Abstract
During the last decades, effective pain reduction and early mobilization were identified as the central priorities in therapy of insufficiency fractures of the pelvis. For operative treatment minimally-invasive stabilization techniques are favored. While there is consensus on the significance of sufficient dorsal stabilization the role of additional fixation of the anterior fracture component stays under discussion. Within the present study we developed an internal ring fixator system (RingFix) with the question whether an in-itself-closed construct can improve stability of the entire ring structure. RingFix was evaluated on an osteoporotic bone model with a standardized FFP IIIc fracture within an established biomechanical setup regarding its primary stabilization potential. Further, it was compared to transiliac–transsacral screw fixation with and without stabilization of the anterior fracture component. The transiliac–transsacral fixation with separate screw fixation of the anterior fracture showed significantly higher stability than the RingFix and the transiliac–transsacral screw fixation without anterior stabilization. Our results show that stabilization of the anterior fracture component relevantly improves the stability of the entire ring construct. As a bridging stabilizer, RingFix shows biomechanical advantages over an isolated dorsal fracture fixation, but inferior results than direct stabilization of the single fracture components.
Similar content being viewed by others
Introduction
The observed increasing incidence of fragility fractures of the pelvis (FFP) led to a special interest in these pathologies over the last decade and opened a wide field of research1,2. With the successful implementation of specific classification systems, treatment recommendations and algorithms were contrived3,4,5. The central objective in treatment of affected, typically frail patients is a sufficient pain control to allow for early remobilization. Analgesic therapy and physiotherapeutic mobilization is regarded as the primary therapy in isolated anterior (FFP I) and non-displaced posterior fractures (FFP II)3. In patients with posteriorly displaced fractures (FFP III and FFP IV) or with prolonged pain and thereof immobilization, surgical treatment is recommended6. The objective of surgical intervention in those patients is not an anatomical reduction but a stable in-situ fixation to achieve sufficient pain control and to allow for immediate postoperative remobilization of the patient. Further, the surgical therapy should be as minimally-invasive as possible to minimize surgically-related complications in this fragile patient group with commonly reduced bone quality. While there is consensus about the necessity of a stable fixation of the posterior pelvic ring, discussion remains controversial about indications and methods for stabilization of the anterior fracture part.
For minimal-invasive stabilization of the posterior pelvic ring in FFP, different percutaneous (trans-) iliosacral7,8,9,10,11,12,13 and transiliac internal fixation techniques14,15 are described. The anterior fracture component is often left without fixation2,16. However, the placement of an internal17,18 or external fixator19, percutaneous screw fixation of the superior pubic ramus16,20,21, or open reduction and plate osteosynthesis22 are common methods used for surgical stabilization of anterior fracture components with overall good results reported but also a relevant number of implant related complications like implant loosening or backing out of screws22,23. Within a biomechanical study on an FFP Type IIIc fracture in an osteoporotic pelvic bone model we could show, that the stabilization of the anterior fracture component in addition to a trans-iliosacral posterior fixation enhances the stability of the entire ring construct significantly16. These results raised the question whether an in-itself-closed ring fixation construct augmenting the pelvic ring might provide higher stability than a separate in-situ fixation of the single fracture components.
Thus, within the present pilot study, we built up an in-itself-closed internal ring fixator for the pelvic ring fixation (RingFix) and evaluated the stability of the RingFix on an FFP Type IIIc fracture in an osteoporotic pelvic bone model within a standardized biomechanical test setup in comparison to transiliac–transsacral screw fixation as an established fixation method with and without fixation of the anterior fracture component.
Material and methods
The test setup and the utilized bone models were similar as in a biomechanical study on FFP Type IIIc fractures recently published by our group16.
For the actual study, 24 explicit osteoporotic synthetic bone models (‘Sawbone Type 1301-1’, Fa. Sawbones, Sawbones Europe AB Servicing Europe, Middle East, and Africa, Malmö, Sweden) were used and subdivided into three groups with different fixation methods.
To simulate a standardized FFP Type IIIc fracture, in all bone models a complete fracture of the sacral ala was generated on the left side lateral to the neuroforamina using a 3D printed sawing template. A standardized vertical-oblique fracture of the superior and inferior pubic ramus was generated also on the left side in the area of the obturator foramen, consistent with a Nakatani II fracture23. In all samples a 3 mm thick felt was placed into the fracture gap to simulate a partially unstable fracture.
To achieve optimum comparability standardized position of the implants was achieved by using customized 3D printed drilling templates which were calculated previously on the CAD data set of the bone model. Eight of the bone models received a trans-iliosacral screw fixation with a cannulated 7.3 mm semi-threaded trans-iliosacral screw (140/32 mm) with washer in S1 and a shorter, oblique 7.3 mm fully-threaded iliosacral screw with washer in S1 ending up in the area of the promontory. Further eight bone models received the same stabilization with an additional cannulated 7.3 mm semi-threaded retrograde transpubic screw for fixation of the anterior pelvic ring.
The remaining eight bone models were stabilized with the RingFix. RingFix was built up on two 5.0 mm Steinmann pins with central thread introduced slightly lateral to the anterior inferior iliac spine, passing the ilium completely intraosseous and ending up after passing the posterior cortex of the ilium in the area of the posterior superior iliac spine. Right and left Steinmann pins were connected posteriorly over a pre-bent 5.0 mm rod as used for dorsal instrumentation in spine surgery and the specific connector clamps for Steinmann pin-rod-connection of the USS II system of DePuy Synthes (USS Universal Spine System, DePuy Synthes, Johnson & Johnson, New Brunswick, New Jersey, USA) (Fig. 1).
After posterior connection, reduction of the anterior fracture part was performed by connecting the anterior ends of the Steinmann pins via another pre-bent 5.0 mm rod and clamps under slight adduction force. The posterior and anterior rods are meant to be located subcutaneously, pins could be introduced in anterior–posterior direction as performed in the lab as well as in posterior-anterior way.
As all peripelvic ligaments are missing within this model, an additive iliosacral screw was used within the RingFix group to compensate the lack of the dorsal ligamentous apparatus and achieve a comparable rotational stability in all three groups. For this propose an additional short oblique 7.3 mm fully-threaded iliosacral screw with washer in S1 ending up in the area of the promontory was introduced following the same screw corridor as in the two other groups.
Groups were named as: trans-iliosacral and iliosacral screw with anterior fixation (SI +), trans-iliosacral and iliosacral screw without anterior fixation (SI−); ring fixator with iliosacral screw (RingFix).
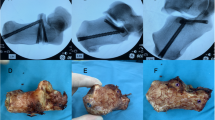
All bones were checked radiographically in ap, inlet and outlet view for correct implant positioning after stabilization (Fig. 2).
Testing was performed on a servo-pneumatic test machine (Fa. SincoTec, Clausthal-Zellerfeld, Germany). The bones were attached to the lever arm of the test machine over a small PMMA block screwed to the endplate of S1 simulating the loading transmission from the lumbar spine to the sacrum, and set on to two fixed bipolar hip protheses building up a counterfort, simulating a standing position with a pelvic tilt of about 15°. In this physiological position axial loading could be performed with a minimum of shear forces. Further details of the test setup are described in a previous study of our group16. Figure 3 shows a picture of the test setup with a SI + specimen attached to the test machine.
As the pelvic ring is loaded with up to two to three times the body weight during standing and normal walking force-controlled axial loading was performed with a pre-load of 50 N followed by cyclic loading with 50–1200 N for a total of 1000 test cycles with a frequency of 0.05 Hz, in order to simulate a two-legged stance24,25. The translation of the lever arm that was needed to generate the demanded forces was measured. Testing was interrupted and finished early in case of breakdown of the bone, or if the measured translation of the level arm exceeded 25 mm according to the existing test protocol16. The number of cyclic axial loading at failure was documented for each pelvic model. Optical tracking of fracture sites was performed separately for the anterior and the dorsal fracture component using a camera system and the Software Simi Motion (Simi Motion 2D/3D, Fa. Simi Reality Motion Systems GmbH, Unterschleißheim, Germany, www.simi.com) observing movements on the fracture sites based on in total eight optical markers. Statistical evaluation was performed using GraphPad Prism (Prism 9.5.1, GraphPad Software, 225 Franklin Street. Fl. 26, Boston, MA 02110, USA, www.graphpad.com) applying Mann–Whitney-U test and Kruskal–Wallis test.
Results
Only two of the 24 bones finished 1000 axial loading cycles according to the protocol. In the SI + group, two of eight bones finished the test. In the SI− and in the RingFix group none of the bones finished the total of 1000 loading cycles. The SI + group showed the highest stability (median of reached cycles 862.5), followed by the RingFix group (median of reached cycles 349.0) and the SI− (median of reached cycles 41.0). Table 1 shows the reached number of loading cycles at failure, Fig. 4 gives a graphic overview on the number of reached loading cycles.
Optical measurements showed a heterogenic pattern throughout the testing. While within the SI + group a rather consistent movement at the fracture sites was observed, the SI− and RingFix group showed an increasing movement at the fracture gap especially of the pubic fracture. Figure 5 illustrates the median measured movements at the fracture site separately for the sacral and the pubic fracture.
Observed interfragmentary movement at the fracture sites under cyclic loading throughout the testing. Interfragmentary movement was observed to be significantly less within the SI + group compared to SI− and RingFix o both fractures sites (SI + vs. SI− p < 0.001; SI + vs. RingFix p < 0.001; SI− vs. RingFix p = 0.7263).
As within the SI− and RingFix group none of the specimen finished the test according to the protocol the respective curve ends early.
Furthermore, we observed new sacral fractures on the contralateral side of the initial fracture. Those new fractures occurred more frequently and earlier during testing in the bone models without anterior fixation.
Discussion
In this study we built up an in-itself closed ring fixator (RingFix) for internal stabilization of pelvic ring fractures. To explore its mechanical properties and therapeutic potential, we performed a biomechanical evaluation of the RingFix on a standardized an FFP Type IIIc fracture in an explicit osteoporotic sawbone model. We observed a significantly lower stability after bridging fixation with the in-itself closed internal pelvic ring fixator (RingFix) than after direct stabilization of all individual fracture components, but a significantly higher stability than after intra-osseous trans-sacral fixation without fixation of the anterior fracture component. Especially at the pubic fracture gap more and increasing movement over time was observed within the groups without direct anterior fracture fixation. The results show that the RingFix system has no advantages as a stand-alone implant for the fixation of FFP Type IIIc fractures or fracture types with a higher instability over existing fixation methods, but point out again the impact of an anterior fixation to the hole construct’s stability.
Discussions on when and how to operate and the question whether both the posterior and anterior pelvic ring should be stabilized are still open and a standardized treatment protocol for FFP is lacking. There is a broad consensus that adequate pain control is crucial in all fracture types to allow for early mobilization of the patient and thereby preventing longer or permanent immobility with its associated complications. There is also consensus on the role of surgical treatment for mechanical stabilization of unstable fracture types as well as for extended pain therapy and pain control. Specific classification systems for osteoporotic and insufficiency fractures of the pelvic ring, such as the FFP classification by Rommens et Hofmann3 and the OF classification following the corresponding classification system for vertebral body fractures5, have led to an enhanced understanding of these fractures and to specific recommendations for an adequate treatment in relation to the fracture type26. The stabilization of the posterior pelvic ring is accepted as the primary goal in the operative treatment of FFP. Different minimally-invasive alternatives are available. As the most common method different intra-osseous sacroiliac and transiliac–transsacral fixation techniques are described1,2,7,8,9,10,12. Cintean et al. showed within a biomechanical study a significantly reduced interfragmentary movement in FFP Type IIc fractures using a transiliac–transsacral fixation method in comparison with unilateral fixation27. Further, Dienstknecht et al. did not find a significant difference in stability between iliosacral screw fixation and transiliac internal fixation (TIFI) of a complete iliosacral separation28. El-Hamalawy et al. did not find a difference in clinical outcome between intra- and extra-osseous sacral fixation of posterior pelvic ring injuries in a young patient cohort29. Within a finite element analysis Salasek et al. performed a biomechanical comparison of a transiliac internal fixator and two iliosacral screws for treatment of transforaminal sacral fractures and found significantly higher stiffness and lower stress in the group of the TIFI model30.
From this data, we may conclude that intra- and extra-osseous bridging fixation of posterior pelvic ring fractures can be considered equivalent. TIFI may be the technique of choice in patients with a dysmorphic sacrum, in which a safe trans-sacral corridor is not available31,32.
The necessity of stabilization of the anterior fracture part remains controversial. Several studies report about good clinical and radiological results after posterior without additional anterior stabilization2,33. In their biomechanical study, Osterhoff et al. emphasized the role of the pectineal ligament as a relevant secondary stabilizer of the anterior pelvic ring34 which might be a reason why conservative treatment of anterior pelvic ring fractures is successful in fractures without relevant dislocation and thereof still intact ligaments. Nevertheless, it remains unclear how the integrity of the anterior ligaments can be ensured in clinical practice.
On the other hand, several authors report about good treatment results and major pain control addressing the anterior fracture component as well. Several minimally invasive techniques are in practice. The supraacetabular external fixator uses the anterior part of the supraacetabular corridor for the placement of its Steinmann pins over small incisions. However, a relatively high complication rate is associated with this method including pin track infection, wound breakdown, and difficult mobilization of the patient due to extensive hardware19,20,35. Internal fixator (INFIX) systems consisting of a subcutaneous, curved rod connecting the supra-acetabular fixator pins or screws were developed to provide comparable stability, and to avoid the complications of the external fixator. In addition, Hack et al. showed within a biomechanical study that there was less plastic deformation and higher stiffness found in the internal fixator group as compared to an external fixator group36. However, literature data also reveal that the internal fixator is associated with a significant complication rate including periarticular ossification, and damage to the femoral vessels and the femoral and lateral femoral cutaneous nerves17,18,37,38. Percutaneous screw fixation of the anterior pelvic ring in antegrade or retrograde direction is another fixation technique. It is described as a sufficient and minimally invasive fixation method for fractures without relevant displacement20,39. Even for displaced fractures, retrograde percutaneous screw fixation is described to be possible using a special manoeuvre21. Nevertheless, the insertion of a straight 7.3 mm screw is only possible in half of the trans-pubic corridors40. Fractures with a relevant displacement or very near to the pubic symphysis are reduced and fixed with open reduction and plate osteosynthesis. In their retrospective analysis of a total of 48 patients with FFP, Herteleer et al. described a high rate of screw loosening with loss of stability22.
The objective of the present study was the development and mechanical evaluation of an in-itself closed ring fixator system (RingFix) for internal stabilization of FFP. The RingFix can be regarded as a merger of the TIFI and the INFIX. It closes the broken pelvic ring indirectly and independent of the fracture location. Fixation of both the posterior and anterior pelvic fracture components is achieved without a direct in-situ stabilization of the fractures. The results of this biomechanical study show that an in-situ stabilization of the single fracture components (SI + group) is significantly superior to an indirect (RingFix group) or incomplete (SI− group) fixation.
Thinking of a potential use in clinical practice there are some further concerns to mention. The insertion of the RingFix would require to change the patient’s position from prone to supine at least once during the surgical procedure which might prolong the surgical procedure relevantly. Further, the reported problems and observed complications of the INFIX involving the femoral vessels and nerves at risk would remain as well as soft tissue complications like potential skin irritation and ulceration18,37,38.
We conclude that the RingFix cannot be used as a stand-alone implant for the stabilization of an unstable FFP type with a posterior and anterior fracture component as it does not provide any advantages over direct fracture fixation. It might have a role as an additive fixation tool in cases of very poor bone quality or bone defects and could be considered as an option in special situations of traumatic pelvic ring fractures with bone defect or extended soft tissue damage.
There are several limitations of the study to state. All tests were performed on synthetic bone models. Even if a specific osteoporotic bone model was used, the artificial bone model does not fully simulate the individual physiological variability of bone mineral density within the pelvic bone. Further, secondary stabilizers such as muscles and especially strong peripelvic ligaments like the pectineal ligament and the iliolumbar ligaments are missing in this model. For this, an additive iliosacral screw was used within the RingFix group to compensate the lack of the dorsal ligamentous apparatus and achieve a comparable rotational stability in all three groups. As the peripelvic ligaments are missing within our model, construct stability might be underestimated in general within this study. Another limitation to mention is that in our test setup an upright stand with only symmetric axial loading was simulated. Tensile forces and asymmetric loading as they occur during walking were not reproduced.
Conclusion
Our observations suggest that:
-
(1)
An in-itself closed internal ring fixator construct (RingFix) does not show any advantages over in-situ stabilization of the posterior and anterior fracture components of an unstable FFP type.
-
(2)
Fixation of the anterior fracture significantly improves the stability of the entire pelvic ring construct.
Data availability
Raw data were generated at University Medical Center of Johannes Gutenberg University Mainz, Germany. Derived data supporting the findings of this study are available from the corresponding author [C.A.] on request.
References
Heiman, E. et al. Fragility fractures of the pelvis and sacrum: Current trends in literature. Hip Pelvis 34(2), 69–78 (2022).
Wilson, D. G. G., Kelly, J. & Rickman, M. Operative management of fragility fractures of the pelvis - a systematic review. BMC Musculoskel. Disord. 22(1), 717 (2021).
Rommens, P. M. & Hofmann, A. Comprehensive classification of fragility fractures of the pelvic ring: Recommendations for surgical treatment. Injury 44(12), 1733–1744 (2013).
Krappinger, D., Kaser, V., Merkel, A., Neururer, S. & Lindtner, R. A. An alphanumeric classification of osteoporotic pelvic ring injuries. Arch. Orthop. Trauma Surg. 141(5), 861–869 (2021).
Ullrich, B. W. et al. OF-Pelvis classification of osteoporotic sacral and pelvic ring fractures. BMC Musculoskel. Disord. 22(1), 992 (2021).
Rommens, P. M. et al. Prospective assessment of key factors influencing treatment strategy and outcome of fragility fractures of the pelvis (FFP). Eur. J. Trauma Emerg. Surg. 48(4), 3243–3256 (2022).
van Zwienen, C. M., van den Bosch, E. W., Snijders, C. J., Kleinrensink, G. J. & van Vugt, A. B. Biomechanical comparison of sacroiliac screw techniques for unstable pelvic ring fractures. J. Orthop. Trauma 18(9), 589–595 (2004).
Hartensuer, R. et al. Safety, effect and feasibility of percutaneous SI-screw with and without augmentation-a 15-year retrospective analysis on over 640 screws. J. Clin. Med. 9(8), 2660 (2020).
Zderic, I. et al. Screw-in-screw fixation of fragility sacrum fractures provides high stability without loosening-biomechanical evaluation of a new concept. J. Orthop. Res. 39(4), 761–770 (2021).
Mehling, I., Hessmann, M. H. & Rommens, P. M. Stabilization of fatigue fractures of the dorsal pelvis with a trans-sacral bar. Operative technique and outcome. Injury 43(4), 446–451 (2012).
Sciubba, D. M. et al. CT fluoroscopically guided percutaneous placement of transiliosacral rod for sacral insufficiency fracture: Case report and technique. AJNR Am. J. Neuroradiol. 28(8), 1451–1454 (2007).
Wagner, D. et al. Trans-sacral bar osteosynthesis provides low mortality and high mobility in patients with fragility fractures of the pelvis. Sci. Rep. 11(1), 14201 (2021).
Rommens, P. M. et al. Minimally invasive stabilization of fragility fractures of the pelvis with transsacral bar and retrograde transpubic screw. Oper. Orthop. Traumatol. 34(2), 153–171 (2022).
Rommens, P. M. et al. Fragility fractures of the pelvis. Unfallchirurg. 122(6), 469–482 (2019).
Muller, F. & Fuchtmeier, B. A systematic review of the transiliac internal fixator (TIFI) for posterior pelvic injuries. SICOT J. 7, 40 (2021).
Arand, C. et al. Do we need to fix the anterior fracture component in insufficiency fractures of the pelvis? A biomechanical comparison on an FFP type IIIc fracture in an osteoporotic pelvic bone model. Injury 54, 111096 (2023).
Yin, Y. et al. Anterior subcutaneous internal fixator (INFIX) versus plate fixation for pelvic anterior ring fracture. Sci. Rep. 9(1), 2578 (2019).
Apivatthakakul, T. & Rujiwattanapong, N. Anterior subcutaneous pelvic internal fixator (INFIX), Is it safe? A cadaveric study. Injury 47(10), 2077–2080 (2016).
Schuetze, K. et al. Short-term outcome of fragility fractures of the pelvis in the elderly treated with screw osteosynthesis and external fixator. Eur. J. Trauma Emerg. Surg. 48(3), 2413–2420 (2022).
Rommens, P. M. et al. Minimal-invasive stabilization of anterior pelvic ring fractures with retrograde transpubic screws. Injury 51(2), 340–346. https://doi.org/10.1016/j.injury.2019.12.018 (2020).
Mosheiff, R. & Liebergall, M. Maneuvering the retrograde medullary screw in pubic ramus fractures. J. Orthop. Trauma 16(8), 594–596 (2002).
Herteleer, M., Boudissa, M., Hofmann, A., Wagner, D. & Rommens, P. M. Plate fixation of the anterior pelvic ring in patients with fragility fractures of the pelvis. Eur. J. Trauma Emerg. Surg. 48(5), 3711–3719 (2022).
Starr, A. J., Nakatani, T., Reinert, C. M. & Cederberg, K. Superior pubic ramus fractures fixed with percutaneous screws: What predicts fixation failure?. J. Orthop. Trauma 22(2), 81–87 (2008).
Dalstra, M. & Huiskes, R. Load transfer across the pelvic bone. J. Biomech. 28(6), 715–724 (1995).
Kramers-de Quervain, I. A. Ganganalyse beim Gehen und Laufen. Schweiz. Z. Sportmed. Sporttraumatol. 56(2), 35–42 (2008).
Rommens, P. M. et al. Clinical pathways for fragility fractures of the pelvic ring: Personal experience and review of the literature. J. Orthop. Sci. 20(1), 1–11 (2015).
Cintean, R. et al. Sacroiliac versus transiliac–transsacral screw osteosynthesis in osteoporotic pelvic fractures: A biomechanical comparison. Eur. J. Trauma Emerg. Surg. 49(6), 2553–2560. https://doi.org/10.1007/s00068-023-02341-6 (2023).
Dienstknecht, T. et al. Biomechanical analysis of a transiliac internal fixator. Int. Orthop. 35(12), 1863–1868 (2011).
El-Hamalawy, A. G., Karim, M. A., Khaled, S. A., Khaled Fawzy, M. & Abdel-Kader, E. K. Minimally invasive stabilization of posterior pelvic ring injuries through transiliac internal fixator versus Iliosacral screw: A prospective comparative cohort study. Injury 54(6), 1677–1686. https://doi.org/10.1016/j.injury.2023.02.042 (2023).
Salasek, M., Jansova, M., Kren, J., Pavelka, T. & Weisova, D. Biomechanical comparison of a transiliac internal fixator and two iliosacral screws in transforaminal sacral fractures: A finite element analysis. Acta Bioeng. Biomech. 17(1), 39–49 (2015).
Wagner, D. et al. Space available for trans-sacral implants to treat fractures of the pelvis assessed by virtual implant positioning. Arch. Orthop. Trauma Surg. 139(10), 1385–1391 (2019).
Wagner, D. et al. Morphometry of the sacrum and its implication on trans-sacral corridors using a computed tomography data-based three-dimensional statistical model. Spine J. 17(8), 1141–1147 (2017).
Hopf, J. C., Krieglstein, C. F., Muller, L. P. & Koslowsky, T. C. Percutaneous iliosacral screw fixation after osteoporotic posterior ring fractures of the pelvis reduces pain significantly in elderly patients. Injury 46(8), 1631–1636 (2015).
Osterhoff, G. et al. The pectineal ligament is a secondary stabilizer in anterior pelvic ring fractures - a biomechanical study. Injury 53(2), 334–338 (2022).
Palmer, S., Fairbank, A. C. & Bircher, M. Surgical complications and implications of external fixation of pelvic fractures. Injury 28(9–10), 649–653 (1997).
Hack, J. et al. Stability of internal versus external fixation in osteoporotic pelvic fractures - a biomechanical analysis. Injury 51(11), 2460–2464 (2020).
Fang, C., Alabdulrahman, H. & Pape, H. C. Complications after percutaneous internal fixator for anterior pelvic ring injuries. Int. Orthop. 41(9), 1785–1790 (2017).
Hesse, D. et al. Femoral nerve palsy after pelvic fracture treated with INFIX: A case series. J. Orthop. Trauma 29(3), 138–143 (2015).
Arduini, M., Saturnino, L., Piperno, A., Iundusi, R. & Tarantino, U. Fragility fractures of the pelvis: Treatment and preliminary results. Aging Clin. Exp. Res. 27(Suppl 1), S61–S67 (2015).
Arand, C. et al. Anatomical evaluation of the transpubic screw corridor based on a 3D statistical model of the pelvic ring. Sci. Rep. 11(1), 16677 (2021).
Acknowledgements
Parts of the present study are also part of the doctoral thesis of Christian Hartung.
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding statement was provided by Mainzer Trauma-Stiftung (Grant No. 2018-01).
Author information
Authors and Affiliations
Contributions
C.A. designed and prepared the study, conducted testing, evaluated and analyzed data and wrote the manuscript. C.H. conducted testing and revised the manuscript. D.M. conducted testing and revised the manuscript. E.G. revised the manuscript. J.W. evaluated and analyzed data statistically and revised the manuscript. D.W. designed and prepared the study, revised the manuscript. P.M.R. designed and prepared the study, revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arand, C., Hartung, C., Mehler, D. et al. Biomechanical evaluation of an experimental internal ring fixator (RingFix) for stabilization of pelvic ring injuries on an osteoporotic bone model. Sci Rep 14, 20823 (2024). https://doi.org/10.1038/s41598-024-71138-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71138-3
Keywords
This article is cited by
-
Iliosacral screw osteosynthesis – state of the art
Archives of Orthopaedic and Trauma Surgery (2025)