Abstract
Sodium–glucose cotransporter 2 (SGLT-2) inhibitors are the only medications that improve clinical outcomes regardless of baseline left ventricular ejection fraction. Despite the recognized effectiveness of SGLT-2 inhibitors, there remains a paucity of research on the discontinuation of these medications. The objective of this study is to analyze the rate of discontinuation of SGLT-2 inhibitors, to evaluate the impact of discontinuation on the clinical outcome, and to identify the factors associated with discontinuation. From 2015 to 2021, 775 heart failure patients prescribed an SGLT-2 inhibitor were retrospectively collated at Samsung Medical Center, Seoul, Republic of Korea. The SGLT-2 inhibitor discontinuation rate and the effect of SGLT-2 inhibitor discontinuation on clinical outcome were analyzed using the Kaplan–Meier survival curve. Factors related to discontinuation were analyzed through Cox regression and competing risk survival analysis. The discontinuation rate of SGLT-2 inhibitors was 7.5% at 1 year and 20% at 5 years. General weakness, over-diuresis and volume depletion, renal dysfunction progression, and urinary tract infections are the major reasons for discontinuing SGLT-2 inhibitors in general medical practice. The group that stopped using SGLT-2 inhibitors had a higher rate of heart failure hospitalization than the control group (adjusted HR 2.600, 95% CI [1.233–5.481], P = 0.012). In multivariable Cox regression analysis, the factors associated with total SGLT-2 inhibitor discontinuation were women (HR 2.478, 95% CI [1.553–3.953], P < 0.001) and lower estimated glomerular filtration rate (eGFR) (HR 0.884 per 10 ml/min/1.73 m2, 95% CI [0.789–0.991], P = 0.034). Patients who discontinued SGLT-2 inhibitors experienced an increased risk of heart failure hospitalization, and the rate of discontinuation was higher in women and those with lower eGFR.
Similar content being viewed by others
Introduction
Medical treatment for heart failure (HF) has recently undergone a significant transformation with the introduction of sodium-glucose cotransporter-2 (SGLT-2) inhibitors. SGLT-2 inhibitors emerged as a new and effective treatment option for heart failure with preserved ejection fraction (HFpEF), which previously had no clear treatment option other than diuretics. SGLT-2 inhibitors are currently the only class I medications that can be used in HF regardless of left ventricular ejection fraction (LVEF). For these reasons, the importance of this medication is being emphasized.
Nevertheless, like any medication, there are factors that hinder the continued use of SGLT-2 inhibitors. Adherence to medication plays an important role in the effective management of HF. The rate of discontinuation of SGLT-2 inhibitors during the 18 month follow-up period in the DAPA-HF trial was 4.9%. However, considering that general population are usually more fragile than patients enrolled in a randomized controlled trial (RCT), real-world situations might be different from those of a clinical trial. Additionally, the reasons for discontinuation of SGLT-2 inhibitors may differ from those of conventional HF medications due to the distinct mechanism of actions1. It remains unclear which side effects specifically lead to discontinuation of SGLT-2 inhibitors among HF patients and the underlying patient characteristics responsible for side effects leading to discontinuation2.
This study aimed to investigate the actual rate of medication discontinuation in medical practice, to evaluate the impact of such discontinuation on clinical outcomes, and to identify the main discontinuation reasons and factors influencing discontinuation among patients with HF.
Methods
The data that support the findings of this study are available from the corresponding authors with reasonable request.
Study population and definition
A retrospective analysis was conducted at Samsung Medical Center in Seoul, Republic of Korea, from 2015 to 2021. During the period, a total of 843 patients prescribed SGLT-2 inhibitors along with a heart failure diagnosis code was extracted. To identify the list of heart failure patients, we utilized the I25.5, I42 and I50 codes. The prescribed SGLT-2 inhibitors were dapagliflozin and empagliflozin. Both outpatient and inpatient patients were included, and all part prescriptions were included. The need for informed consent to participate was waived by Institutional Review Board as patients were retrospectively enrolled and data were analyzed after being anonymized. The study protocols were approved by Institutional Review Board and this study was conducted according to the principles of the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations (Institutional Review Board 2024-04-029-001 Samsung medical center, Korea, Republic of).
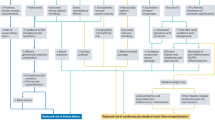
Among these 843 patients, those who did not meet the HF definition outlined in the 2021 European Society of Cardiology HF guideline or the 2022 American Heart Association clinical practice guideline were excluded3,4. In addition, patients with a follow-up period less than 3 months and those who used SGLT-2 inhibitors for less than 3 days during their hospital stay were excluded. Additionally, patients who had undergone heart transplantation or had left ventricular assist device insertion before SGLT-2 prescription were also excluded from the analysis. Finally, 775 HF patients who were prescribed SGLT-2 inhibitors were included in the study (Fig. 1).
To assess the etiology of HF, individual chart reviews were conducted and the cases were classified into six categories: ischemic cardiomyopathy (ICMP), dilated cardiomyopathy, restrictive cardiomyopathy (RCMP), atrial cardiomyopathy, valvular heart disease (VHD), and comorbidity-related HFpEF. ICMP was defined as a history of percutaneous coronary intervention or coronary artery bypass graft or identification of a significant lesion in one or more coronary arteries. A diagnosis of RCMP included hypertrophic cardiomyopathy, sarcoidosis, amyloidosis, mitochondrial encephalopathy-lactic acidosis/stroke-like episodes syndrome with restrictive physiology, and idiopathic RCMP. Atrial cardiomyopathy was defined as HFpEF or heart failure with mildly reduced ejection fraction (HFmrEF) with enlargement of the left or right atrium due to atrial fibrillation. VHD was defined as a greater than moderate case of valve operation, significant valvular stenosis, or regurgitant lesion. Comorbidity-related HFpEF was defined as HFpEF occurring due to an underlying disease such as hypertension or end-stage renal disease, in the absence of intrinsic myocardial disease or coronary disease.
Diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease (CKD), and atherosclerosis were verified by the disease code prior to SGLT-2 inhibitor prescription. Atrial fibrillation was confirmed with disease code or ECG records. HF medication prescribed at the time of SGLT-2 inhibitor was also included in the analysis. For laboratory values and echocardiographic parameters, the one closest to the date of initial SGLT-2 prescription was selected.
Discontinuation was defined as cessation and persistence of SGLT-2 inhibitor therapy due to side effects, except for cases where it was temporarily stopped and resumed. The reasons for discontinuation were meticulously investigated by reviewing the medical charts. In cases where the reasons were not explicitly stated in the medical charts, additional information was gathered through phone interviews. All discontinuations due to both adverse events and other than adverse events were included in the discontinuation group. Patients who were using SGLT-2 inhibitor until just before censoring were included in the continuation group.
Statistics
Baseline characteristics are presented as mean ± standard deviation or median [interquartile range] as appropriate based on normality assumption for continuous variables; and are described as numbers and relative frequencies (%) for categorical variables. Group comparisons were performed using student’s t-test or Mann Whitney U test for continuous variables, and the Chi-square test or Fisher’s exact test for categorical variables. Shapiro–Wilk test was used for normality assumption of continuous variables.
The start of the follow-up period was defined as the initiation date of the SGLT-2 inhibitor in patients with existing heart failure. For patients who were diagnosed with heart failure after starting the SGLT-2 inhibitor, the date of the first heart failure diagnosis was used as the starting point. The data locking date for analyses was November 2022. Patients were censored at last follow-up date or data locking date (November 2022).
To avoid immortal time bias, we performed landmark analysis of time to occurrence of heart failure hospitalization after discontinuation and set the median time from date of initiating SGLT-2 inhibitor to the date of discontinuation (252 days) as a landmark time point. Hereby, patients who experienced heart failure hospitalization or censoring within 252 days were excluded for the analyses. In the discontinuation group, the follow-up period was defined as the time from the discontinuation date to either a heart failure hospitalization event or censoring. In the continuation group, the follow-up period was defined as the time from the 252nd day after starting the SGLT-2 inhibitor to either a heart failure hospitalization event or censoring. The cumulative rate of each of SGLT-2 inhibitor discontinuation and heart failure hospitalization after discontinuation was estimated by Kaplan–Meier method. Group comparison in Kaplan–Meier curves was performed using the log-rank test. To evaluate the effect of clinical outcomes on whether SGLT-2 inhibitor treatment is discontinued, Cox proportional hazards regression model used, calculating hazard ratio (HR) and 95% confidence interval (CI). Multivariable analysis also was performed including clinically relevant variables.
The change in N-terminal pro B-type natriuretic peptide (NT-proBNP) levels between the final value and the baseline value was compared between the continuation and discontinuation groups using the Mann–Whitney U test. Additionally, within the discontinuation group, the change in NT-proBNP levels before and after discontinuation was compared using the paired t-test. To account for the potential influence of renal dysfunction on NT-proBNP interpretation, patients with an initial NT-proBNP level exceeding 35,000 pg/mL were excluded from the analysis.
We also used Fine and Gray’s regression model to investigate the effect of discontinuation due to specific reason. For cause-specific model of each discontinuation reason, the competing risks due to the discontinuation for the other reasons exist. There were no patients having more than one of the discontinuation reasons. In these risk factors leading to discontinuation analysis, we conducted the sensitivity analysis including an additional 22 patients who were lost to follow-up within three months.
All P-values were two-sided, and P-values < 0.05 were considered statistically significant. Statistical analyses were performed using R Statistical Software (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics of the study population and specific reasons for discontinuation
For a total of 775 HF patients who were prescribed SGLT-2 inhibitors, mean age at first prescription was 66 years with 198 women (25.5%) (Table 1). For HF category, 58.0% of patients had heart failure with reduced ejection fraction (HFrEF), 13.7% had HFmrEF, and 28.3% had HFpEF. The main etiologies of HF were ICMP (53.9%) and dilated cardiomyopathy (26.5%) while 2.3% (18/775) had RCMP, 7.1% (55/775) had atrial cardiomyopathy, 4.1% (32/775) had VHD, and 2.7% had comorbidity-related HFpEF (21/775). The remaining 28 patients were diagnosed with tachycardia-induced cardiomyopathy, congenital heart disease, pacing-induced cardiomyopathy, stress-induced cardiomyopathy, or myocarditis. For comorbidities, 70.6% (547/775) of the population was diagnosed with diabetes with 5.3 [0.3–11.4] years of average duration of being diagnosed. Among 547 diabetes patients, the duration of being diagnosed in 220 patients (40.2%) had remained unknown. In addition, 50.6% and 35.0% of the population had coexisting hypertension and atrial fibrillation (AF), respectively.
For concomitant treatment, 89.5%, 48.8%, and 91.5% of the patients were using b-blockers, angiotensin receptor-neprilysin inhibitors (ARNI), and Renin-angiotensin-system (RAS) blockers at the time of starting SGLT-2 inhibitor. 6.1% (47/775) of the patients were initially prescribed the medication for diabetes control, while the remaining patients were prescribed the medication primarily for heart failure control from the beginning.
Among 775 patients, 690 used an SGLT-2 inhibitor without discontinuation and 85 had discontinued such treatment. Table 2 shows the reasons of discontinuation for these 85 patients. The main reasons of discontinuation were general weakness (eight patients), over-diuresis and volume depletion (10 patients), progression of renal dysfunction (19 patients), and urinary tract infection (UTI) (17 patients). The remaining 31 patients discontinued the treatment due to cost issues or patient complaints of side effects such as hypoglycemia and constipation. Proportions of general weakness and UTI were higher in women. And time from initiation of medication to discontinuation due to general weakness (median 91.0 [59.0–128.0] days) was significantly shorter than other causes (P = 0.037). For other causes, time to discontinuation due to over-diuresis and volume depletion, renal dysfunction progression, and UTI was median 332.5 [49.0–512.0], 334.0 [228.0–467.5], and 312.0 [127.0–476] days, respectively. (Supporting Information, Fig. S1). The average time to medication discontinuation for all 85 patients was mean 370.2 days, median 252.0 [110.0–484.0] days, with the shortest discontinuation period 4 days and the longest 1973 days.
SGLT-2 inhibitor discontinuation rates and clinical effect of discontinuation
The cumulative rate of SGLT-2 inhibitor discontinuation at 1 and 5 years were 7.5% 20.0%, respectively (Fig. 2). The median follow-up period for all 775 patients was 567 days, the follow-up period for the 85 patients who discontinued the medication was 616 days, and the follow-up period for the 690 patients who did not discontinue was 564 days, showing no statistical difference (P = 0.413).
The HF hospitalization rate was higher in the discontinuation group than the continuation group (HR 2.922, 95% CI [1.427–5.982], P = 0.003). Higher rate of HF hospitalization in the discontinuation group was significant even after adjusting for age, gender, and eGFR (HR 2.600, 95% CI [1.233–5.481], P = 0.012) (Fig. 3).
When examining the effect of SGLT-2 inhibitors on changes in NT-proBNP levels, patients with an initial NT-proBNP level exceeding 35,000 pg/mL were excluded from the analysis due to the significant impact of NT-proBNP levels on kidney function. As for the change in NT-proBNP level from baseline, the NT-proBNP levels in the discontinuation group exhibited an increasing trend, whereas the NT-proBNP levels in the continuation group showed a decreasing tendency (Fig. 4A, P = 0.001). There was no significant difference between two groups in the time from the time of initiation to last NT-proBNP follow-up (611.9 [505.9–885.4] days in the continuation group vs. 567.4 [469.3–811.4] days in the discontinuation group, P = 0.094). For discontinuation group, NT-proBNP level was decreased from baseline to the time of discontinuation while increased from the time of discontinuation to the last follow-up date, which showed no significance between two periods (Fig. 4B, P = 0.181).
Factors that contributed to total discontinuation and caused specific discontinuation
There was no difference in the discontinuation rate according to HF categories (P = 0.570) or presence of ICMP (P = 0.330). The discontinuation rate was significantly higher in women (P < 0.001) and patients with eGFR less than 60 ml/min/1.73 m2 (P = 0.002) (Fig. 5).
Differences in SGLT-2 inhibitor discontinuation rate according to baseline characteristics. (A) According to HFrEF, HFmrEF, and HFpEF. (B) According to underlying etiology. (C) According to gender. (D) According to baseline eGFR. eGFR estimated glomerular filtration rate, HFmrEF heart failure with mildly reduced ejection fraction, HFpEF heart failure with preserved ejection fraction, HFrEF heart failure with reduced ejection fraction, ICMP ischemic cardiomyopathy.
Table 3 shows the result of risk factor analyses for discontinuation. The risk of discontinuation was significantly associated with older patients (HR 1.038, 95% CI [1.018–1.059], P < 0.001), women (HR 2.816, 95% CI [1.836–4.318], P < 0.001), lower body mass index (HR 0.931 per kg/m2, 95% CI [0.883–0.981], P = 0.008), and lower eGFR (HR 0.832 per 10 mL/min/1.73 m2, 95% CI [0.754–0.919], P < 0.001). In multivariable regression analysis, women (HR 2.478, 95% CI [1.553–3.953], P < 0.001) and lower eGFR (HR 0.884 per 10 mL/min/1.73 m2, 95% CI [0.789–0.991], P = 0.034) were still significantly associated with the risk of discontinuation (Table 3).
For subgroup analyses, we performed risk factor analyses in discontinuation group according to the reason of discontinuation. For those who discontinued the treatment due to general weakness, old age (HR 1.072, 95% CI [1.005–1.144], P = 0.036), women (HR 8.839, 95% CI [1.789–43.68], P = 0.008), lower BMI (HR 0.858 per kg/m2, 95% CI [0.762–0.965], P = 0.011) and lower LVEF (HR 0.562 per 10%, 95% CI [0.410–0.771], P < 0.001) were significantly associated with the risk of discontinuation. For discontinuation due to over-diuresis and volume depletion, RCMP (HR 11.21, 95% CI [2.308–54.42], P = 0.003) was significantly associated with the risk of discontinuation. For discontinuation due to renal dysfunction progression, only eGFR level was significantly associated with the risk of discontinuation (HR 0.613 per 10 mL/min/1.73 m2, 95% CI [0.486–0.773], P < 0.001). And for discontinuation due to UTI, women (HR 5.778, 95% CI [2.150–15.53], P < 0.001) and lower eGFR (HR 0.770 per 10 mL/min/1.73 m2, 95% CI [0.600–0.988], P = 0.040) were significantly associated with the risk of discontinuation. (Supporting Information, Table S1).
A sensitivity analysis was conducted including 22 patients who had a follow-up period of less than three months (Table S2). The multivariable analysis indicated that women (HR 2.456, 95% CI [1.553–3.884], P < 0.001), lower BMI (HR 0.940 per kg/m2, 95% CI [0.890–0.994], P = 0.030), and lower eGFR (HR 0.893 per 10 mL/min/1.73 m2, 95% CI [0.798–0.998], P = 0.046) were significantly associated with the risk of drug discontinuation. In the cause-specific discontinuation analysis, it was observed that discontinuation due to general weakness was more frequent among old age (HR 1.072, 95% CI [1.004–1.144], P = 0.036), women (HR 8.765, 95% CI [1.140–43.31], P = 0.008), lower BMI (HR 0.860 per kg/m2, 95% CI [0.765–0.967], P = 0.011) and lower LVEF (HR 0.563 per 10%, 95% CI [0.410–0.772], P < 0.001). Discontinuation due to volume depletion was more common in patients with RCMP (HR 11.21, 95% CI [2.308–54.42], P = 0.003). Discontinuation due to renal dysfunction progression was more prevalent in patients with lower eGFR (HR 0.613 per 10 mL/min/1.73 m2, 95% CI [0.486–0.774], P < 0.001), and discontinuation due to UTI was more frequent in women (HR 6.262, 95% CI [2.366–16.57], P < 0.001), and those with lower eGFR (HR 0.754 per 10 mL/min/1.73 m2, 95% CI [0.592–0.959], P = 0.021) (Table S3).
Discussion
This study yielded several novel findings. First, the primary causes of SGLT-2 inhibitor discontinuation in routine medical practice were identified as general weakness, over-diuresis and volume depletion, renal dysfunction progression, and UTI. The discontinuation rate was 7.5% at 1 year and 20% at 5 years. Second, discontinuation of SGLT-2 inhibitors was associated with an increased risk of HF admission. Finally, women and those with lower eGFR had significantly higher rates of discontinuation.
Discontinuation rate of SGLT-2 inhibitors in the real world
In our study, the discontinuation rate of SGLT-2 inhibitors at 1 year was 7.5%, which is comparatively low compared to the discontinuation rates of beta-blockers (24%) and ARNI (27%) at 12 months in a multinational observational study5. Additionally, the discontinuation rate of SGLT-2 inhibitors was much lower than the 40% discontinuation rate of the mineralocorticoid receptor antagonists in the same study. These findings suggest that SGLT-2 inhibitors can be consistently maintained as they do not significantly affect blood pressure or cause electrolyte imbalances. The discontinuation rates in our study were higher than those in the DAPA-HF trial6, where adverse events leading to medication discontinuation occurred at a rate of 4.7% during the 18 month follow-up period. This disparity can be attributed to the typically less healthy patients enrolled in RCTs than encountered in real-world clinical practice.
The relationship between maintenance of SGLT-2 inhibitors and prognosis
In our study, we observed a higher rate of HF hospitalization following discontinuation of SGLT-2 inhibitors. However, due to the retrospective nature of the study, it is challenging to determine whether the increased hospitalization rate in the discontinuation group was directly caused by the discontinuation itself or if there were hidden confounding factors that led to both discontinuation and increased hospitalization. Nonetheless, considering the increase in NT-proBNP levels following SGLT-2 inhibitor discontinuation, it can be speculated that the cessation of volume reduction and cardio-protective effects provided by SGLT-2 inhibitors contributed to the subsequent increase in hospitalizations.
Achievement and maintenance of target doses have been widely recognized as important factors in prognosis in various studies involving ACE inhibitors, ARBs, beta-blockers, and ARNI7,8,9,10,11,12. However, the significance of maintenance has not been discussed in relation to SGLT-2 inhibitors, as they are relatively new compared to other HF medications. Our findings clearly demonstrate that discontinuation of SGLT-2 inhibitors is associated with a notable increase in HF hospitalization. This corresponds to recent research findings that demonstrate the exacerbation of heart failure symptoms and NT-proBNP levels following blinded withdrawal of SGLT-2 inhibitors13. By establishing the potential harm of discontinuation, our study underscores the significance of maintaining SGLT-2 inhibitor treatment to optimize patient outcomes and to reduce the burden of HF-related complications.
It may be not appropriate to promptly resume SGLT2 inhibitor treatment in patients who have discontinued it due to adverse effects. However, considering the potential harm that may arise from discontinuing SGLT2 inhibitors, it might be worthwhile to cautiously restart the medication, taking into account the risk factors, once the patient's condition has improved.
Main reasons for discontinuation and their contributing factors
Over-diuresis and volume depletion and UTI, which were major side effects leading to discontinuation in this study, were also major adverse events of interest in the SGLT-2 inhibitor RCT6,14,15,16. Also, in real-world data, urinary tract infection occurs in approximately 7% and dehydration in around 3% of diabetic patients treated with SGLT2 inhibitors17. Our study identified women and lower eGFR as the most influential factors associated with the discontinuation of SGLT-2 inhibitors. These findings align with observations from other studies, which have also reported higher discontinuation rates of HF medications among women and CKD patients5,18. Difficulties in titration of conventional HF medications in these patient populations have been attributed to factors such as hypotension, hyperkalemia, and worsening renal function. However, the specific reasons for discontinuation of SGLT-2 inhibitors differed from those commonly observed with HF medications targeting the renin–angiotensin–aldosterone system.
Factors contributing to discontinuation by specific reasons are as follows. Patients who discontinued SGLT-2 inhibitors due to over-diuresis and volume depletion were found to have a higher prevalence of restrictive physiology. The osmotic diuretic effect of SGLT-2 inhibitors, which promotes sodium and glucose excretion from the proximal tubule, is beneficial for patients requiring volume control19,20,21. However, limited ventricular filling in RCMP is likely vulnerable to volume depletion. Therefore, SGLT-2 inhibitors need to be used with caution in RCMP patients without signs of congestion.
The discontinuation rate due to renal dysfunction progression was notably high in patients with a low baseline eGFR, possibly due to the lack of demonstrated benefits of SGLT-2 inhibitors in HF patients with an eGFR less than 20 mL/min/1.73 m26,16,22,23.
In this study, patients with a high risk of UTI were women and those with CKD. In women, the risk of UTI is further increased due to anatomical factors such as a shorter urethra compared to men. Additionally, increased chronic inflammation in patients with CKD could increase the risk of UTI24,25.
Through this study, we have identified risk factors that are likely to lead to discontinuation. It is important to closely monitor patients who possess these risk factors with relatively short-term follow-ups to effectively manage and mitigate adverse effects.
Limitation
The study acknowledges several limitations. First, we acknowledge the limitations inherent in our retrospective study design, which makes it challenging to determine whether the observed prognostic effects of discontinuation of SGLT-2 inhibitors are solely due to the discontinuation itself or are influenced by hidden confounding factors in the discontinuation group. Therefore, the possibility of reverse causality cannot be ruled out. Also, loss to follow-up in certain patients and missing values could have impacted the results and introduced uncertainty. Second, there are the limitations of a single-center study. This study may have reflected the characteristics of a tertiary general hospital that mainly treats seriously ill patients. Generalizability to other populations and other healthcare settings is limited due to this inherent limitation. Also, admissions to other healthcare institutions were not captured in the outcomes unless they were reported, potentially leading to incomplete data. Third, the study had a higher proportion of diabetic patients (71%) compared to the national HF registry in Korea, where diabetes accounts for 30% of cases26. This discrepancy is attributed to lack of coverage of SGLT-2 inhibitors by insurance for HF patients without diabetes in Korea, which restricts their prescription. Therefore, the generalizability of the findings to the overall HF population may be limited. Forth, symptoms leading to the discontinuation of the drug may not merely be side effects but could also represent early signs of worsening cardiac function, thereby potentially contributing to reverse causality. Despite these limitations, the significance of this study lies in its in-depth analysis of risk factors leading to SGLT-2 inhibitor discontinuation and finding the clinical impact of discontinuing SGLT-2 inhibitors in real-world.
Conclusion
In the real world, SGLT-2 inhibitors exhibited a discontinuation rate of 7.5% in 1 year. Given the worse clinical outcomes observed in patients who discontinued SGLT-2 inhibitors, careful consideration is needed before treatment cessation. Furthermore, special consideration is needed for patients with specific characteristics associated with adverse side effects.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Abbreviations
- ARNI:
-
Angiotensin receptor/neprilysin inhibitor
- ARB:
-
Angiotensin receptor blocker
- CKD:
-
Chronic kidney disease
- HFmrEF:
-
Heart failure with mildly reduced ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- ICMP:
-
Ischemic cardiomyopathy
- LVEF:
-
Left ventricular ejection fraction
- NT-proBNP:
-
N-terminal pro B-type natriuretic peptide
- RCMP:
-
Restrictive cardiomyopathy
- SGLT-2:
-
Sodium–glucose cotransporter-2
- UTI:
-
Urinary tract infection
- VHD:
-
Valvular heart disease
References
Lopaschuk, G. D. & Verma, S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: A state-of-the-art review. JACC Basic Transl. Sci. 5, 632–644 (2020).
Halimi, S. & Verges, B. Adverse effects and safety of SGLT-2 inhibitors. Diabetes Metab. 40, S28–S34 (2014).
Writing Committee Members, ACC AHA Joint Committee Members. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J. Card. Fail. 28, e1–e167 (2022).
McDonagh, T. A. et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42, 3599–3726 (2021).
Savarese, G. et al. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: A multinational observational study (US, UK and Sweden). Eur. J. Heart Fail. 23, 1499–1511 (2021).
McMurray, J. J. V. et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381, 1995–2008 (2019).
Olsen, S. L. et al. Carvedilol improves left ventricular function and symptoms in chronic heart failure: A double-blind randomized study. J. Am. Coll. Cardiol. 25, 1225–1231 (1995).
Bristow, M. R. et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation 94, 2807–2816 (1996).
Packer, M. et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Gr. Circ. 100, 2312–2318 (1999).
Konstam, M. A. et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): A randomised, double-blind trial. Lancet 374, 1840–1848 (2009).
Corrado, E. et al. Low- vs high-dose ARNI effects on clinical status, exercise performance and cardiac function in real-life HFrEF patients. Eur. J. Clin. Pharmacol. 78, 19–25 (2022).
Halliday, B. P. et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): An open-label, pilot, randomised trial. Lancet 393, 61–73 (2019).
Packer, M. et al. Blinded withdrawal of long-term randomized treatment with empagliflozin or placebo in patients with heart failure. Circulation 148, 1011–1022 (2023).
Packer, M. et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 383, 1413–1424 (2020).
Solomon, S. D. et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 387, 1089–1098 (2022).
Anker, S. D. et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 385, 1451–1461 (2021).
Goldman, A. et al. The real-world safety profile of sodium-glucose co-transporter-2 inhibitors among older adults (≥ 75 years): A retrospective, pharmacovigilance study. Cardiovasc. Diabetol. 22, 16 (2023).
Garred, C. H. et al. Adherence and discontinuation of optimal heart failure therapies according to age: A Danish nationwide study. J. Am. Heart Assoc. 11, e026187 (2022).
Tang, J., Ye, L., Yan, Q., Zhang, X. & Wang, L. Effects of sodium–glucose cotransporter 2 inhibitors on water and sodium metabolism. Front. Pharmacol. 13, 800490 (2022).
Heise, T. et al. Acute pharmacodynamic effects of empagliflozin with and without diuretic agents in patients with type 2 diabetes mellitus. Clin. Ther. 38, 2248–2264 (2016).
Wilcox, C. S., Shen, W., Boulton, D. W., Leslie, B. R. & Griffen, S. C. Interaction between the sodium–glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J. Am. Heart Assoc. 7, e007046 (2018).
EMPA-KIDNEY Collaborative Group. Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol. Dial. Transplant. 37, 1317–1329 (2022).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446 (2020).
Shankar, M., Narasimhappa, S. & Madhura, N. S. Urinary tract infection in chronic kidney disease population: A clinical observational study. Cureus 13, e12486 (2021).
Balmaceda, N. et al. Infection risks in multiple myeloma: A systematic review and meta-analysis of randomized trials from 2015 to 2019. BMC Cancer 21, 730 (2021).
Park, J. J. & Choi, D. J. Current status of heart failure: Global and Korea. Korean J. Intern. Med. 35, 487–497 (2020).
Author information
Authors and Affiliations
Contributions
Minjung Bak collected and analyzed the data, and writed the manuscript. Kina Jeon collected the data David Hong prepared the figure Heayoung Shin prepared the table. Darae Kim did supervision Jin-oh Choi did Conceptualization and Design and supervision Sang Ah Chi helped data analysis and writed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bak, M., Chi, S.A., Jeon, K. et al. Discontinuation rates, clinical effects and provocation factors of SGLT-2 inhibitor in the real world. Sci Rep 14, 30653 (2024). https://doi.org/10.1038/s41598-024-71231-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71231-7