Abstract
Identifying mechanisms that drive population divergence under varying geographic and ecological scenarios can inform our understanding of evolution and speciation. In particular, analysis of genetic data from island populations with known colonisation timelines allows us to identify potential source populations of diverging island subspecies and current relationships among populations. Silvereyes (Zosterops lateralis) are a small passerine that have served as a valuable study system to investigate evolutionary patterns on both large and small geographic scales. We examined genetic relatedness and diversity of two silvereye subspecies, the mainland Z. l. cornwalli and island Z. l. chlorocephalus, and used 18 077 single nucleotide polymorphisms (SNPs), to compare locations across southeast Queensland, Australia. Although silvereyes are prolific island colonisers our findings revealed population divergence over relatively small spatial scales was strongly influenced by geographic isolation mediated by water barriers. Strong genetic connectivity was displayed between mainland sites, but minimal inter-island connectivity was shown despite comparable sampling distances. Genetic diversity analysis showed little difference in heterozygosity between island and mainland populations, but lower inbreeding scores among the island populations. Our study confirmed the range of the Z. l. chlorocephalus subspecies throughout the southern Great Barrier Reef. Our results show that water barriers and not geographic distance per se are important in driving incipient divergence in island populations. This helps to explain the relatively high number of phenotypically differentiated, but often geographically proximate, island silvereye subspecies compared to a lower number of phenotypically less well-defined Australian continental subspecies.
Similar content being viewed by others
Introduction
How patterns of intraspecific population genetic structure develop over time and space can be informative about incipient stages of speciation1. Any process or feature that reduces the rate of between versus within population genetic exchange generates population structure2. These can include physical barriers, limits to dispersal capacity of organisms whether physical or mediated by climate or competitive exclusion, and differences in local selective regimes3. Empirical and theoretical population genetics studies have allowed us to gain a deep level of understanding of many of these processes, especially through the examination of spatial genetic patterns4. However, very early stages of divergence likely involve stochastic elements that are difficult to reconstruct by studying long-diverged populations5. Recent natural establishment of new populations provide opportunities to investigate these stages empirically. Additionally, when coupled with information on spatial genetic structure of neighbouring populations, studying newly established populations can reveal how intraspecific geographic variation is generated and maintained.
Historically, islands have been prime natural settings to explore population-level differences within species because geographic allopatry is clearly defined. This provides a convenient framework to study the effects of isolation as measured by geographic distance of water6,7. However, the extent to which water barriers per se versus geographic distance influence population divergence requires an understanding of how different types of matrices affect gene flow. Application of isolation-by-resistance models that determine least cost path based distances among populations can resolve such questions8,9. Population divergence in insular settings can also be strongly affected by stochastic fluctuations in levels of genetic diversity10,11. Genetic diversity can be lost during population founding and establishment (‘founder effects’), which can influence adaptive potential of the new population12. Nevertheless, these effects vary with the size of naturally colonising populations, with some studies showing minimal founding effects when many individuals are involved in the colonisation process and populations recover rapidly13. This means that not all colonising species are likely to experience strong founder effects, though loss of genetic variation and increases in genetic differentiation can result from gradual drift within small, isolated populations over time14,15. Detailed information on colonisation timing and histories can therefore help to tease out the processes that have contributed to the divergence of island populations from other island or mainland populations.
Among avian taxa, silvereyes (Zosterops lateralis) are renowned for their dispersal abilities, colonising multiple locations and habitat types especially in Oceania and Southeast Asia, thus providing an ideal model in which to study connectivity and divergence16. There are 17 subspecies of silvereye across Australasia and the southwest Pacific, five of which occur on the Australian mainland and twelve that have exclusively insular distributions17,18. Studies on island silvereyes show that single founder events have a limited effect on allelic diversity or heterozygosity and degree of genetic divergence, instead showing that sequential founding events and longer timescales of island dwelling have a greater effect on diversity and divergence15,19. This is because silvereyes tend to colonise islands in medium to large flocks and quickly increase population size on arrival15. It is well known empirically and theoretically that two features that serve to limit diversity loss and avoid rapid neutral divergence during population bottlenecks are the width of the bottleneck (number of founders) and the length of the bottleneck (how quickly a population recovers)15.
One of the most recent natural colonisation events of silvereyes has been on Lady Elliot Island (LEI), the southernmost island of the Great Barrier Reef (Fig. 1). LEI was strip mined for guano in the 1800’s removing all vegetation and associated fauna. Revegetation efforts commenced in the 1969, facilitating the colonisation of the silvereyes at the time. They have since become one of the most common land birds on the island20,21. An early genetic fingerprinting study indicated that the LEI population is likely the Zosterops lateralis chlorocephalus subspecies, as found on other islands in the southern Great Barrier Reef (GBR)20. Clear genetic and morphological differences exist between the GBR Z.l. chlorocephalus and mainland Zosterops lateralis cornwalli subspecies, with Z. l. chlorocephalus exhibiting larger body sizes22. However, questions remain regarding the likely origin of the colonising individuals to LEI. Some aspects that could be explored include whether admixture has occurred with the mainland subspecies, facilitated by the close proximity of mainland adjacent K’gari (Fraser Island) (85 km from LEI), or if founder effects resulted in a depauperate genetic population. Moreover, the level of divergence of LEI from other populations (both mainland Z. l. cornwalli and other GBR islands) is unknown. To test levels of divergence and admixture history, we used next generation sequencing technologies to provide the required level of bioinformatic and statistical power to a) establish the contemporary relationship between the LEI population and three surrounding populations, including confirmation of subspecies; b) infer genetic history of the LEI population and identify any founder effects; and c) determine levels of genetic diversity of mainland and island populations to comment on evolutionary and divergence potential.
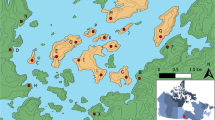
The four study sites across Queensland, including the two mainland sites (Sunshine Coast and Fraser Coast (Maryborough)) and the two island sites (Lady Elliot Island and Heron Island). Inset highlights the study area locations in the red rectangle. Image created by aradu using ArcMap 10.7 https://desktop.arcgis.com.
Results
The DArT pipeline23,24 generated 62 454 SNPs across 90 individuals. One individual from the Sunshine Coast has considerably high missing data and low heterozygosity and was removed from the dataset (S1, S2—supplementary materials). The final dataset after filtering was 18 077 SNPs and contained 89 individuals (S3—supplementary materials). The test for loci under selection did not reveal any statistical outliers and all loci were retained. A pair of individuals from Maryborough were found to have very high genetic similarity correlative to known duplicates, and one individual was then removed from subsequent analysis (Table 1).
The first axis on the Principal Coordinates Analysis (PCoA) separates island from mainland populations, and the second separates the two island populations (Fig. 2). The existence of three genetic clusters is further supported by the admixture analysis, with K = 3 having the lowest cross-entropy value of K-values tested25. A single individual from LEI clustered with the Heron Island group and likely represented an admixed individual. Results showing K = 2 support the major split between the mainland and island subpopulations; and K = 4 (S5—supplementary materials) potentially highlights other island variants in the Capricorn Bunker Group (Islands and reefs along the Tropic of Capricorn) that were not screened as part of this study.
The cross-entropy criterion of the snmf results support K = 3 (Fig. 3), clustering the two mainland locations as a single population. Admixture values are lower between LEI and Heron Island, highlighting one LEI individual sharing a large amount of admixture with Heron Island (Fig. 3) (proportions for this individual: HI = 0.687, LEI = 0.263, SC = 0.05).
Pairwise FST results (Table 2) indicate significant genetic differentiation between all locations (p = < 0.001) with the lowest differentiation between the two mainland populations, Sunshine Coast (‘SC’) and Maryborough (‘M’) (FST = 0.021) followed by the two island sites Heron Island (‘HI’) and LEI (FST = 0.048). Highest levels of differentiation were observed in pairwise comparisons of each island (HI and LEI) and each mainland location (SC and M) (FST ranging from 0.211 to 0.250) (Table 2).
Across the four locations, the lowest inbreeding coefficient (FIS) was found in the HI individuals followed by LEI, M and SC (Table 3). These results showed higher genetic diversity scores and less inbreeding in the island sites and specifically the recently colonised LEI. Heterozygosity scores reveal SC as the most heterozygous population, followed by HI, LEI and M (Table 3).
The BayesAss analysis shows that there is very little contemporary migration among the four populations (Table 4). SC to M on the mainland is the only comparison where the 95% credible values do not overlap zero.
Discussion
We investigated silvereye population genetic structure across the Southern Great Barrier Reef (GBR) Capricorn Bunker group and mainland Queensland, with a focus on the recently colonised LEI. By comparing geographically equidistant populations separated by water and land, we show the amplifying effect of water barriers on genetic divergence beyond simple isolation by distance effects. This could be related to a number of factors that reduce dispersal, establishment and breeding opportunities, including the costs of overwater dispersal, intraspecific competition and reduced reproductive compatibility26. The closer genetic relationship between LEI and Heron Island compared to the mainland populations confirms that LEI was colonised from a Capricorn Bunker Island and not the mainland. Dense clustering of the two mainland sampling locations indicates that there is little genetic structure within the mainland populations, likely due to frequent, ongoing gene flow. The mainland sites can therefore be viewed as admixed populations, though the two sampling locations do not span the full range of this geographically widespread subspecies, and directional migration or gene flow may either be asymmetric or difficult to determine based on our sample sizes27.
The LEI population is genetically closer to the Heron Island population than the mainland, confirming the subspecies origin as Z. l. chlorocephalus. Contemporary movement from Heron Island to Lady Elliot is supported by a single individual that is either a migrant or a result of recent admixture of a migrant with a local LEI individual. However, we also report moderate, but statistically significant levels of divergence between the Heron Island population and that on LEI, also seen in the previous fingerprinting study20, suggesting that the LEI population may be sourced from another one of the Bunker group islands—whether solely, or in addition to Heron Island. Given that Z. l. chlorocephalus has a large range (422 km) along the southern GBR from Bushy Island in the north (20.9540° S, 150.0726° E) to LEI in the south28,29, and that there is some, but limited evidence from banding studies of movement between islands (less than 1% of the Heron Island population having been found breeding on other islands22,29), there is likely opportunity for genetic divergence among Z. l. chlorocephalus populations to accrue over time. A genetic study across the entire range of the subspecies would be needed to determine if a closer genetic match to the LEI population exists in the region. A more complete investigation of genetic relationships across the range, including islands that could act as stepping-stones between LEI and Heron Island could also reveal previously unknown patterns of interisland connectivity. For example, silvereyes have been documented across neighbouring islands with gaps as little as 40 km from LEI, including Lady Musgrave, One Tree, Hoskyn and Fairfax Islands28,30. In particular, Fairfax Island has similar distances from LEI to Heron Island and has documented sightings of three Heron Island colour banded individuals28,31. However, silvereye surveys across the entire Capricorn Bunker were last conducted in 199232, hence there has been no opportunity for documenting contemporary movement of Heron Island banded birds.
If a population genetic study of the Capricorn Bunker Island revealed that Heron Island remained the most likely source of the LEI population, then our data would indicate that within 50 years, it has sufficiently diverged to be a separate population within the Capricorn Bunker, which represents a rapid rate of divergence. Rapid divergence at both neutral and selected loci could be the result of a founder effect resulting in a non-representative suite of variants, along with strong drift in generations immediately post-colonisation if the population remains small and isolated15. Genetic diversity measures for both the Heron Island and LEI populations were comparable to the mainland populations and did not suggest a founder effect had occurred on LEI. Genetic evidence and modelling of founder population sizes in other recently colonised silvereye populations concluded that a combination of substantial founding flock sizes and rapid population recovery insulates silvereye populations from founder effects15,19,33. Thus, although founder effects and drift may have contributed to some of the patterns we have uncovered, these subpopulations do not appear to be subject to major losses of diversity seen in some other cases where founder effects and low population sizes interact19,34,35.
Interestingly, both island populations had lower inbreeding scores than the mainland sites. This was unexpected as mainland populations tend to be larger and less inbred than island populations, with increased connectivity between habitat patches9,36. The higher levels of inbreeding observed at mainland sites could be due to the timing of sampling (between February and July) following the breeding season (September and December). Site attachments are common among silvereyes and fledglings have been observed following their parents whilst foraging, often until independence is reached37. Therefore, it is possible that predominantly closely related individuals (i.e., related juveniles) were sampled, consequently leading to high inbreeding scores on the mainland. Additionally, one mainland site had limited samples (four only in Maryborough) and those from the Sunshine Coast were from a single flock, both of which could have affected the intra-population inbreeding calculations. Other explanations of higher inbreeding levels could be related to factors such as population declines, habitat fragmentation or general matrix quality on the mainland sites38.
Rapid divergence from a Heron Island source population could also be explained by a selective response to a novel environment19. LEI’s revegetation process since being stripped during decades of guano mining has meant that its relative successional ecosystem is younger and more highly managed than other Bunker group islands39. However, it seems unlikely that this explains genetic divergence between the Heron and LEI populations seen here. This is because the process would only apply to loci under selection and our data likely includes a substantial number of neutral loci, and secondly, the two populations are phenotypically similar: weight averages 13 g, (20% heavier than their mainland counterparts, with 10 g average (Table 1)). Weight in silvereyes is highly representative of overall body size measurements, and these (particularly bill size) have been found to be under directional selection in island silvereyes15,22, an expectation of the island rule40,41. Therefore, divergent selective regimes as an explanation for rapid divergence seems unlikely and LEI’s divergence may reflect that they come primarily from another already diverged Bunker population.
Overall, examining population connectivity among conspecifics is vital for understanding a species’ persistence and evolution, contributing to our knowledge of evolutionary processes42. This study further indicates that the isolation via a long-distance water barrier is likely a much more important factor than overall distance in disrupting gene flow. This supports the isolation by resistance model which predicts a positive relationship between genetic differentiation and the resistance distance8,9. Furthermore, intermixing among mainland populations was evident, even though the mainland sites are slightly further apart geographically than the island sites (i.e., mainland groups are 150 km apart, compared to the islands being 110 km apart and the island-mainland sites 80 km apart). Thus, more rapid divergence potential can be observed among insular silvereyes, due to isolation via long distance water barrier. It is likely that future studies could investigate whole-genome level variation and the relative effects of distance, habitat connectivity, founder effects and drift on these subpopulations, including potentially functional or adaptive genetic changes19.
In summary, this study provides valuable insights into how distance and water barriers drive relative levels of genetic divergence among southeast Queensland silvereyes. Furthermore, findings provide new information regarding the most recently colonised population on LEI, highlighting how geographically localised population structure is influenced by barriers to gene flow and confirming the LEI subspecies a Z l. chlorocephalus. Not only does this inform our body of knowledge about current versus historical patterns of divergence but also allows a broader understanding around the maintenance of isolated and potentially genetically distinct populations among avifauna.
Methods
Study sites
Genetic samples of resident silvereyes were collected across four locations in southeast Queensland (Fig. 1): two from the mainland, Maryborough and Sunshine Coast, and two Great Barrier Reef Capricorn Bunker coral cay islands, Heron Island and Lady Elliot Island (LEI) (Table 1). The resident mainland subspecies is Z. l. cornwalli, and the resident Heron Island and LEI subspecies is Z. l. chlorocephalus. Population sampling formed a longitudinal transect with each site separated by up to 160 km with the two island populations roughly 85 km from the mainland. Fieldwork was conducted in both rural and coral cay habitat types, sampling resident silvereye populations. Whilst the approximately equidistant nature of the four study sites was the main driver for site selection, silvereye abundance and resource availability were also considered when selecting the sites.
The subspecies Z. l. chlorocephalus occurs across multiple islands of the Capricorn Bunker group and exists as a metapopulation with limited movement among island populations20. The two Capricorn Bunker silvereye populations sampled in this study, LEI and Heron Island, have very different demographic histories. LEI (Fig. 1) is a 49-hectare coral cay that was continually cleared of vegetation due to guano mining and goat over-grazing between the years of 1863 and 196939. This effectively removed all habitat for terrestrial birds and it was not until after revegetation efforts commenced in 1969 that silvereyes (re)colonised LEI20,21,43. This was shown by a survey in the 1970s that found no silvereyes43 and then subsequent surveys between 1984 and 1986 recording up to 200 resident silvereyes20,21.
In contrast, Heron Island is a smaller (17-hectare) wooded coral cay, just over 100 km north of LEI, which likely has had a silvereye population for as long as the island has been vegetated (approximately between 3000 and 4000 years)19,22,44. The breeding population size is between 200 and500 adult individuals of the the Z. l. chlorocephalus subspecies22,45. Long term studies since the 1960s show that less than 1% of banded Heron Island individuals move to other Capricorn Bunker islands, even those that are geographically close (e.g. Wilson and One Tree islands, only 17 km away)28. Despite intermittent arrival of mainland vagrant silvereyes45,46, they do not form breeding pairs with the Z.l. chlorocephalus subspecies on Heron Island, with no evidence of gene flow with any mainland subspecies19,29,47.
Blood sampling field methods
Blood samples from silvereyes were collected at the LEI, Sunshine Coast and Maryborough sites between February and July 2021 (Table 1). Samples from Heron Island individuals were collected as part of a prior study in 201522 (Table 1). Birds were captured with a mist net and banded with a numbered Australian Bird and Bat Banding Scheme (ABBBS) band (and a series of colour bands on Heron Island). A small blood sample (~ 10–40 μL) was then taken from the brachial vein with a 26G needle, collecting the resulting blood droplet with a heparinized haematocrit tube. Blood was stored either in 95% ethanol, lysis buffer48 or on filter paper until DNA extraction.
DNA extraction, sequencing and SNP discovery
DNA was extracted using a QIAGEN DNeasy blood and tissue extraction kit, following the manufacturer’s protocol (DNeasy® Blood & Tissue Handbook)49 with some modifications. Prior to extraction blood samples were homogenised using the subsequent steps: vortexed at full speed (10 rpm) for 5–15 min; 400 μl pipetted into a 1.5 Eppendorf tube; centrifuged at 20,000 g for 1 min; and the blood was separated from ethanol using a pipette.
DNA was eluted from the extraction column with 100 μl of AE buffer49. To concentrate the DNA, the buffer was run through the column a second time and the volume of the elute reduced to 50–60 μl in an Eppendorf’s Vacfuge Vacuum Concentrator. Samples were sequenced using restriction-associated digest protocols at Diversity Arrays Technology Pty Ltd (DArT) (http://www.diversityarrays.com/), using the DArTseq™ method24. DartSeq is a genotyping by sequencing approach that reduces genome complexity through digestion of genomic DNA with pairs of restriction enzymes. The restriction enzymes, in this study PstI and SphI, have site-specific adaptors to enable high throughput sequencing in an Illumina Genome Analyzer IIx (Illumina Inc., San Diego, CA). Samples are processed using the proprietary DArTsoft analytical pipeline50. Reads were then mapped to a 64.0 × coverage silvereye subspecies genome (Zosterops lateralis melanops) (1.036 Gb) (NCBI Assembly GCA_001281735.1) as described by Cornetti et al.51, using the National Center for Biotechnology Information’s (NCBI) Basic Local Alignment Search Tool (BLAST)52. This resulted in 62 454 SNPs called with an average density of 16.77 reads per locus (69 bp length).
To assure high quality of our SNP data set for downstream analyses to address our questions, additional filtering was done using the radiator package53 and dartR23 in R (version 2022.02.2)54. Multiple parameter sets were investigated to maximise the number of individuals and loci retained in the dataset while reducing noise and missing data. The final filtering followed the order and threshold criteria of: reproducibility = 0.85, individual call rate = 0.6, SNP callrate = 0.9, random removal of secondary SNPs, read depths between 5 and 50, minor allele frequency = 0.01, and Hardy–Weinberg equilibrium at 0.05 with false rate discovery adjustment for multiple p-value comparisons (not applied to the Maryborough location which had fewer than 5 samples). Putative loci under selection were investigated with Outflank55 implemented in dartR23, which uses a likelihood approach to identify detect Fst outliers. The default false discovery rate threshold (0.05) was used for calculating q-values, as well as default proportion (0.05) of loci trimmed at the upper and lower end of the range of Fst prior to applying the likelihood function.
Population structure and admixture
To assess population structure, Principal Coordinate Analysis (PCoA) plots were visualised with ggplot256 and the axes selected based on which eigenvectors explained the most variation in the SNP data.
Weir–Cockerham’s Fst parameter with 10,000 bootstrap values was calculated in the Stampp v1.6.3 package in R54. These measures give an indication of genetic differentiation on a scale of 0–1, where 0 represents complete panmixia and 1 is fixation of alternative alleles42.
We examined admixture with the sparse non-negative matrix factorisation algorithm using the snmf function implemented in the R package LEA25. This method is similar to programs such as Admixture57 and Structure58, but is optimised for large SNP data sets as it is computationally faster and robust to departures from traditional population genetic model assumptions such as Hardy–Weinberg equilibrium25. The optimal number of ancestral populations (K) was assessed based on the cross-entropy criterion through examining a range of K from 1 to 10 with 10 replicates for each scenario25. This was to account for any potential structure that was unaccounted for by the location assignment. Individual assignment admixture was visualised using the pophelper package59.
Genetic diversity and demographic estimates
Expected and observed homozygosity, heterozygosity and inbreeding coefficients (FIS) were calculated using the gl.report.heterozygosity function in dartR23. Heterozygosity measurements reflect allele frequencies as a percentage per individual and averages for each population, with those closer to 1 being more heterozygous. Inbreeding coefficient (FIS) outputs closer to 1 represent higher levels of inbreeding.
Lastly, recent migration rates between the four populations were assessed using BA3-SNPs v3.0.460, a modification of BayesAss61 that allows handling of large SNP datasets. First, the optimal mixing parameters for migration rates (deltaM = 0.1563) were assessed, allele frequencies (delta = 0.325), and inbreeding coefficients (deltaF = 0.0188) by running ten repetitions of BA3-SNP-autotune v3.0.4 as recommended by Mussmann et al.60 Subsequently, BA3-SNPs was run with the predefined mixing parameters for 50 million generations, sampling every 100th generation. The first ten million generations were discarded as burn-in and chain convergence was assessed using Tracer v1.7.262. Following author recommendations61, migration rates were deemed significant if 95% credible sets (μ ± σ × 1.96) did not contain zero. Estimation of migration rates was performed on a subset of 7 859 SNPs where all samples were genotyped (i.e. sample missingness was zero).
Data availability
The datasets generated and analysed during the current study are available in the OSF repository: https://osf.io/g4m7p/?view_only=08a01674cde54a679a3612505c3d7f52.
Change history
24 October 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-76124-3
References
Samarasin, P., Shuter, B. J., Wright, S. I. & Rodd, F. H. The problem of estimating recent genetic connectivity in a changing world. Conserv. Biol. 31, 126–135. https://doi.org/10.1111/cobi.12765 (2017).
Slatkin, M. Gene flow and the geographic structure of natural populations. Science 236, 787–792 (1987).
Sexton, J. P., McIntyre, P. J., Angert, A. L. & Rice, K. J. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 40, 415–436 (2009).
Kisel, Y. & Barraclough, T. G. Speciation has a spatial scale that depends on levels of gene flow. Am. Nat. 175, 316–334 (2010).
Excoffier, L., Foll, M. & Petit, R. J. Genetic consequences of range expansions. Annu. Rev. Ecol. Evol. Syst. 40, 481–501 (2009).
Grant, P. R. Reconstructing the evolution of birds on islands: 100 years of research. Oikos 92, 385–403. https://doi.org/10.1034/j.1600-0706.2001.920301.x (2001).
Parent, C. E., Caccone, A. & Petren, K. Colonization and diversification of Galápagos terrestrial fauna: A phylogenetic and biogeographical synthesis. Philos. Trans. Royal Soc. B Biol. Sci. 363, 3347–3361. https://doi.org/10.1098/rstb.2008.0118 (2008).
Kikkawa, J. Range extension of the Capricorn Silvereye ‘Zosterops lateral’s chlorocephala’, with a note on the birds of Bushy Island. Sunbird J. Qld. Ornithol. Soc. 27, 45–48. https://doi.org/10.3316/informit.162170991065125 (1997).
McRae, B. H. Isolation by resistance. Evolution 60, 1551–1561. https://doi.org/10.1111/j.0014-3820.2006.tb00500.x (2006).
Allendorf, F. W. Genetic drift and the loss of alleles versus heterozygosity. Zoo Biol. 5, 181–190. https://doi.org/10.1002/zoo.1430050212 (1986).
Whittaker, R. J. & Fernández-Palacios, J. M. Island Biogeography: Ecology, Evolution, and Conservation (Oxford University Press, 2007).
Shirk, R. Y., Hamrick, J. L., Zhang, C. & Qiang, S. Patterns of genetic diversity reveal multiple introductions and recurrent founder effects during range expansion in invasive populations of Geranium carolinianum (Geraniaceae). Heredity 112, 497–507. https://doi.org/10.1038/hdy.2013.132 (2014).
Clegg, S. Evolutionary changes following island colonization in birds. in The Theory of Island Biogeography Revisited, 293–325 (2010).
Mundy, N. I., Winchell, C., Burr, T. & Woodruff, D. S. Microsatellite variation and microevolution in the critically endangered San Clemente Island loggerhead shrike (Lanius ludovicianus mearnsi). Proc. Royal Soc. Lond. Series B Biol. Sci. 264, 869–875. https://doi.org/10.1098/rspb.1997.0121 (1997).
Clegg, S. M. et al. Genetic consequences of sequential founder events by an island-colonizing bird. Proc. Natl. Acad. Sci. 99, 8127–8132. https://doi.org/10.1073/pnas.102583399 (2002).
Moyle, R. G., Filardi, C. E., Smith, C. E. & Diamond, J. Explosive Pleistocene diversification and hemispheric expansion of a “great speciator”. Proc. Natl. Acad. Sci. 106, 1863–1868. https://doi.org/10.1073/pnas.0809861105 (2009).
Gill, F., Donsker, D. & Rasmussen, P. Sylviid babblers, parrotbills & white-eyes. World Bird List Version 5 (2017).
Menkhorst, P., Rogers, D., Clarke, R. & Sullivan, P. The Australian Bird Guide (Csiro Publishing Melbourne, 2017).
Sendell-Price, A. T., Ruegg, K. C., Robertson, B. C. & Clegg, S. M. An island-hopping bird reveals how founder events shape genome-wide divergence. Mol. Ecol. 30, 2495–2510. https://doi.org/10.1111/mec.15898 (2021).
Degnan, S. M. Genetic variability and population differentiation inferred from dna fingerprinting in silvereyes (aves: zosteropidae). Evolution 47, 1105–1117. https://doi.org/10.1111/j.1558-5646.1993.tb02139.x (1993).
Walker, T. The birds of Lady Elliot Island. Sunbird J. Qld. Ornithol. Soc. 16, 73–82. https://doi.org/10.3316/informit.694775841509513 (1986).
Clegg, S. M., Frentiu, F. D., Kikkawa, J., Tavecchia, G. & Owens, I. P. 4000 years of phenotypic change in an island bird: Heterogeneity of selection over three microevolutionary timescales. Evol. Int. J. Org. Evol. 62, 2393–2410. https://doi.org/10.1111/j.1558-5646.2008.00437.x (2008).
Gruber, B., Unmack, P. J., Berry, O. F. & Georges, A. DARTR: An r package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Mol. Ecol. Resour. 18, 691–699. https://doi.org/10.1111/1755-0998.12745 (2018).
Kilian, A. et al. Diversity arrays technology: A generic genome profiling technology on open platforms. Data Prod. Anal. Popul. Genom. https://doi.org/10.1007/978-1-61779-870-2_5 (2012).
Frichot, E. & François, O. LEA: An R package for landscape and ecological association studies. Methods Ecol. Evol. 6, 925–929 (2015).
Robertson, E. P. et al. Isolating the roles of movement and reproduction on effective connectivity alters conservation priorities for an endangered bird. Proc. Natl. Acad. Sci. 115, 8591–8596. https://doi.org/10.1073/pnas.1800183115 (2018).
Little, G., Little, J., Kyte, R. & Stuart, A. Silvereye subspecies on Broughton Island New South Wales. 44, 38–43 (2020).
Kikkawa, J. The Capricorn white-eye ‘Zosterops chlorocephalu’. Sunbird J. Qld. Ornithol. Soc. 33, 64–76. https://doi.org/10.3316/informit.881763393810023 (2003).
Brook, B. W. & Kikkawa, J. Examining threats faced by island birds: A population viability analysis on the Capricorn silvereye using long-term data. J. Appl. Ecol. 35, 491–503. https://doi.org/10.1046/j.1365-2664.1998.3540491.x (1998).
Booth, J. Birds of Fairfax island (Bunker group) and Wreck island (Capricorn group), Great Barrier reef. Sunbird J. Qld. Ornithol. Soc. 1, 85–92 (1970).
Verhoeven, M. A. et al. Age-dependent timing and routes demonstrate developmental plasticity in a long-distance migratory bird. J. Anim. Ecol. 91, 566–579 (2022).
Domma, S. & Reciter, H. The birds of One Tree Island with notes on their yearly cycle and feeding ecology. Sunbird J. Qld. Ornithol. Soc. 4, 63–77 (1973).
Estoup, A. & Clegg, S. M. Bayesian inferences on the recent island colonization history by the bird Zosterops lateralis lateralis. Mol. Ecol. 12, 657–674. https://doi.org/10.1046/j.1365-294x.2003.01761.x (2003).
Hunt, D. A. G. A., Dibattista, J. D. & Hendry, A. P. Effects of insularity on genetic diversity within and among natural populations. Ecol. Evol. 12, e8887. https://doi.org/10.1002/ece3.8887 (2022).
Duncan, R. P. & Blackburn, T. Causes of extinction in island birds. Anim. Conserv. 10, 149–150. https://doi.org/10.1111/j.1469-1795.2007.00110.x (2007).
Frankham, R. Inbreeding and extinction: Island populations. Conserv. Biol. 12, 665–675. https://doi.org/10.1111/j.1523-1739.1998.96456.x (1998).
Mayr, E. Evolution as a Process. eds. Huxley, J., Hardy, AC & Ford, EB. (1954).
Wilson, S. M. & Kikkawa, J. Post-fledging parental investment in the Capricorn silvereye. Emu 88, 81–87 (1988).
Rae, N. L. & Carter, S. J. B. Rock to riches: The revegetation of Lady Elliot Island. Aust. J. Multi-Discip. Eng. 5, 33–38. https://doi.org/10.1080/14488388.2007.11464754 (2007).
Benítez-López, A. et al. The island rule explains consistent patterns of body size evolution in terrestrial vertebrates. Nat. Ecol. Evol. 5, 768–786. https://doi.org/10.1101/2020.05.25.114835 (2021).
Potvin, D. A. Larger body size on islands affects silvereye Zosterops lateralis song and call frequency. J. Avian Biol. 44, 221–225. https://doi.org/10.1111/j.1600-048x.2012.05820.x (2013).
Weir, B. S. & Goudet, J. A unified characterization of population structure and relatedness. Genetics 206, 2085–2103 (2017).
Fien, I. A note on the Avifauna of Lady Elliot island. Sunbird J. Qld. Ornithol. Soc. 2, 63–67 (1971).
Hopley, D. The Geomorphology of the Great Barrier Reef (Wiley, 1982).
Kikkawa, J. Birds recorded at Heron island. Sunbird J. Qld. Ornithol. Soc. 1, 34–48 (1970).
Clegg, S. M. et al. Microevolution in island forms: The roles of drift and directional selection in morphological divergence of a passerine bird. Evolution 56, 2090–2099 (2002).
Estandia, A. et al. Candidate gene length polymorphisms are linked to dispersive behaviour: Searching for a mechanism behind the “paradox of the great speciators”. BioRxiv https://doi.org/10.1101/2023.01.19.524190v1.abstract (2023).
Seutin, G., White, B. N. & Boag, P. T. Preservation of avian blood and tissue samples for DNA analyses. Can. J. Zool. 69, 82–90 (1991).
DNeasy, Q. Blood & tissue handbook. in Purification of Total DNA from Animal Tissues (Spin-Column Protocol), 28–30. (2006).
Cruz, V. M. V., Kilian, A. & Dierig, D. A. Development of DArT marker platforms and genetic diversity assessment of the US collection of the new oilseed crop lesquerella and related species. PloS One 8, e64062. https://doi.org/10.1371/journal.pone.0064062 (2013).
Cornetti, L. et al. The genome of the “great speciator” provides insights into bird diversification. Genome Biol. Evol. 7, 2680–2691. https://doi.org/10.1093/gbe/evv168 (2015).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2 (1990).
Gosselin, T., Lamothe, M., Devloo-Delva, F. & Grewe, P. Radiator: RADseq data exploration, manipulation and visualization using R. R Package Version 1 (2020).
R Development Core Team. A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021).
Whitlock, M. C. & Lotterhos, K. E. Reliable detection of loci responsible for local adaptation: Inference of a null model through trimming the distribution of F ST. Am. Nat. 186, S24–S36 (2015).
Wickham, H. ggplot2: Elegant graphics for data analysis. https://doi.org/10.1080/15366367.2019.1565254 (2016).
Rosenberg, N. A. et al. Genetic structure of human populations. Science 298, 2381–2385 (2002).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959. https://doi.org/10.1534/genetics.116.195164 (2000).
Francis, R. M. Pophelper: An R package and web app to analyse and visualize population structure. Mol. Ecol. Resour. 17, 27–32. https://doi.org/10.1111/1755-0998.12509 (2017).
Mussmann, S. M., Douglas, M. R., Chafin, T. K. & Douglas, M. E. BA3-SNPs: Contemporary migration reconfigured in BayesAss for next-generation sequence data. Methods Ecol. Evol. 10, 1808–1813 (2019).
Wilson, G. A. & Rannala, B. Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163, 1177–1191 (2003).
Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901–904 (2018).
Acknowledgements
We begin by acknowledging the Traditional Custodians of the land on which we conducted our study, the Butchulla people of Badtjala and recognise their continued connection to the land. We also pay my respects to their Elders past and present and extend that respect to Aboriginal and Torres Strait Islander peoples and the continuation of their cultural, spiritual, and educational practices. We thank the Maleny Botanic Gardens, Cecile Espigole, and the Lady Elliot Island Eco-Resort for land access to sample silvereyes; Asia Haines and the Leaf to Reef team for collaborative efforts and field assistance. This project was funded by the University of the Sunshine Coast and the Reef Islands Initiative, a Great Barrier Reef Foundation program, supported by Lendlease, the Australian Government’s Reef Trust, the Queensland Government and the Fitzgerald Family Foundation.
Author information
Authors and Affiliations
Contributions
C.D., S.M.C., K.A.T. and D.A.P. conceived the manuscript; A.R., C.D., S.M.C. and D.A.P. wrote the manuscript, A.R., A.T.S.-P. and Y.F. performed formal data analyses, K.A.T. and C.D. secured funding and resources and A.R., C.D., S.M.C. and D.A.P. edited and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Samples from Lady Elliot Island, Maryborough and the Sunshine Coast were collected with the approval of the University of the Sunshine Coast Animal Ethics committee (project ANA20173), the Great Barrier Reef Marine Park (Permit G21/45947.1), and Queensland Government Wildlife Authority (Research Permit WA0029696). Heron Island samples were collected with the approval of the University of Queensland animal ethics committee (project ZOO/ENT/002/05/NERC), and the Queensland Government (Scientific Purposes Permit WITK02736805). All methods were carried out in accordance with the relevant guidelines and regulations, and in compliance with the ARRIVE guidelines (https://arriveguidelines.org).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article omitted an affiliation for Dominique A. Potvin. Full information regarding the correction made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Radu, A., Dudgeon, C., Clegg, S.M. et al. Genetic patterns reveal geographic drivers of divergence in silvereyes (Zosterops lateralis). Sci Rep 14, 20426 (2024). https://doi.org/10.1038/s41598-024-71364-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71364-9