentobarbital with an overdose of anesthesia. The choroid was isolated, and the total protein contained was extracted. After electrophoresis, membrane transfer, and sealing, the membrane was successively incubated with VEGFA antibody (1:1000, PA1-16948, Thermo, USA) and species-specific HRP-conjugated secondary antibodies (diluted 1:5000, CoWin Biosciences, USA). Finally, grayscale analysis was performed on the basis of chemiluminescence. GAPDH (1:1000, 2118S, Cell Signaling Technology, USA) served as an internal reference.
Statistics
Statistical analysis was performed using SPSS 25.0 software, and the results were recorded as the means ± SEs. Independent sample t tests or one-way ANOVA were selected for statistical analysis between groups. The correlation between VEGFA content and AL and between VEGFA content and SE was analyzed using Pearson analysis. P < 0.05 was considered significant.
Ethics approval and consent to participate
This study followed the tenets of the Declaration of Helsinki and was approved by the Ethical Committee of Jinshan Hospital of Fudan University (IEC 2020-S41, JIEC 2022-S09). The Ethics Committee of the Shanghai Public Health Clinical Center (2022-A009-01) approved the study, which followed the ARVO Statement for the use of animals in ophthalmic and vision research. All methods were performed in accordance with the ARRIVE guidelines. All methods were carried out in accordance with relevant guidelines and regulations.
Results
SE and ocular biometric parameters of EM, non-HM and HM groups
There were no statistically significant differences in gender composition, average age and intraocular pressure among the EM, Non HM, and HM groups (Table S1). As is shown in Fig. 1, the SE of the HM group was significantly lower than those of the EM and non-HM groups (P < 0.001) (Fig. 1A). Since axial myopia accounts for most cases of myopia, AL was measured in our study. The AL of the non-HM group was longer than that of the EM group, whereas the AL of the HM group was the longest among the three groups (P < 0.001) (Fig. 1B). However, only the ACD of the HM group was significantly higher than those of the EM and non-HM groups (P < 0.05) (Fig. 1C). As an important parameter for myopia, VCD was detected and displayed similar results: HM > non-HM > EM (P < 0.001) (Fig. 1D). Meanwhile, AL/CR (Fig. 1E) and VCD/AL (Fig. 1F) also showed similar tendencies, i.e., HM > non-HM > EM (P < 0.001).
Alterations in choroid thickness and choriocapillaris vessel density in the EM, non-HM and HM groups
Figure S1 was the choroidal thickness map detected by OCTA. The results obtained via OCTA (Fig. 2) showed that pCTs and pCTi of HM were significantly less than those of the non-HM and EM groups (P < 0.01). Moreover, the pCTt of the three groups displayed an HM < non-HM < EM pattern (P < 0.01). The pCTn of the HM group was also less than that of the EM group (P < 0.05). The SFCT of the HM group was significantly less than those of the non-HM and EM groups (P < 0.01).
Figure S2 was the choriocapillaris vessel density map detected by OCTA. Similar results were discovered in all macular measurements; that is, macular choriocapillaris vessel density in the HM group was significantly less than those in the non-HM and EM groups (P < 0.01) (Fig. 3A). On the other hand, as Fig. 3B shown: PCVDc in the HM group was lower than that in the EM group (P < 0.05). There was no significant difference in PCVDs among the three groups. PCVDi in the HM and non-HM groups was significantly lower than that in the EM group (P < 0.05). PCVDt and PCVDn in the HM group were lower than those in the non-HM and EM groups (P < 0.001).
Measurement of the choriocapillaris vessel density with OCTA. (A) The differences in MCVDc, MCVDs, MCVDi, MCVDt, and MCVDn among EM, Non-HM and HM groups. (B) The differences in PCVDc, PCVDs, PCVDi, PCVDt, and PCVDn among EM, Non-HM and HM groups. *p < 0.05, **p < 0.01, *** p < 0.001 compared with the EM group; ##p < 0.01, ###p < 0.001 compared with the Non-HM group.
Alterations in the levels of VEGFA, VEGFB, VEGFC and PlGF in the tears of the EM, non-HM and HM groups
The levels of VEGFA, VEGFB, VEGFC and PlGF were measured via ELISA. The demographic characteristics, VEGFA, VEGFB, VEGFC and PIGF of the EM, Non-HM and HM groups are presented in Table S2. As shown in Fig. 4A, VEGFA level demonstrated significant differences among the groups: HM < non-HM < EM (P < 0.001). There was no significant difference in the level of VEGFB among the three groups. The level of VEGFC in the HM group was lower than those in the EM and HM groups (P < 0.05). The level of PlGF in the EM group was higher than those in the HM and non-HM groups (P < 0.01). In addition, analysis of the correlation revealed a negative correlation between VEGFA levels and AL (Fig. 4B), whereas VEGFA levels were positively correlated with SE (Fig. 4C). Therefore, VEGFA levels may be related to the degree of myopia.
Tear concentration of VEGFA, VEGFB, VEGFC and PIGF and correlations between VEGFA levels and SE and AL. (A) The differences in tear concentration of VEGFA, VEGFB, VEGFC and PIGF among EM, Non-HM and HM groups. (B) Tear concentrations of VEGFA and the axial length. (C) Tear concentrations of VEGFA and Spherical equivalent. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the EM group; ##p < 0.01, ###p < 0.001 compared with the Non-HM group.
Alterations in SE and AL in the FDM guinea pig group
There were no significant differences in SE and AL between the control and FDM groups before form deprivation (0 w) (P > 0.05). However, 4 weeks after modeling, SE and AL of the Control and FDM groups presented in Table S3, SE in the FDM group was significantly lower than that in the control group (P < 0.001) (Fig. 5A), whereas AL in the FDM group was much longer than that in the control group (P < 0.001) (Fig. 5B). Moreover, the alterations in the SE and AL of the FDM group were greater than those of the control group (P < 0.001) (Fig. 5C,D).
Change in the choroidal vessel in the FDM group
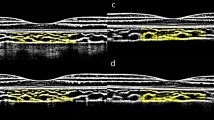
Choroidal sections dissected from the eyeballs of guinea pigs were labeled with α-SMA before being observed with a confocal microscope (Fig. 6A–F). Then, the vascular area fraction score was obtained via ImageJ, which was used to reflect the vascular density in the choroid. As shown in Fig. 6G, the vascular area fraction score in the FDM group was significantly less than that in the control group (p < 0.001), which suggested less vascular density in the choroid after onset of myopia in the FDM group.
Immunofluorescence image of choroidal vessels. (A, D) Red staining the choroidal vessels labeled by α-SMA; (B, E) Blue staining represents DAPI-stained choroidal vessels; (C, F) were the Merge images. (scale bar: 250 μm); (G) The differences in vascular area fraction in the control and FDM groups. ***p < 0.001 compared with the Control group.
Alterations in VEGFA expression in the FDM group
The expression of VEGFA in the choroid was assessed by immunohistochemistry (n = 3 per group) and Western blotting (n = 6 per group). As shown in Fig. 7A–D, VEGFA was expressed in the cytoplasm of endothelial cells in the choroidal vessels. Meanwhile, Western blotting was applied to detect alterations in the expression of VEGFA in the choroid, which revealed lower expression of VEGFA in FDM guinea pigs than in the control group (P < 0.01) (Fig. 7E ).
The effects of conbercept on SE, AL and choroidal vessels in the FDM group
After form deprivation for 4 w and 6 w, there were significant differences in SE, AL, and the change in SE and AL between the FDM and control groups (P < 0.001) (Fig. 8A–D). After form deprivation for 4 weeks, conbercept was injected intravitreally into the guinea pigs. Two weeks after the injection, it was observed that SE was significantly lower in the FDM + conbercept group than those in the control and FDM + vehicle groups (P < 0.001) (Fig. 8A), and AL was significantly longer in the FDM + conbercept group than those in the control and FDM + vehicle groups (P < 0.001) (Fig. 8B). Alterations in the changes in SE and AL in the FDM + conbercept group compared with the FDM + vehicle and control groups were also observed (P < 0.001) (Fig. 8C,D). On the other hand, the vascular area fraction score was shown in Table S7 and Fig. 8E, which represented the choroidal vascular density of the FDM + conbercept group was less than those of the other two groups, and the FDM + vehicle group showed less choroidal vascular density than the control group (P < 0.001) (Fig. 8F–N). Therefore, intravitreal injection of conbercept may aggravate myopia and reduce vascular density in the choroid.
SE and AL in the right eyes of the Control, FDM + Conbercept and FDM + Vehicle groups. The differences of SE (A) and AL (B) before, after 4 weeks and 6 weeks form-deprivation among the three groups; The differences in changes of SE (C) and AL (D) before, after 4 weeks and 6 weeks form-deprivation among the three groups; (E) The differences in vascular area fraction among the three groups; (F, I, L) Red staining represents the choroidal vessels labeled by α-SMA; (G, J, M) Blue staining represents DAPI-stained choroidal vessels; (H, K, N) were the Merge images (scale bar: 250 μm). **p < 0.01, ***p < 0.001 compared with the Control group; ###p < 0.001 compared with the FDM + Vehicle group.
Discussion
OCTA application makes it possible to perform a more refined observation of the choroid24,25. Del et al.5 observed 198 eyes of 121 children aged 3 to 16 years and discovered that myopic eyes showed a trend of SFCT thinning. Wei et al.26 studied 3468 adults and revealed that SFCT was decreased by 15 μm with every 1.0 D of myopia progression. Shen et al.27 found that the progression of myopia is closely related to SFCT. Deng et al.28 also found a trend of thinning pCT in 340 myopic children. Song et al.29 analyzed refractive and AL changes with pCT in 82 myopic patients and concluded that myopic refraction increases with a corresponding thinning of the pCT. Moreover, Jiang et al.30 also found that the pCT decreased by 5 μm with every 1.0 D increase in myopia in a study of 3468 subjects.Therefore, our results related to the change in choroidal thickness in HM patients are consistent with the results of most relevant studies, which indicates that myopia is closely associated with choroidal thickness changes and that choroidal thinning may be a pathological alteration of myopia11,15 and act as predictive biomarkers of myopia control efficacy31.
As the choroid is a highly vascularized tissue structure, blood vessels are one of the main components that make up the tissue32. Therefore, choroidal thickness is closely related to choroidal perfusion33,34. When Wu et al.11 used OCTA found that choroidal thinning in myopia was accompanied by a decrease in choroidal perfusion. Meanwhile, Gupta et al.35 observed choroidal thinning in myopia was associated with decreased choroidal vascularity. As expected, our present study discovered that choroidal thinning in patients with high myopia was accompanied by a significant decrease in blood flow density in the choroidal capillary layer. To further demonstrate that the reduction in blood flow density in the choroidal capillary layer in severe myopia is due to a reduction in the choroid, we observed choroidal flatmounts in FDM guinea pigs. As the guinea pig retina is completely avascular36, there are no retinal vessels to observe, and it naturally becomes a good choice for observing choroidal vessels. The choroidal vessels include three layers: the large, middle and capillary layers. Choroidal capillaries cannot be well labeled at present, but α-SMA is positive in the large and middle choroidal vessels37, which mainly affect the choroidal thickness, so we used α-SMA to label them. We found that the number of choroidal vessels was significantly lower in myopic guinea pigs. These results confirm that the reduced number of choroidal vessels in myopia is responsible for less vascular density in the choroidal layer, which in turn leads to thinning of the myopic choroid.
Senge et al.38 found that VEGFA is an important proangiogenic factor39, and its expression level influences blood flow in tissues40. Intraocular VEGFA is mainly derived from retinal pigment epithelium (RPE)41 and can promote choroidal neovascularization42. Moreover, Jonas43 and Hu et al.44 observed a significant decrease in VEGFA content in the aqueous humor of myopic eyes. In the present study, it was observed that the VEGFA content in the tears of highly myopic patients was significantly lower than that of patients in the EM group. Although it is still controversial whether the VEGFA content in the tears of myopic patients can accurately reflect the VEGFA levels in intraocular structures, including the retina and choroid, the change in VEGFA levels in the tears in the current study was used to reflect the change in VEGFA expression in the choroid after onset of myopia to some degree45,46,47,48,49. To demonstrate that the reduction in choroidal blood flow in patients with high myopia is caused by a decrease in VEGFA expression in the choroid, we replicated the FDM guinea pig model and observed a significant decrease in VEGFA expression in the choroid of myopic guinea pigs, and choroidal flatmounts also showed a significant decrease in the number of choroidal vessels. Since conbercept competes with the VEGF receptor for VEGFA, it was used as an antagonist against VEGFA in the current study. On this basis, we injected conbercept into the vitreous cavity of FDM guinea pigs to block VEGF receptors and found a significant decrease in myopic refraction and a significantly increased axial length accompanied by a further decrease in the number of choroid vessels. These results suggest that a decrease in choroidal VEGFA levels or blockage of its function may cause a decrease in choroidal vascularity and blood flow, which in turn may affect the development of myopia.
Why does VEGFA expression in the choroid decrease in the progression of myopia? We assume that it may be related to decreased numbers of RPE cells. On the one hand, it has been established that VEGF in the choroid is derived from RPE cells41 and that VEGF is also involved in the development and regulation of the choroidal vasculature50. Moreover, myopia development causes degenerative changes in RPE cells and results in a decrease in the number of RPE cells51,52. Therefore, when myopia develops, fewer RPE cells cause a decrease in choroidal VEGFA expression, leading to a decline in choroidal vascularity and blood flow, which in turn causes choroidal thinning. In addition, since RPE cells are supplied by choroidal blood vessels32, decreased choroidal blood flow in turn aggravates the degeneration of RPE cells, further promoting the development of myopia. Therefore, exploring the interrelationship between choroidal blood flow changes and altered VEGFA expression in the choroid after the onset of myopia may help further explain the progression of myopia and offer some novel ideas for the clinical treatment of myopia in the future. While, increasing choroidal blood flow may have a certain effect on myopia prevention and control53. On the other hand, in the clinic, conbercept is widely used in the treatment of choroidal neovascularization, diabetic retinopathy with macular edema and other diseases, and its efficacy has been confirmed. In our study, we found that conbercept may lead to the reduction of choroidal vessels by blocking VEGFA and lead to a higher degree of myopia.
Therefore, our study on the effect of VEGFA expression on the changes of choroidal blood vessels, blood flow and thickness in myopia will help to further understand the progression of myopia, which may also become a new target for the treatment of myopia in the future. we suggest that in clinical applications, we may also need to monitor the effect of conbercept on diopter.
Conclusions
In this study, the down-regulation of VEGFA expression may be an important reason for the reduction of choroidal blood flow density and choroidal thickness after myopia. Further research is needed to explore the specific mechanisms by which VEGFA influences myopic development, potentially providing new targets for clinical intervention.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Xiao, O. et al. Distribution and severity of myopic maculopathy among highly myopic eyes. Invest. Ophthalmol. Vis. Sci. 59, 4880–4885 (2018).
Holden, B. A. et al. Global Prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123, 1036–1042 (2016).
Morgan, I. G. et al. The epidemics of myopia: Aetiology and prevention. Prog. Retin. Eye Res. 62, 134–149 (2018).
Dolgin, E. The myopia boom. Nature 519, 276–278 (2015).
Del-Prado-Sanchez, C. et al. Choroidal, macular and ganglion cell layer thickness assessment in Caucasian children measured with spectral domain optical coherence tomography. Eur. J. Ophthalmol. 31, 3372–3378 (2021).
Xiong, S. et al. Changes in choroidal thickness varied by age and refraction in children and adolescents: A 1-year longitudinal study. Am. J. Ophthalmol. 213, 46–56 (2020).
Jin, P. et al. Longitudinal changes in choroidal and retinal thicknesses in children with myopic shift. Retina 39, 1091–1099 (2019).
Xiuyan, Z. et al. Thickness, vessel density of retina and choroid on OCTA in young adults (18–24 years old). Microvasc. Res. 136, 104169 (2021).
Wang, X. Q., Zeng, L. Z., Chen, M. & Liu, L. Q. A meta-analysis of alterations in the retina and choroid in high myopia assessed by optical coherence tomography angiography. Ophthalmic. Res. 64, 928–937 (2021).
Pan, M. et al. PPARgamma modulates refractive development and form deprivation myopia in Guinea pigs. Exp. Eye Res. 202, 108332 (2021).
Wu, H. et al. Assessment of choroidal vascularity and choriocapillaris blood perfusion in anisomyopic adults by SS-OCT/OCTA. Invest. Ophthalmol. Vis. Sci. 62, 8 (2021).
Corvi, F., Su, L. & Sadda, S. R. Evaluation of the inner choroid using OCT angiography. Eye (Lond.) 35, 110–120 (2021).
Fernandez-Vigo, J. I. et al. Reproducibility of macular and optic nerve head vessel density measurements by swept-source optical coherence tomography angiography. Eur. J. Ophthalmol. 1120672119834472 (2019).
Borrelli, E., Sarraf, D., Freund, K. B. & Sadda, S. R. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog. Retin. Eye Res. 67, 30–55 (2018).
Moon, J. Y. et al. Wide-field swept-source optical coherence tomography angiography in the assessment of retinal microvasculature and choroidal thickness in patients with myopia. Br. J. Ophthalmol. (2021).
Liu, X. et al. Choroidal thickness and choriocapillaris vascular density in myopic anisometropia. Eye Vis. (Lond.) 8, 48 (2021).
Bill, A., Sperber, G. & Ujiie, K. Physiology of the choroidal vascular bed. Int. Ophthalmol. 6, 101–107 (1983).
Summers, J. A. The choroid as a sclera growth regulator. Exp. Eye Res. 114, 120–127 (2013).
Ohno-Matsui, K. et al. International photographic classification and grading system for myopic maculopathy. Am. J. Ophthalmol. 159, 877–883 (2015).
Ohno-Matsui, K., Lai, T. Y., Lai, C. C. & Cheung, C. M. Updates of pathologic myopia. Prog. Retin. Eye Res. 52, 156–187 (2016).
Lin, E. et al. Are choriocapillaris flow void features robust to diurnal variations? A swept-source optical coherence tomography angiography (OCTA) study. Sci. Rep. 10, 11249 (2020).
Bennett, A. G., Rudnicka, A. R. & Edgar, D. F. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefes Arch. Clin. Exp. Ophthalmol. 232, 361–367 (1994).
Rust, R. et al. A practical guide to the automated analysis of vascular growth, maturation and injury in the Brain. Front. Neurosci. 14, 244 (2020).
Castellino, N. et al. Morphological parameters of myopic choroidal neovascularization as predictive factors of anti-VEGF treatment response. Sci. Rep. 12, 10435 (2022).
Wang, J. C. & Miller, J. B. Optical coherence tomography angiography: Review of current technical aspects and applications in chorioretinal disease. Semin. Ophthalmol. 34, 211–217 (2019).
Wei, W. B. et al. Subfoveal choroidal thickness: The Beijing eye study. Ophthalmology 120, 175–180 (2013).
Shen, M. et al. Longitudinal changes in choroidal thickness varied with refractive progression in myopic and non-myopic children: A two-year cohort study. Invest. Ophthalmol. Vis. Sci. 65, 17 (2024).
Deng, J. et al. Distribution pattern of choroidal thickness at the posterior pole in chinese children with myopia. Invest. Ophthalmol. Vis. Sci. 59, 1577–1586 (2018).
Song, A., Hou, X., Zhuo, J. & Yu, T. Peripapillary choroidal thickness in eyes with high myopia. J. Int. Med. Res. 48, 300060520917273 (2020).
Jiang, R., Wang, Y. X., Wei, W. B., Xu, L. & Jonas, J. B. Peripapillary choroidal thickness in adult chinese: The Beijing eye study. Invest. Ophthalmol. Vis. Sci. 56, 4045–4052 (2015).
Wu, H. et al. Choroidal vasculature act as predictive biomarkers of long-term ocular elongation in myopic children treated with orthokeratology: A prospective cohort study. Eye Vis. (Lond.) 10, 27 (2023).
Puyo, L., Paques, M., Fink, M., Sahel, J. A. & Atlan, M. Choroidal vasculature imaging with laser Doppler holography. Biomed. Opt. Express 10, 995–1012 (2019).
Nickla, D. L. & Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 29, 144–168 (2010).
Roskal-Walek, J., Laudanska-Olszewska, I., Biskup, M., Gierada, M. & Odrobina, D. Choroidal thickness in women with uncomplicated pregnancy: Literature review. Biomed. Res. Int. 2017, 5694235 (2017).
Gupta, P. et al. Characterization of choroidal morphologic and vascular features in young men with high myopia using spectral-domain optical coherence tomography. Am. J. Ophthalmol. 177, 27–33 (2017).
Yu, D. Y. & Cringle, S. J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 20, 175–208 (2001).
Haddad, A., Laicine, E. M., Tripathi, B. J. & Tripathi, R. C. An extensive system of extravascular smooth muscle cells exists in the choroid of the rabbit eye. Exp. Eye Res. 73, 345–353 (2001).
Senger, D. R. et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985 (1983).
Melincovici, C. S. et al. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 59, 455–467 (2018).
Shibata, Y. et al. Direct regulation of prostate blood flow by vascular endothelial growth factor and its participation in the androgenic regulation of prostate blood flow in vivo. Endocrinology 145, 4507–4512 (2004).
Klettner, A., Westhues, D., Lassen, J., Bartsch, S. & Roider, J. Regulation of constitutive vascular endothelial growth factor secretion in retinal pigment epithelium/choroid organ cultures: p38, nuclear factor kappaB, and the vascular endothelial growth factor receptor-2/phosphatidylinositol 3 kinase pathway. Mol. Vis. 19, 281–291 (2013).
Yi, X. et al. Vascular endothelial growth factor expression in choroidal neovascularization in rats. Graefes Arch. Clin. Exp. Ophthalmol. 235, 313–319 (1997).
Jonas, J. B., Tao, Y., Neumaier, M. & Findeisen, P. VEGF and refractive error. Ophthalmology 117, 2234 (2010).
Hu, Q. et al. Intravitreal vascular endothelial growth factor concentration and axial length. Retina 35, 435–439 (2015).
She, M., Li, T., Shi, W., Li, B. & Zhou, X. AREG is involved in scleral remodeling in form-deprivation myopia via the ERK1/2-MMP-2 pathway. FASEB J 36, e22289 (2022).
Hagan, S., Martin, E. & Enriquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: Potential use for predictive, preventive and personalised medicine. EPMA J. 7, 15 (2016).
Ang, W. J. et al. Evaluation of vascular endothelial growth factor levels in tears and serum among diabetic patients. PLoS One 14, e0221481 (2019).
Sun, L., Liu, X. & Zuo, Z. Regulatory role of miRNA-23a in diabetic retinopathy. Exp. Ther. Med. 22, 1477 (2021).
Waszczykowska, A. et al. The role of angiogenesis factors in the formation of vascular changes in scleroderma by assessment of the concentrations of VEGF and sVEGFR2 in blood serum and tear fluid. Mediat. Inflamm. 2020, 7649480 (2020).
Marneros, A. G. et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am. J. Pathol. 167, 1451–1459 (2005).
Bai, H. X. et al. Bruch s membrane thickness in relationship to axial length. PLoS One 12, e0182080 (2017).
Jonas, J. B., Ohno-Matsui, K., Holbach, L. & Panda-Jonas, S. Retinal pigment epithelium cell density in relationship to axial length in human eyes. Acta Ophthalmol. 95, e22–e28 (2017).
Jeong, H. et al. Topical application of Bunazosin hydrochloride suppresses myopia progression with an increase in choroidal blood perfusion. Invest. Ophthalmol. Vis. Sci. 64, 15 (2023).
Wan, T., Shi, W. Q., Liang, R. B., Li, T., Li, B. & Zhou, X. D. VEGFA may be a potential marker of myopic choroidal thickness and vascular density changes. preprint, https://doi.org/10.21203/rs.3.rs-2349925/v1.
Acknowledgements
A preprint has previously been published54.
Funding
This work was supported by Project of Shanghai Shenkang Hospital Development Center, Grant/Award Number: SHDC2020CR1043B-004 and Project of Shanghai Jinshan District Health Commission, Grant/Award Number: JSKJ-KTQN-2021-02.
Author information
Authors and Affiliations
Contributions
T.W. wrote the main manuscript text and have made the acquisition, analysis, and interpretation of data, W.S. have made the analysis of data, R.L. collected the date, T.L. have revised the manuscript, B.L. and X.Z. have made substantial contributions to the design of the work and revised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wan, T., Shi, W., Liang, R. et al. VEGFA may be a potential marker of myopic choroidal thickness and vascular density changes. Sci Rep 14, 20514 (2024). https://doi.org/10.1038/s41598-024-70616-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70616-y