Abstract
Improved understanding of mosquito–plant feeding interactions can reveal insights into the ecological dynamics of pathogen transmission. In wild malaria vectors Anopheles gambiae s.l. and An. funestus group surveyed in selected dryland ecosystems of Kenya, we found a low level of plant feeding (2.8%) using biochemical cold anthrone test but uncovered 14-fold (41%) higher rate via DNA barcoding targeting the chloroplast rbcL gene. Plasmodium falciparum positivity was associated with either reduced or increased total sugar levels and varied by mosquito species. Gut analysis revealed the mosquitoes to frequently feed on acacia plants (~ 89%) (mainly Vachellia tortilis) in the family Fabaceae. Chemical analysis revealed 1-octen-3-ol (29.9%) as the dominant mosquito attractant, and the sugars glucose, sucrose, fructose, talose and inositol enriched in the vegetative parts, of acacia plants. Nutritional analysis of An. longipalpis C with high plant feeding rates detected fewer sugars (glucose, talose, fructose) compared to acacia plants. These results demonstrate (i) the sensitivity of DNA barcoding to detect plant feeding in malaria vectors, (ii) Plasmodium infection status affects energetic reserves of wild anopheline vectors and (iii) nutrient content and olfactory cues likely represent potent correlates of acacia preferred as a host plant by diverse malaria vectors. The results have relevance in the development of odor-bait control strategies including attractive targeted sugar-baits.
Similar content being viewed by others
Introduction
In nature, mosquitoes interact with biotic and abiotic factors, in multiple ways in the ecosystem affecting their adaptation and vectorial capacity—a measure of the transmission potential of a pathogen1,2. Plants are an important resource for adult mosquitoes and are commonly used as a resting site, or nutrient source especially sugars which is an essential nutritional requirement. Plant-derived sugars provide energy for flight but is also converted and stored as metabolic reserves that include lipids and glycogen that support survival and reproduction3,4,5,6. Previous studies have shown that plant feeding in Anopheles gambiae and An. arabiensis, affects their fitness and survival, which differs by plant type and correlates with sugar content4,7,8. Plant feeding could also be influenced by the pathogen infection status of the vector. For instance, Plasmodium falciparum infected An. gambiae females were more attracted and probed more on suitable host plants than non-infected mosquitoes and translated into increased sugar uptake9. Such trophic related effects imposed by parasite infection are yet to be investigated in the natural field situation and could vary between vector species depending on their metabolic needs.
Evidently, few studies have linked plant feeding to vectorial attributes and Plasmodium transmission in nature. Provision of sugar through plant meals can increase mosquito lifespan and potentially pathogen transmission risk. In manipulative field experiments, removal of flowering branches of the invasive shrub Prosopis juliflora, a supposed sugar rich plant, resulted in a three-fold drop in Anopheles female survivorship10. A related study showed substantial and consistent reduction in population size and survival of Anopheles sergentii in a sugar-poor oasis compared to an area with abundance of flowering Acacia raddiana that provided plentiful sugar11. Thus, availability of plants in the local environment could be a major driver of mosquito population dynamics and vector potential. Noteworthy, such fitness related effects could be dependent on specific plant species foraged upon and the environment.
The objective of this study was to characterise plant-feeding ecology of wild anopheline females with a focus on An. gambiae s.l. and An. funestus group which are among malaria vectors in dryland ecosystems of Kenya12. Malaria transmission in these landscapes is seasonal and may be intense during the rainy season13,14. We hypothesized that plant exploitation could offset survival constraints of local vectors in such an environment. Specifically, the degree of plant feeding among wild female populations was estimated and specific plants utilized profiled to test inference on foraging range. The association between energetic content (total sugars and lipid) in the mosquitoes with P. falciparum infection positivity or plant feeding was also documented. Additionally, whether utilization of specific plants is dependent on its sugar content/diversity or volatile organic profiles was determined. An integrative approach was adopted to generate concomitant data from a single mosquito specimen (e.g., sibling species identity, parasite infectivity, fructose and energetic content, plant DNA and host plant identification). This approach allowed for better assessments of inter-relationships and test for correlations between the different biological and ecological parameters.
Materials and methods
Study sites
The study was carried out at three sites that are generally categorized as dryland ecologies. The first two study sites were Kerio Valley (Baringo County) and Nguruman (Kajiado County) (Supplementary Fig. S1). Kerio Valley is characterized by dry landscapes with heterogeneous savannah vegetation predominantly Acacia plant, grass, and discontinuous woodland. Malaria in this area has been described as seasonal15. The area (36.277° E, 0.723° N) lies within the Great Rift Valley at an altitude range between 870 and 2499 m above sea level. The area receives ~ 745 mm of rainfall annually with average daily temperatures varying between 18 and 33 °C. Also situated along the Great Rift Valley is Nguruman which lies between 2.0981° S, 36.7820° E at an altitude of 500 m above sea level receiving an annual rainfall of about 570 mm. The annual daily temperatures range between 11 and 27 °C and malaria in this area is also seasonal13,15. A large part of this area is covered with thorny bushes such as Prosopis juliflora, Acacia and Cactus trees. Rabai (Kilifi County), an additional site, in the coastal region, approximately 19 km northwest of Mombasa city, is considered endemic for malaria. It lies between 3.9107° S, 39.6093° E and receives an annual rainfall of about 900–1100 mm and temperatures between 16 and 32 °C. Of note, rainfall can be erratic and unpredictable, sometimes resulting in prolonged dry spells and drought. The landscape is characterised by heterogeneous vegetation with mango and coconut plantations being more predominant.
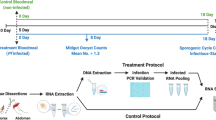
Mosquito collection and processing
Adult host-seeking anophelines were surveyed using CDC light traps baited with dry ice (CO2), placed outdoors about 10–15 m apart in randomly selected homesteads from dusk to dawn between August 2019 and May 2020. Details of the trapping design, handling, and transportation of samples from field to the laboratory at icipe (International Centre of Insect Physiology and Ecology) and then cold storage (− 80 °C) have been previously described14. Published taxonomic keys16,17 were used to identify the anopheline mosquitoes. Each mosquito was dissected into head/thorax and abdomen. DNA from the head/thorax were processed by conventional PCR to identify sibling species of An. gambiae s.l. and An. funestus group, using a cocktail of primers18,19. High resolution melting (HRM) profiles from real-time PCR products of non-coding mitochondrial sequence (ncMS) were used to detect P. falciparum sporozoite infection. Details on the methodologies are described in Ref.14 (Supplementary Fig. S2).
The abdomen of each specimen was processed for total sugar and lipids, and plant DNA analysis as described previously20. Briefly, the abdomens were individually crushed in microcentrifuge tubes using sterile polypropylene pestles and 200 µl of absolute ethanol added and incubated at − 20 °C for 30 min. Thereafter, the samples were centrifuged at 12,000 revolutions per min (rpm) at 4 °C for 10 min. The supernatant was collected carefully into separate microcentrifuge tubes and analysed for total sugars and lipids (described below). The remaining pellets were left to dry overnight in a biosafety cabinet and processed for plant DNA re-extraction and amplification (see below).
To separate sugars from lipids, 200 µl of chloroform was added directly to the supernatant to extract lipids followed by centrifugation at 1000 rpm at 4 °C for 1 min. To enhance phase separation, 100 µl of PCR water was added and the mixture further centrifuged at 1000 rpm at 4 °C for 1 min. The upper alcohol layer containing sugars and the lower layer containing lipids (chloroform) were collected and processed separately for sugar and lipid analysis.
Evidence of recent plant feeding in field-derived anopheline mosquitoes
The cold anthrone test that detects fructose was used as described previously20. The cold anthrone is a colorimetric test that detects presence of fructose as evidence of recent plant sugar feeding21. If present, it is catalysed by the concentrated acid (sulphuric acid) in the antrone reagent to form hydroxyl furfural which then condenses with two molecules of naphthol from the anthrone reagent to form a blue-green complex. Presence of this color complex is indicative of a positive result whose quantity can be estimated by spectrophotometric measurement of the absorbance at 620 nm wavelength. Briefly, 200 µl aliquots of the supernatants were transferred to test tubes, followed by addition of 1ml of anthrone reagent and incubation of the mixture at room temperature (25 °C) for 75 min. Glucose dissolved in 70% ethanol was used as positive control. A color change of yellow to blue or green in the sample was scored as positive indicating the presence of fructose.
Analysis of lipids and total sugars in mosquito
The vanillin phosphoric method was used to test for presence of lipids21. The procedure is described in Ref.20. Two hundred microlitres of lipid standards were transferred into test tubes alongside the samples and heated at 100 °C for 5 min to vaporize the chloroform. Two hundred microlitres of concentrated sulphuric acid were added followed by incubation in a heating block for 10 min and then allowed to cool, after which 1 ml of vanillin phosphoric acid reagent was added and incubated at room temperature for 5 min. Sulphuric acid aids conversion of unsaturated lipids to soluble sulfonic acid derivatives resulting in a red coloration, an indication of presence of lipids, after adding vanillin phosphoric acid reagent. The solutions were mixed thoroughly by vortexing. This was followed by quantifying total lipids using a microplate reader (Epoch, Biotek) and the Gen5 software (Biotek) on a 96-well plate. Lemon grass oil20 served as positive control run at different concentrations spanning the range of the analyte concentration. Calibration curves and linear equations were generated from these samples, and they were used to estimate the total amounts of lipids in each specimen.
The sugar fraction was processed using the hot anthrone method by adding 1 ml of anthrone reagent and heating the mixture at 110 °C for 17 min. For sugar content, glucose dissolved in 70% ethanol served as positive control.
Plant DNA extraction and amplification by polymerase chain reaction (PCR)
Plant DNA was extracted from the abdominal pellets using ISOLATE II Plant DNA Kit (Bioline, London, UK) following the manufacturer’s instructions. PCR was used to amplify plant DNA by targeting the ribulose-1,5 bisphosphate carboxylase (rbcL) gene as described previously20,22. Ten microliter PCR reaction volume using the My Taq DNA Polymerase Kit (Bioline, London, UK) were prepared comprising 5× My Taq reaction buffer, 0.4 µM final concentrations for each primer, 0.125 µl of My Taq DNA polymerase and 2 µl of DNA template. The thermal cycling conditions were initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 1 min and final extension at 72 °C for 10 min. The amplicons were visualized on a 1.5% agarose gel stained with ethidium bromide (Sigma-Aldrich, GmbH, Germany) against a 1 kb DNA ladder (HyperLadder, Bioline, London, UK). Amplicons (expected band size 400-600bp) were cleaned using ExoSAP IT (Affymetrix Inc., Santa Clara, CA, USA.) rapid clean-up kit following the manufacturer’s instructions and outsourced for bidirectional Sanger sequencing to Macrogen (Inc Europe Laboratory). Sequences were cleaned using MEGA v 723 and compared to GenBank reference sequences using Basic Local Alignment Search Tool (BLAST, “Highly similar” option) and those with > 98% percentage identity were scored as the host plants.
Processing plant and wild mosquitoes for sugar profiling
The aerial parts of randomly selected acacia plants were harvested, wrapped up in newspapers and transported to icipe laboratory. These plants are interspersed around homesteads in the study locality (Kerio Valley). The collected plants were air-dried at room temperature for two weeks and crushed into fine powder using an electric grinder (Retsch GmbH, Haan, Germany). Twenty (20) mg of each of the ground material were extracted in 1 ml of absolute ethanol followed by vortexing for 10 s after which the samples were sonicated for 10 min. After sonication, homogenates were centrifuged at 14,000 rpm for 5 min and the supernatant was filtered using a filter paper. The filtrate was stored carefully for sugar analysis using gas chromatography coupled to mass spectrometry (GC–MS) (described below). Both plant and mosquito samples were analyzed in triplicate each.
For sugar profiling, 40 µl of the filtrate was placed in a GC vial insert and left to evaporate to dryness, followed by addition of 10 µl of silylation reagent (Tri-sil HTP, Thermo Fischer, Scientific, Waltham, Massachusetts) and incubation for 15 min at 85 °C. Two microliters of each sample were analysed by splitless injection using a 7890 gas chromatograph (Agilent Technologies) coupled to inert mass spectrometer (597C, EL, 70eV, Agilent Palo Alto, C.A, U.S.A) (GC–MS) equipped with an HP-5 column (30 m × 0.25 mm ID × 0.25 μm film thickness, Agilent, Palo Alto, California, USA). The carrier gas was helium with a flow rate of 1.2 ml/min. The oven temperature was held at 70 °C for 3 min, then increased at 10 °C/min to 280 °C. Plant sugars were identified by comparison of spectra of their trimethylsilyl derivatives with library data (Adams2.L, Chemecol.L and NIST05a.L) and with those of authentic standards.
Collection and analysis of plant volatiles
Volatiles released from the aerial vegetative parts of intact acacia plants (frequently fed upon; see “Results” section) were collected over an enclosed airtight oven bag (Reynolds, Richmond, VA, USA)22, and passing charcoal-filtered air through (flow rate = 350 ml/min) HayeSep Q adsorbents (30 mg, Sigma Scientific, LLC, Micanopy, Florida, USA). Volatile trapping was from randomly selected acacia plants interspersed around homesteads in the study locality (Kerio Valley) some used for sugar profiling. A portable system (Clean Air Delivery System (CADS)-Field Unit, Sigma Scientific, LLC, Micanopy, Florida, USA) was used in trapping volatiles during the day (6:00–17:59H) and night (18:00–5:59H the following day) to investigate whether profile differences can provide a clue into potential activity period that mosquitoes seek sugar. Trapping from enclosed surrounding air served as negative controls. One adsorbent was used for each replicate trapping and eluted with 200 µl GC-grade dichloromethane (DCM) (Burdick & Jackson, Muskegon, Michigan, USA) and the eluate (1 µl) analysed by GC–MS as described above. Compounds present in controls were excluded from compositional profiles in each sample. Where available, the identities of volatile organic compounds (VOCs) were confirmed by comparison of mass spectral data with authentic standards and retention indices as described previously24.
Chemical standards
Synthetic sugar standards used included %), d-glucose (Fischer Scientific, Bishop Meadow Rd, Loughborough, UK, 99.5%), d-fructose (Sigma-Aldrich, 99%), d-(+)-sucrose (Fischer Scientific, 99.5%), d-(+)-galactose (Sigma-Aldrich, 99%), d-(+)-xylose (Sigma-Aldrich, St. Louis, Missouri, USA, 99%), l-arabinose (Sigma-Aldrich, 99%), l-rhamnose (Sigma-Aldrich, 99%) and d-turanose (Sigma-Aldrich, 98%). Lemon gras oil (98%) was sourced locally (Cinnabar Green Ltd, Nanyuki, Kenya).
Ethics statement
The study received approvals from the Scientific Ethics and Review Committee of the Kenya Medical Research Institute (SERU-KEMRI) (Protocol numbers 2787 and 4592). Before data collection, the local administration (chief) was briefed on the purpose of the study, procedures and associated risks and benefits. In addition, informed written consent was obtained from household heads to trap mosquitoes around homesteads.
Statistical analysis
Logistic regression was used to test effect of energetic content (total sugar, lipid) and evidence of plant feeding (presence of Plant DNA) as predictors of P. falciparum infection. Mosquito species, site, total sugar, lipid and plant DNA were specified as explanatory variables. Total sugar and lipid contents were compared between infected and non-infected mosquitoes for overall mosquito data and separately for An. longipalpis C (had an adequate sample size), by Kruskall–Wallis test (data was non-normally distributed based on Shapiro–Wilk test). The same test was implemented to test the effect of site or species on the total sugar or lipid content. Mean total sugar or lipid content (± standard error) per mosquito aggregated by infection status was plotted for overall mosquito and individually for An. longipalpis C. All analyses were performed in R v 4.2.1 and results considered significant at p ≤ 0.05. Differences in the chemical composition between day- and night-time volatile profiles of acacia plants were evaluated using analysis of similarity (ANOSIM), performed in PAST v4.06b25. Additionally, the differences in the chemical composition between day and nighttime acacia volatiles were visualised using heatmap implemented in GraphPad Prism v10.1.2 (GraphPad Software, Inc., San Diego, CA).
Results
Rates of plant feeding vary among field collected anopheline species
The results from the different analytical methods are described hereafter while accounting for variation in species composition, physiological status, P. falciparum rates and spatial heterogeneity. A total of 680 individual mosquito samples were processed by the cold-anthrone singly recording an overall low fructose positivity rate of 2.8% (19/680). The rates varied by species: An. longipalpis C (2.75%, 10/364), An. rivulorum (3.13%, 2/64), An. arabiensis (3.85%, 2/52) and morphologically scored An. funestus s.l. that failed to amplify by PCR (3.07%, 5/163) (Table 1). Fifty-five percent (371/680) of cold-anthrone processed samples were analyzed by PCR to amplify plant DNA of the rbcL gene. These included the fructose positive (n = 19) and negative samples (n = 352). One hundred and fifty-two samples amplified successfully for rbcL gene which translated to an overall PCR success rate of 41% (152/371) (Table 2). The PCR success rate varied among the fructose positive 63.2% (12/19) and fructose negative samples 39.7% (140/352). The data indicates frequent plant feeding in these mosquitoes but likelihood of gross underestimation of the extent of feeding by the cold-anthrone test.
Energetic reserves (total sugar and lipid content) among wild malaria vectors change with Plasmodium falciparum positivity
Table 3 captures a summary of P. falciparum infection detected in the different species across the three study sites. Concurrent data derived from each of the 297 samples (all species and sites) on amount of total sugars, lipids, presence of plant DNA based on rbcl gene (positive/negative) was explored to test for effect of species/site on nutrient levels and their influence on P. falciparum positivity.
The amount of total sugar varied significantly by species (Kruskal–Wallis χ2 = 60.08, df = 4, p < 0.0001) and site (Kruskal–Wallis χ2 = 9.18, df = 2, p = 0.01). A similar trend showed effect of species (Kruskal–Wallis χ2 = 24.608, df = 4, p = 0.0001) and site (Kruskal–Wallis χ2 = 41.157, df = 2, p < 0.0001) on lipid content. Further analysis showed no variation in the amount of total sugar (Kruskal–Wallis χ2 = 1.03, df = 1, p = 0.31) and lipid (Kruskal–Wallis χ2 = 0.20, df = 1, p = 0.66) between infected (n = 56) and uninfected (n = 241) mosquitoes (Fig. 1a). In contrast An. longipalpis C in Kerio Valley, infected with P. falciparum had significantly less sugar amount than their uninfected counterparts (Fig. 1b; Kruskal–Wallis χ2 = 5.83, df = 1, p = 0.016) but not for lipids (Kruskal–Wallis χ2 = 0.02, df = 1, p = 0.90). The data indicates a likely species-specific effect in energetic utilization in the presence of parasite infection.
Energetic comparisons between Plasmodium falciparum (Pf) infected and non-infected wild anophelines, (a) overall and (b) An. longipalpis C. Overall mosquito dataset included 297 samples (infected = 56, non-infected = 241) and 148 An. longipalpis C (infected = 18, non-infected = 130). Analysis was by Kruskall–Wallis test in R at at p ≤ 0.05.
Logistic regression analysis for the overall mosquito data revealed that the odds of P. falciparum infection was not associated with total sugar, lipid content or evidence of plant feeding (Table 4). However, disaggregating the data for An. longipalpis C alone (dominant species) and in Kerio Valley (comprising > 99% occurrence), showed an influence of total sugar (estimate = − 0.21 ± 0.09; Z value = − 2.43; p = 0.015) but not lipid content (estimate = 0.11 ± 0.07; Z value = 1.65; p = 0.10).
Wild anophelines feed frequently on acacia plants
The rbcL amplicons were sequenced for the different mosquito species of which 74 were successful (success rate = 48.7%, 74/152; Table 5). Four samples (n = 4) that had multiple bands (> 1) potentially indicating multiple plant hosts, were unsuccessfully sequenced. The sequence chromatograms of the remainder samples were poor or messy and could not be cleaned or analyzed. Nine plant species were identified as host plants after analyzing the 74 rbcL sequences, majority (89.2%, 66/74) were acacia plants (family Fabaceae) (Fig. 2) largely represented by Vachellia tortilis. The other species represented in low proportion were Cynodon dactylon (grass, family Poaceae) Dichrostachys cinerea (family Fabaceae), Musca ornata (family Musaceae), Newtonia dupaarquetiana (family Fabaceae), Senna tora (family Fabaceae), Parkia filicoides (family Fabaceae), Terminalia guyaensis (family Combretaceae), Stryphnodendron adstringens (family Fabaceae). Both infected (11/66; 16.7%) and non-infected mosquitoes (55/66; 83.3%) had fed on acacia.
Diverse sugar types detected in acacia plants than in mosquitoes
To verify whether the observed propensity for acacia plants could be related to its nutrient content, the vegetative parts of acacia (collected from Kerio Valley only) were analyzed for sugars using GC–MS. Eleven monosaccharides were identified in the plants: arabinose, ribose, allulose, fructose, mannose, glucose, allose, galactose, lyxose, talose and xylose. In addition, three disaccharides: sucrose, maltose, gentiobiose; two sugar alcohols: inositol and pinitol were identified (Supplementary Table 1). However, these sugar types detected varied across the replicate acacia plants with the common ones being glucose, ribose, sucrose, talose and inositol. Glucose was commonly detected in the mosquito (An. longipalpis C from Kerio Valley only) crop followed by talose, fructose and mannose (Supplementary Table S1).
Acacia plants emit volatile organic compounds known to attract mosquitoes
The headspace volatile constituents of the vegetative part of selected acacia plants from Kerio Valley only, were analyzed to determine whether the apparent proclivity towards acacias could be attributed to emission of volatile constituents that attract the mosquitoes for feeding. GC–MS analysis of headspace volatiles identified 43 volatile organic compounds (VOCs) (Fig. 3) belonging to the chemical classes: alcohols (31.4%), aldehydes (~ 6.4%), ketones (~ 6.5%), terpenes (~ 17.5%), hydrocarbons (~ 29.0%) and esters (~ 9.2%) (Fig. 3). Overall chemical composition and abundance differed significantly between day and night (ANOSIM, R = 0.16, p = 0.02) with most of the VOCs highly enriched in the day- than nighttime (Fig. 3). Thus, relative differences for specific constituents were evident (Fig. 3). The major constituents included 1-octen-3-ol (29.9%), α-cedrene (9.1%), acetophenone (3.9%).
Discussion
Determining outdoor use of environmental resources, specifically plants is critical to improving our understanding of factors that contribute to vector sustenance and malaria transmission. In this study, we characterized the plant-feeding association among wild anopheline female mosquitoes in a dryland ecosystem in Kenya. The proportion of plant-fed mosquitoes was unexpectedly low following biochemical analysis by the cold anthrone test. However, much higher plant-feeding rates were detected when molecular assay of the rbcl gene was employed. The difference could be attributed to the higher sensitivity of DNA-based approaches in profiling plant species in sugar meals20,22,26,27. The cold anthrone test provides only a conservative estimate of the extent of plant feeding3; absorbed sugars could be converted to products such as trehalose, glycogen, fat, and CO2 that do not readily react with reagents used in cold anthrone test. Since a higher plant DNA was detected among fructose negative samples, focusing on fructose-positive samples alone to identify putative host plants of disease vectors20,22,28 can obscure the true picture of plant feeding rates. Notably, amplification was not 100% successful in all the fructose positive samples which indicates that use of rbcl gene alone is inadequate, and the efficiency could be improved by incorporating other targets (e.g., MatK, ITS) or next-generation sequencing approaches16,27 Overall, the data indicates frequent plant feeding among diverse wild malaria vectors. Variation in the proportion of plant feeding among the species could indicate inherent differences in degree of utilization or relative importance in their biology in nature, given the same method of sample collection and testing employed.
In nature, mosquitoes primarily derive sugars from host plants that provide immediate (ready-to-use) source of flight energy allowing them to seek blood hosts, mates and oviposition site and even plants3,29. Sugar meals can also contribute to accumulation of reserves (lipids, glycogen) which is important for survival for resting mosquitoes and less likely for flight3. Our findings showed marked variation in the amount of sugar detected among the different mosquito species, which suggests species-specific differences in sugar requirements or activities with regards to dispersal and flight capabilities. Our study sites had effect on the amount of sugar detected in captured mosquitoes; this could signify difference in physiological adaptation under varying environmental influences and perhaps locally adapted plants foraged upon often with heterogeneous nutrient content30. Variation in sugar levels could be affected by insect age and physiological status of the source plant, or if feeding includes diverse plants known to vary in sugar content4,7,8. The lipid content differed by study site and mosquito species, confirming that malaria vectors may be flexible in the proportional allocation of resources to enhance their survival6,8 especially during unpredictable circumstances such as drought. Different plants confer different survival rewards to mosquitoes by virtue of accumulated energy reserves as demonstrated in laboratory feeding experiments in An. gambiae4,8 and Culex pipiens6: If the foraging behaviour includes diverse plants, then local scale or site-specific differences in lipid content as observed would not be unexpected.
In controlled experiments, Plasmodium infections have been known to exert energetic costs to their mosquito host vectors9,31. In the present study, when mosquito data was combined (i.e., all species), those infected with P. falciparum were more likely to have a higher amount of total sugar but not lipid in their gut than uninfected cohort. In contrast to other species considered, P. falciparum infection was associated with reduced levels of total sugars in An. longipalpis C, suggesting that the physiological effect of malaria parasite infection on energetic budget could be species-specific. Increased or reduced sugar uptake after plant feeding by P. falciparum infected An. gambiae was found to be dependent on parasite stage (oocyst vs sporozoite)9. The identical processing and testing of head/thorax samples preclude any likely effect of parasite infection stage on our observed data. However, our observation is in accordance with a previous demonstration that Aedes aegypti and An. stephensi infected with P. gallinaceum and P. cynomolgi, respectively, had reduced levels of glucose than the uninfected group32,33. In contrast to the present data for An. longipalpis C, Rivero and Ferguson31 observed that An. stephensi infected with P. chaubadi was associated with increased levels of glucose. The available evidence thus far, indicates that any upward or downward direction of energetic changes in mosquitoes attributed to parasite infection is non-trivial lending support for influence of other factors. Although associative, our results provide the first report on this observation in the field situation. Other factors inherent in mosquito vector species such as reallocation of the energetic resources for other fitness attributes or needs (e.g. survival, fecundity34), parasite loads, dispersal/flight ability could contribute to variation in the effect seen in nature and should be investigated further. Thus, the plant species, spatial heterogeneity and mosquito infection status could be important predictive factors that affect energetics reserve of wild anopheline vectors.
Profiling plant sources of sugar meal in the gut surprisingly revealed > 90% feeding on Fabaceae plants, dominated by acacia plants by all the mosquito samples across the three studied sites. Acacias (family Fabaceae) are particularly adapted to dryland ecosystems (Nguruman and Kerio Valley). Other plant species in the family Fabaceae were also represented, consistent with previous plant meal studies implicating this family among suitable host plants of anophelines28,35, Ae. aegypti20 and other blood feeding insects like sandflies22,26. The absence of data on relative abundance or biomass in relation to other plant distribution in the study localities makes direct inference on preference not plausible from the current data. However, the notable utilization of acacia plants by a multitude of anopheline species could depict a strong and close adaptive ecological interaction, especially in dry ecosystems, as also reported in the present study. Selective plant-sugar sources among available plants by mosquitoes seem to correlate strongly with sugar content as demonstrated in An. gambiae7. Among the sugars detected were glucose, fructose and sucrose known to comprise > 95% of nectar sugars36. Consistent with previous findings22,35, the vegetative part (i.e., nonflowing leaves/stems) of acacia plants has a high sugar richness, in the Kerio Valley environment. In the field, feeding on acacia as a sugar rich plant have been shown to correlate with longer lifespan of the malaria vector An. sergentii and predicted to lead to higher malaria transmission potential11.
Could the overwhelming foraging habit on acacia contribute to higher malaria transmission? Any naturally occurring resource like plants that influence lifespan has the potential to impact pathogen transmission. Further studies addressing how acacia affects the survival and other life history traits (e.g., fecundity) in malaria vectors are warranted. Surprisingly, far fewer sugars were detected in the midgut of An. longipalpis C than in the acacia plants considered in the analysis. The survival benefits and nutrient rewards likely could depend on the composition and the level of specific sugar type derived from plants. Ingestion of sugars from plants such as sucrose, glucose and gulose appears to favor mosquito survival7; two (sucrose, glucose) of which were common in the mosquito gut and likely indicating common sugar constituents in the diet of this and other malaria vectors. On the other hand, arabinose, lactose, and cellulose were found to be toxic to Ae. aegypti and their consumption reduced survival37,38. Such laboratory-based results may not simulate the natural situation where mosquitoes are more likely to ingest a blend of sugars. Sucrose is the most preferred carbohydrate by mosquitoes, however, the analysis of sugar profiles from mosquito abdomen did not record any sucrose because the sugar is readily digested by alpha-glucosidases39. The role of diverse sugars in a plant meal in mosquito biology deserves further investigations. We note plant feeding on acacia plants by infected and non-infected mosquitoes indicative of potential stronger influence of plant nutrition on the physiology and life history attributes of many vectors. However, this is likely to be dependent on specific acacia plant species given that certain species (e.g. Acacia nilotica) have been found to have repellent or larvicidal activity on mosquito species (e.g. Cx. pipiens)40.
The headspace volatile of acacia (from Kerio Valley only) was dominated by constituents such as 1-octen-3-ol (29.9%), acetophenone (3.9%), α-cedrene (9.1%). These VOCs are well-known mosquito attractants including malaria vectors28,41,42, which suggests they could play a role in mosquito host plant finding. Of note, our findings indicate higher emissions of VOCs during the day than night and for specific compounds (Fig. 3) although it remains unclear how this observation relates to the peak attraction or activity period of anopheline mosquitoes for plant host-seeking. Plant feeding normally precedes blood feeding in nature; there is value in adaptation to plant feeding earlier than blood feeding that occurs at night, although activity periods for plant feeding are poorly described for most anophelines. Together, given that presence of specific attractant cues in host odor may lead to an increase in attractiveness to mosquitoes, our data indicates that apparent proclivity towards acacias could be ascribed to their volatile emission. Further studies need to investigate the full profile of constituents that mosquitoes detect in the volatile profiles and to confirm behavioral impact to the individual mosquitoes. From the public health perspective, this knowledge of widespread vector plant feeding and associated determinants, lend strong support for exploration in developing new ways for surveillance and controlling vector populations including attractive toxic sugar baits and improved attractants9,35. Estimating the significance of plant feeding for pathogen transmission should also be pursued43,44.
One limitation of the study design is that it lacked seasonality and focused on specimens collected using a single trapping method and limited to outdoors. The latter factor impacted adequate representation of highly indoor species (e.g., An. funestus s.s.) to allow for comparative assessment. Also, differential occurrence of the vector species in the study areas precluded firm comparisons of the effect of each site to the observed pattern hampering generalizations that can be drawn.
Another limitation is the lack of standardization of plant parameters such as age and physiological status which may have influenced the variation in sugar content and volatile profiles. Nonetheless, the heterogeneity in plants parameters also mirrors what happens in nature. Further studies should characterize the plant population structure to establish potential preference and importance of specific acacia species as well as comparative profiling of sugar content of other plants that are less utilized in the environment of the study areas. Also, mosquito age or prior effect of a blood meal could not be controlled. Blood meal will, however, contribute largely to egg development and not energy supply (carbohydrate and lipids) because blood protein gets broken down and absorbed much slower than sugar and yields only a fraction of the amount of lipids that an equivalent quantity of sugar can produce21. Regardless of these limitations, our findings provide insights into plant-feeding associations among malaria vectors thriving in dryland ecosystem.
In conclusion, cold anthrone that detects fructose commonly used as a proxy for inferring plant feeding in disease vectors could grossly underestimate the extent of feeding when compared to DNA-based approaches. Presence of P. falciparum infection may be associated with either reduced or increased levels of total sugars depending on the species. The apparent selectivity in plant feeding in the wild malaria vectors examined probably reflect the high representation of acacia plants that appear to dominate the landscape of dry ecosystems. Both the nutrient content in the vegetative parts and olfactory emission profiles could represent potential correlates of suitability of acacia as a host plant by diverse malaria vectors.
Data availability
The datasets used and/or analysed during the current study are available in the icipe data repository (https://dmmg.icipe.org/dataportal/dataset/malaria-vectors-plant-interaction).
References
Lefevre, T. et al. Transmission traits of malaria parasites within the mosquito: Genetic variation, phenotypic plasticity, and consequences for control. Evol. Appl. 11, 456–469 (2018).
Tchouassi, D. P., Torto, B., Sang, R., Riginos, C. & Ezenwa, V. O. Large herbivore loss has complex effects on mosquito ecology and vector-borne disease risk. Transbound. Emerg. Dis. 68(4), 2503–2513 (2021).
Van Handel, E. Metabolism of nutrients in the adult mosquito. Mosq. News 44, 573–579 (1984).
Nyasembe, V. O., Teal, P. E. A., Mukabana, W. R., Tumlinson, J. H. & Torto, B. Behavioral response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasit. Vectors 5, 234 (2012).
Fernandes, L. & Briegel, H. Reproductive physiology of Anopheles gambiae and Anopheles atroparvus. J. Vector Ecol. 30(1), 11–26 (2005).
Yu, B. T., Hu, Y., Ding, Y. M., Tian, J. & Mo, J. Feeding on different attractive flowering plants affects the energy reserves of Culex pipiens pallens adults. Parasitol. Res. 117, 67–73 (2018).
Manda, H. et al. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malar J. 6, 113 (2007).
Nyasembe, V. O. et al. The invasive American weed Parthenium hysterophorus can negatively impact malaria control in Africa. PLoS ONE 10, e0137836 (2015).
Nyasembe, V. O. et al. Plasmodium falciparum infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Curr. Biol. 24, 217–221 (2014).
Muller, G. C. et al. The invasive shrub Prosopis juliflora enhances the malaria parasite transmission capacity of Anopheles mosquitoes: A habitat manipulation experiment. Malar J. 16, 237 (2017).
Gu, W., Müller, G., Schlein, Y., Novak, R. J. & Beier, J. C. Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PLoS ONE 6(1), e15996 (2011).
Ogola, E. O. et al. Insights into malaria transmission among Anopheles funestus mosquitoes, Kenya. Parasit. Vectors 11, 577 (2018).
Omondi, C. J. et al. Perennial transmission of malaria in the low altitude areas of Baringo County, Kenya. Malar J. 16, 257 (2017).
Kinya, F. et al. Outdoor malaria vector species profile in dryland ecosystems of Kenya. Sci. Rep. 12(1), 7131 (2022).
Division of National Malaria Programme-DNMP, Kenya National Bureau of Statistics-KNBS, ICF. Kenya Malaria Indicator Survey 2020 (DNMP/ICF, 2021).
Coetzee, M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 19, 70 (2020).
Gillies, M. T. & Coetzee, M. A. supplement to the Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). Publ. S. Afr. Inst. Med. Res. 55, 1–146 (1987).
Scott, J. A., Brogdon, W. G. & Collins, F. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 49, 520–529 (1993).
Koekemoer, L., Kamau, L., Hunt, R. & Coetzee, M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 66, 804–811 (2002).
Wanjiku, C., Tchouassi, D. P., Sole, C. L., Pirk, C. W. & Torto, B. Biological traits of wild-caught populations of Aedes aegypti in dengue endemic and non-endemic regions of Kenya. J. Vector Ecol. 46(1), 19–23 (2021).
Van Handel, E. Rapid determination of glycogen and sugars in mosquitoes. JAMCA 1, 299–301 (1985).
Hassaballa, I. B., Sole, C. L., Cheseto, X., Torto, B. & Tchouassi, D. P. Afrotropical sand fly-host plant relationships in a leishmaniasis endemic area, Kenya. PLoS Negl. Trop. Dis. 15(2), e0009041 (2021).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33(7), 1870–1874 (2016).
Hassaballa, I. B., Torto, B., Sole, C. L. & Tchouassi, D. P. Exploring the influence of different habitats and their volatile chemistry in modulating sand fly population structure in a leishmaniasis endemic foci, Kenya. PLoS Negl. Trop Dis. 15(2), e0009062 (2021).
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: Paleontological Statistics software package for education and data analysis. Palaeont. Electr. 4(1), 9 (2001).
Lima, L. H. G. M. et al. DNA barcode for the identification of the sand fly Lutzomyia longipalpis plant feeding preferences in a tropical urban environment. Sci. Rep. 20(6), 29742 (2016).
Abbasi, I. et al. Plant-feeding phlebotomine sand flies, vectors of leishmaniasis, prefer Cannabis sativa. Proc. Natl. Acad. Sci. U.S.A. 115(46), 11790–11795 (2018).
Nyasembe, V. O., Tchouassi, D. P., Pirk, C. W. W., Sole, C. L. & Torto, B. Host plant forensics and olfactory-based detection in Afrotropical mosquito disease vectors. PLoS Negl. Trop. Dis. 12, e0006185 (2018).
Clements, A. N. The Biology of Mosquitoes: Development Nutrition and Reproduction 509 (Chapman and Hall, 1992).
Paré, P. S. et al. The paradox of plant preference: The malaria vectors Anopheles gambiae and Anopheles coluzzii select suboptimal food sources for their survival and reproduction. Ecol. Evol. 14(3), e11187 (2024).
Rivero, A. & Ferguson, H. The energetic budget of Anopheles stephensi infected with Plasmodium chabaudi: Is energy depletion a mechanism for virulence? Proc. R. Soc. Lond. B Biol. Sci. 270, 1365–1371 (2003).
Schiefer, B. A., Ward, R. A. & Eldridge, B. F. Plasmodium cynomolgi: Effects of malaria infection on laboratory flight performance of Anopheles stephensi mosquitoes. Exp. Parasitol. 41(2), 397–404 (1977).
Maier, W. A., Becker-Feldman, H. & Seitz, H. M. Pathology of malaria-infected mosquitoes. Parasitol. Today 3, 216–218 (1987).
Zhao, Y. O. et al. Enhanced survival of Plasmodium-infected mosquitoes during starvation. PLoS ONE 7, e40556 (2012).
Müller, G. C. et al. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar J. 20(9), 262 (2010).
Baker, H. G. & Baker, I. Floral nectar constituents in relation to pollinator type. In Handbook of Experimental Pollination Biology (eds Jones, C. E. & Little, R. J.) 117–141 (Van Nostrand Reinhold, 1983).
Nayar, J. K. & Sauerman, D. M. Jr. Physiological effects of carbohydrates on survival, metabolism, and flight potential of female Aedes taeniorphynchus. J. Insect Physiol. 17(11), 2221–2233 (1971).
Airs, P. M., Kudrna, K. E. & Bartholomay, L. C. Impact of sugar composition on meal distribution, longevity, and insecticide toxicity in Aedes aegypti. Acta Trop. 191, 221–227 (2001).
Souza-Neto, J. A., Machado, F. P., Lima, J. B., Valle, D. & Ribolla, P. E. Sugar digestion in mosquitoes: Identification and characterization of three midgut alpha-glucosidases of the neo-tropical malaria vector Anopheles aquasalis (Diptera: Culicidae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 147(4), 993–1000 (2007).
Baz, M. M. et al. Larvicidal activity of Acacia nilotica extracts against Culex pipiens and their suggested mode of action by molecular simulation docking. Sci. Rep. 14(1), 6248 (2024).
Laporta, G. Z. & Sallum, M. A. M. Effect of CO2 and 1-octen-3-ol attractants for estimating species richness and the abundance of diurnal mosquitoes in the southeastern Atlantic forest, Brazil. Mem. Inst. Oswaldo Cruz. 106(3), 279–284 (2011).
Zhang, H. et al. A volatile from the skin microbiota of flavivirus-infected hosts promotes mosquito attractiveness. Cell 185, 2510–2522 (2022).
Hien, D. F. et al. Plant-mediated effects on mosquito capacity to transmit human malaria. PLoS Pathog. 12(8), e1005773 (2016).
Ayele, S. et al. Maize pollen diet enhances malaria mosquito longevity and infectivity to Plasmodium parasites in Ethiopia. Sci. Rep. 13, 14490 (2023).
Acknowledgements
The authors thank Gilbert Rotich for his assistance during field work. They are grateful to two anonymous persons and Dr Caroline Wanjiku for suggestions regarding the manuscript.
Funding
This study was funded by (i) a Wellcome Trust International Intermediate Fellowship awarded to DPT (222005/Z/20/Z) and (ii) Norwegian Agency for Development Cooperation (Norad) through the project Combatting Arthropod Pests for better Health, Food and Climate Resilience (CAP-Africa) (Grant Number: RAF-3058 KEN-18/0005). The authors gratefully acknowledge the financial support for this research by the following organizations and agencies: Swedish International Development Cooperation Agency (Sida), Swiss Agency for Development and Cooperation (SDC), Australian Centre for International Agricultural Research (ACIAR), the Norwegian Agency for Development Cooperation (Norad), the German Federal Ministry for Economic Cooperation and Development (BMZ) and the Government of the Republic of Kenya. The views expressed herein do not necessarily reflect the official opinion of the donors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
D.P.T. conceived the study; F.K., T.K.M. performed experiments; F.K., T.K.M., D.P.T. analysed the data; C.M.M, C.S.W., B.T., D.P.T. contributed to resources and funding acquisition; F.K., T.K.M., B.T., D.P.T. wrote the draft manuscript; All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kinya, F., Milugo, T.K., Mutero, C.M. et al. Insights into malaria vectors–plant interaction in a dryland ecosystem. Sci Rep 14, 20625 (2024). https://doi.org/10.1038/s41598-024-71205-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71205-9