Abstract
This study aimed to investigate the cutoff values of standardized uptake values (SUVs) and their accuracy using single-photon emission computed tomography–computed tomography (SPECT–CT) for temporomandibular joint (TMJ) osteoarthritis (OA) based on magnetic resonance imaging (MRI) and clinical examination. We included 106 joints of 53 patients with TMJ OA. SUVmax and SUVpeak of each TMJ was measured. SUVref was set as the SUV at the clivus. The diagnostic performance, including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy, were evaluated. SUVmax was 5.15, with a sensitivity of 59.375, specificity of 100.000, PPV of 100.000, NPV of 61.765, and accuracy of 75.472. The cutoff value for SUVpeak was 3.635, with sensitivity of 56.250, specificity of 100.000, PPV of 100.000, NPV of 60.000, and accuracy of 73.585. SUVmax was 3.286 ± 0.780 and 6.623 ± 3.442 in the non-OA and OA groups, respectively (p < 0.001). SUVpeak was 2.324 ± 0.688 and 4.913 ± 2.749 in the non-OA and OA groups, respectively (p < 0.001). SPECT–CT can be helpful for the diagnosis of patients clinically suspected of having OA. It is also recommended that clinicians keep in mind that patients with SUVmax values higher than the cutoff value should be managed with a higher possibility of OA.
Similar content being viewed by others
Introduction
Temporomandibular joint (TMJ) osteoarthritis (OA) is a degenerative disease that is accompanied by articular tissue deterioration, underlying subchondral bone remodeling, immobility, and pain1,2. According to previous studies, the overall prevalence of TMJ OA is reportedly 18.0–84.7% among all patients with temporomandibular disorder (TMD)3. Computed tomography (CT) or cone beam CT has been widely used in the diagnosis of OA to observe bone changes4,5.
Recently, with the development of various imaging techniques, several methods have been used to diagnose TMJ OA, including panoramic view, ultrasonography, magnetic resonance imaging (MRI) and nuclear imaging6,7,8,9. Among these, nuclear imaging, such as bone scintigraphy, provides relatively early detection compared with other radiographical modalities that show changes only after decalcification has progressed to some extent10.
Single-photon emission computed tomography (SPECT)–CT, another type of nuclear imaging, can be useful for simultaneously diagnosing and predicting the prognosis of TMD by using SPECT and CT images simultaneously11,12. In particular, technetium-99 m methylene diphosphonate (99mTc-MDP) reflects the rate of local osteometabolic activity and provides more localized information on bone metabolism related to bone changes10.
The standardized uptake value (SUV) is used to quantify the degree of uptake on the SPECT–CT images. However, in clinical practice, there are some discrepancies between the clinical diagnosis and SUV. In other words, some patients are diagnosed with TMJ OA but do not have a high SUV, whereas others are not thought to have TMJ OA but have a high SUV. Moreover, exact diagnostic criteria for TMJ OA using SPECT–CT have not yet been established.
This study aimed to investigate the cutoff value and accuracy of using SPECT–CT for TMJ OA diagnosis based on MRI and clinical examination.
Materials and methods
Participants
This retrospective study was approved by the hospital’s institutional review board (approval No. #3-2023-0320), and a waiver of written informed consent was granted. This study was also complied with the tenets of the Declaration of Helsinki. All patients visited the Department of Oral and Maxillofacial Surgery, Gangnam Severance Hospital, Yonsei University (Seoul, Korea) with complaints of discomfort and pain in the TMJ between January 2018 and April 2022. After clinical examination, the patients underwent MRI and SPECT–CT at the Department of Radiology and Nuclear Medicine of the same hospital. Patients were excluded if they met the following criteria: (1) scan interval of > 6 months between MRI and SPECT–CT; (2) history of radiation therapy in the head and neck area; (3) a diagnosis of rheumatoid arthritis; or (4) history of trauma or surgery on the maxillofacial bone, including the TMJ. Finally, 56 patients (112 joints) were included in this study.
MRI
MRI was performed using a 3.0-T Magnetom scanner (Achieva; Philips Medical Systems, Best, The Netherlands) with 3-inch surface coils for the TMJs. For T1-weighted imaging, the following parameters were used: repetition time, 450 ms; echo time, 20 ms; slice thickness, 3 mm; field of view, 120 mm; and acquisition matrix size, 240 × 240. The parameters for T2-weighted imaging were as follows: repetition time, 2900 ms; and echo time, 90 ms13.
Clinical diagnosis of TMJ OA
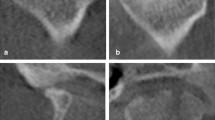
Clinically, TMJ OA was diagnosed based on previous studies14,15. Patients who fall into stage IV (intermediate/late) or stage V (late) of the Wilkes classification are diagnosed with OA16,17. In brief, TMJ OA was diagnosed when structural changes in the TMJ condyle were observed on MRI, and the patients experienced pain. Patients with structural changes without pain or structural changes were diagnosed as non-OA.
SPECT–CT
SPECT–CT examinations were performed using a Symbia Intevo16 (Siemens Healthineers, Erlangen, Germany). SPECT was performed 3 h after intravenous administration of 740 MBq of 99mTc-HDP from the top of the head to the lung apex with the following parameters: 3° rotation per step, 20 s acquisition time per projection, and 512 × 512 matrix size. Low-dose CT images were acquired at 110 kV and 120 reference mA, using adaptive dose modulation. The images were reconstructed using xSPECT Quant and xSPECT Bone (Siemens Healthineers, Erlangen, Germany) for high-resolution analysis and quantification.
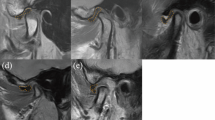
Image analysis was performed by two nuclear medicine physicians (J.H. Lee and Y.H. Ryu) using an open-source LIFEx software version 7.4.618. The SUV was calculated as follows: SUV = (decay-corrected activity [kBq] per mL of tissue volume)/(injected activity [kBq] per gram of body mass). A spherical region of interest (ROI) was manually placed centered on the mandibular condyle and adjusted to encompass all relevant TMJ structures based on axial and coronal CT images. SUVmax was defined as the maximum voxel value within the ROI. SUVpeak was defined as the average SUV within a 1 cm3 sphere centered on the maximum value voxel of the ROI. To account for variations between patients, SUVmax and SUVpeak were normalized by the average SUV of the clivus (SUVref), producing SUVmax/SUVref and SUVpeak/SUVref, respectively.
Statistical analysis
McNemar’s test was used to determine whether there was a difference in the rates of diagnosis using SPECT–CT and clinical diagnoses. Cohen’s kappa was calculated to confirm the overall agreement between SPECT–CT and clinical diagnosis. Using clinical diagnosis as the outcome variable, each cutoff value was calculated using the Youden index, and diagnostic performance was evaluated. The area under the ROC curve, sensitivity, specificity, accuracy, PPV, and NPV was calculated. A Generalized estimating equation (GEE) was used to adjust the estimate for correlated observations and to determine the differences in SPECT–CT variables between patients with and without OA based on clinical diagnosis. Finally, a GEE for all variables was used to determine how SPECT–CT values affected the diagnosis. All statistical analyses were performed by statistical experts using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
In total, 53 patients (106 joints) were included in this study. Of these, five were male and 48 were female, and the average age was 45.47 ± 16.30 years. Among the 106 joints, 42 (39.62%) were diagnosed as non-OA and 64 (60.38%) were diagnosed as OA based on clinical examination and MRI. Among 42 joints diagnosed as non-OA, 28 joints showed neither pain nor bone change, 12 joints showed bone change without pain, and two joint showed pain without bone change. Forty joints (37.74%) were diagnosed with non-OA and 66 joints (62.26%) were diagnosed as OA based on SPECT–CT examination. There was no significant difference between the clinical and SPECT–CT diagnoses (p = 0.6374). The results are summarized in Table 1. Cohen’s kappa value between the two diagnoses was 0.642 (95% confidence interval [CI] 0.492–0.792).
The SUVmax cutoff value was 5.15. The area under the curve (AUC) was 0.797 (95% CI 0.736–0.858) with sensitivity, 59.375 (95% CI 47.342–71.408); specificity, 100.000 (95% CI 100.000–100.000); accuracy, 75.472 (95% CI 67.281–83.663); positive predictive value (PPV), 100.000 (100.000–100.000); and negative predictive value (NPV), 61.765 (95% CI 50.214–73.315). The cutoff value for SUVpeak was 3.635. AUC was 0.781 (95% CI 0.720–0.842) with sensitivity, 56.250 (95% CI 44.096–68.404); specificity, 100.000 (95% CI 100.000–100.000); accuracy, 73.585 (95% CI 65.192–81.978); PPV, 100.000 (100.000–100.000); and NPV, 60.000 (95% CI 48.523–71.477) (Table 2).
There were significant differences between the non-OA and OA groups in all investigated variables (Table 3). SUVmax was 3.286 ± 0.780 and 6.623 ± 3.442 in the non-OA and OA groups, respectively (p < 0.0001). SUVpeak was 2.324 ± 0.688 and 4.913 ± 2.749 in the non-OA and OA groups, respectively (p < 0.0001). Thirty-two of the 42 (76.19%) joints were diagnosed as non-OA both on SPECT–CT and clinically. Similarly, 56 of 64 (87.50%) joints were diagnosed as OA both on SPECT–CT and clinically. Among the patients clinically diagnosed as non-OA, none showed SUVmax and SUVpeak greater than the cutoff values of 5.15 and 3.635, respectively.
In Generalized estimating equation (GEE), the odds ratio (OR) was 3.184 (95% CI 2.040–4.969) for SUVmax and 3.679 (95% CI 2.260–5.990) for SUVpeak (p < 0.0001). When SUVmax was greater than the cutoff value (5.15), the OR was 66.307 (95% CI 7.880–557.920), which was statistically significant (p = 0.001). Similarly, the OR was 46.884 (95% CI 6.909–318.180) when the SUVpeak was greater than the cutoff value (3.635), which was significant (p < 0.0001). The results are summarized in Table 4.
The receiver operating characteristic (ROC) curves for all variables are shown in Fig. 1. There was no significant difference between SUVmax and SUVpeak (p = 0.3684), and SUVmax/SUVref and SUVpeak/SUVref (p = 0.6096), respectively. Other variables such as SUVmax vs SUVmax/SUVref (p = 0.0005), and SUVpeak vs SUVpeak/SUVref (p = 0.0007) showed significant difference.
Discussion
SPECT–CT has been widely used to diagnose TMJ OA, and many studies have been conducted9,19. Recently, Jeon et al. reported significantly higher value of 99Tc-MDP uptake ratio in OA compared with a normal group20. Although changes in the mandibular condyle can also be determined with CT, SPECT can reflect functional aspects21. Therefore, SPECT–CT has the advantage of being able to identify functional information and specify areas of disease. In addition, we believe it is helpful for determining whether to undergo surgery and identify the surgical site more precisely because SPECT–CT can reflect bone inflammation.
In the present study, the average value of SUVmax was also significantly higher (6.623 ± 3.442) in patients with OA compared with non-OA individuals (3.286 ± 0.780). The SUVmax value in the non-OA group was similar with that observed by Ogura et al22. (3.57 ± 0.46 in normal mandibles). Although there is a significant difference in SUVmax values in cases of OA compared with non-OA, the range is wide, making diagnosis occasionally difficult. Accordingly, this study was conducted to determine the cutoff value of SPECT–CT and to analyze its accuracy in diagnosing TMJ OA.
In this study, OA was diagnosed when the morphology of the condyle was changed with joint pain on palpation, based on the diagnostic criteria for temporomandibular disorders reported in 2014 14. According to the result of this study, the cutoff value of SUVmax for diagnosis of TMJ OA was 5.15, with 59.375 (95% CI 47.342–71.408) sensitivity, 100.000 (95% CI 100.000–100.000) specificity, 100.000 (95% CI 100.000–100.000) PPV, and 61.765 (95% CI 50.214–73.315) NPV. This means that even if the patient has joint pain and bony changes on MRI, the SUVmax on SPECT–CT may show relatively diverse values. However, if the patient's SUVmax exceeds 5.15, it can be interpreted that the patient is likely to have OA. In addition, as shown by the 100% specificity, no patients had SUVmax value exceeding 5.15 on SPECT–CT when clinically diagnosed as non-OA (Table 3).
A Generalized estimating equation was performed to determine how SPECT–CT values affected the diagnosis (Table 4). In this study, the OR of SUVmax was 3.184, with statistical significance (p < 0.0001), indicating that SUVmax affects clinical diagnosis. When the SUVmax increased by 1, the possibility of clinical OA increased by 3.184 times. Likewise, if SUVmax and SUVpeak are greater than the cutoff value (5.15 and 3.635, respectively), we can interpret that the possibility of clinical OA increases by 66.307 and 46.884 times, respectively. Arvola et al. reported a strong correlation between SUVmax, SUVpeak and SUVmean23. Thus, we believe that SUVmax and SUVpeak showed similar results.
In this study, the sensitivity and NPV were relatively low, whereas the specificity and PPV were high for all variables, which may be related to the location of the pain. Even if there is pain in the joint, this pain may originate from surrounding tissues such as in the cases of capsulitis or retrodiscitis, or may be referred pain originating from muscles such as the masseter or temporalis24. Obvious masticatory muscle pain can be distinguished from joint pain, but the distinction may not be easy when the pain becomes chronic. According to Palconet et al.25, the degree of bone change in patients with TMJ OA has a poor correlation with pain. In addition, radiographic imaging of TMD does not necessarily reflect aggressive disease activity26. Therefore, joint pain itself may not necessarily be related to condyle problems.
OA is known to be a self-limiting disease; however, in some cases, patients suffer from pain and may also develop skeletal deformities, including malocclusion, anterior open bite, and facial asymmetry26,27,28. We believe that early detection and treatment can reduce secondary problems that may occur. Using bone scintigraphy, it is possible to detect bone changes earlier than with conventional radiography, because the former demonstrates bone metabolism29,30. In addition, the fusion image of SPECT–CT can increase both sensitivity and specificity compared with SPECT alone or planar scintigraphy31.
This study had some limitations. First, sex and age were not considered in this analysis. It has been reported that the value may vary due to differences in bone metabolic rate depending on age and sex32. In this study, most patients were women, so we believe that the influence of sex is relatively small. However, further research considering age is needed in a larger number of patients. Second, the composition of the non-OA group was another limitation. In this study, we categorized contralateral TMJ to the non-OA group. However, the authors also recognize that such cases cannot be true negative controls.
Despite these limitations, this study attempted to determine a cutoff SUV for the diagnosis of TMJ OA, and the cut-off SUV showed high sensitivity and PPV. Conducting a prospective study with more patients and considering onset of pain in the future will help in a more accurate diagnosis of TMJ-OA.
In conclusion, SPECT–CT can be helpful for the diagnosis of patients clinically suspected of having OA. Clinicians should also keep in mind that patients with SUVmax values higher than the cutoff value should be managed with a higher possibility of OA.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Massilla Mani, F. & Sivasubramanian, S. S. A study of temporomandibular joint osteoarthritis using computed tomographic imaging. Biomed. J. 39, 201–206 (2016).
Zhao, Y. P., Zhang, Z. Y., Wu, Y. T., Zhang, W. L. & Ma, X. C. Investigation of the clinical and radiographic features of osteoarthrosis of the temporomandibular joints in adolescents and young adults. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 111, e27-34 (2011).
Pantoja, L. L. Q. et al. Prevalence of degenerative joint disease of the temporomandibular joint: A systematic review. Clin. Oral Investing. 23, 2475–2488 (2019).
Meng, J. H., Zhang, W. L., Liu, D. G., Zhao, Y. P. & Ma, X. C. Diagnostic evaluation of the temporomandibular joint osteoarthritis using cone beam computed tomography compared with conventional radiographic technology. Beijing Da Xue Xue Bao Yi Xue Ban 39, 26–29 (2007).
Comert Kilic, S., Kilic, N. & Sumbullu, M. A. Temporomandibular joint osteoarthritis: Cone beam computed tomography findings, clinical features, and correlations. Int. J. Oral Maxillofac. Surg. 44, 1268–1274 (2015).
Choi, E., Kim, D., Lee, J. Y. & Park, H. K. Artificial intelligence in detecting temporomandibular joint osteoarthritis on orthopantomogram. Sci. Rep. 11, 10246 (2021).
Yura, S., Harada, S. & Kobayashi, K. Diagnostic accuracy on magnetic resonance imaging for the diagnosis of osteoarthritis of the temporomandibular joint. J. Clin. Diagn. Res. 9, ZC95–ZC97 (2015).
Tanaka, E. et al. Effectiveness of low-intensity pulsed ultrasound on osteoarthritis of the temporomandibular joint: A review. Ann. Biomed. Eng. 48, 2158–2170 (2020).
Kim, Y. H. et al. Protective effects of extracorporeal shockwave on rat chondrocytes and temporomandibular joint osteoarthritis; preclinical evaluation with in vivo(99m)Tc-HDP SPECT and ex vivo micro-CT. Osteoarthritis Cartil. 27, 1692–1701 (2019).
Coutinho, A., Fenyo-Pereira, M., Dib, L. L. & Lima, E. N. The role of SPECT/CT with 99mTc-MDP image fusion to diagnose temporomandibular dysfunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 101, 224–230 (2006).
Suh, M. S., Lee, W. W., Kim, Y. K., Yun, P. Y. & Kim, S. E. Maximum standardized uptake value of (99m)Tc hydroxymethylene diphosphonate SPECT/CT for the evaluation of temporomandibular joint disorder. Radiology 280, 890–896 (2016).
Munakata, K. et al. The use of SPECT/CT to assess resorptive activity in mandibular condyles. Int. J. Oral Maxillofac. Surg. 51, 942–948 (2022).
Kim, J. Y., Jeon, K. J., Kim, M. G., Park, K. H. & Huh, J. K. A nomogram for classification of temporomandibular joint disk perforation based on magnetic resonance imaging. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 125, 682–692 (2018).
Schiffman, E. et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the international RDC/TMD consortium network* and orofacial pain special interest groupdagger. J. Oral Facial Pain Headache 28, 6–27 (2014).
Kim, J. H. et al. Effectiveness of bone scans in the diagnosis of osteoarthritis of the temporomandibular joint. Dentomaxillofac. Radiol. 41, 224–229 (2012).
Wilkes, C. H. Internal derangements of the temporomandibular joint. Pathological variations. Arch. Otolaryngol. Head Neck Surg. 115, 469–477 (1989).
Dimitroulis, G. The prevalence of osteoarthrosis in cases of advanced internal derangement of the temporomandibular joint: A clinical, surgical and histological study. Int. J. Oral Maxillofac. Surg. 34, 345–349 (2005).
Nioche, C. et al. LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 78, 4786–4789 (2018).
Park, K. S. et al. Open-mouth bone scintigraphy is better than closed-mouth bone scintigraphy in the diagnosis of temporomandibular osteoarthritis. Nucl. Med. Mol. Imaging 50, 213–218 (2016).
Jeon, S. H. et al. The clinical effectiveness of fused image of single-photon emission CT and facial CT for the evaluation of degenerative change of mandibular condylar head. Maxillofac. Plast. Reconstr. Surg. 45, 33 (2023).
Ahmadzadehfar, H., Biersack, H.-J. & Herrmann, K. Clinical Applications of SPECT-CT 2nd eds. 165–208 (Springer International Publishing, 2022).
Ogura, I. et al. Tc-99m hydroxymethylene diphosphonate SPECT/CT for the evaluation of osteonecrosis of the jaw: Preliminary study on diagnostic ability of maximum standardised uptake value. Clin. Radiol. 75, 46–50 (2020).
Arvola, S. et al. Comparison of standardized uptake values between 99m Tc-HDP SPECT/CT and 18 F-NaF PET/CT in bone metastases of breast and prostate cancer. EJNMMI Res. 9, 1–9 (2019).
Okeson, J. P. Management of Temporomandibular Disorders and Occlusion 8th edn. (Elsevier, 2013).
Palconet, G., Ludlow, J. B., Tyndall, D. A. & Lim, P. F. Correlating cone beam CT results with temporomandibular joint pain of osteoarthritic origin. Dentomaxillofac. Radiol. 41, 126–130 (2012).
Song, H., Lee, J. Y., Huh, K. H. & Park, J. W. Long-term changes of temporomandibular joint osteoarthritis on computed tomography. Sci. Rep. 10, 6731 (2020).
Das, S. K. TMJ osteoarthritis and early diagnosis. J. Oral Biol. Craniofac. Res. 3, 109–110 (2013).
Matsumoto, R. et al. Relationship between the unilateral TMJ osteoarthritis/osteoarthrosis, mandibular asymmetry and the EMG activity of the masticatory muscles: A retrospective study. J. Oral Rehabil. 37, 85–92 (2010).
Epstein, J. B., Rea, A. & Chahal, O. The use of bone scintigraphy in temporomandibular joint disorders. Oral Dis. 8, 47–53 (2002).
Samuel, A. M. & Jain, H. Scintigraphic changes of osteoarthritis: An analysis of findings during routine bone scans to evaluate the incidence in an Indian population. Indian J. Nucl. Med. 27, 73–80 (2012).
Koppula, B. R. et al. SPECT/CT in the evaluation of suspected skeletal pathology. Tomography 7, 581–605 (2021).
Kang, J. H., An, Y. S., Park, S. H. & Song, S. I. Influences of age and sex on the validity of bone scintigraphy for the diagnosis of temporomandibular joint osteoarthritis. Int. J. Oral Maxillofac. Surg. 47, 1445–1452 (2018).
Acknowledgements
We thank Soyoung Jeon, PhD (biostatistician), and Hyejin Yang, MS (biostatistician) of the Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Republic of Korea, for their help with the statistical analyses.
Author information
Authors and Affiliations
Contributions
J.-Y. Kim and C. Lee wrote the original draft; J.-Y. Kim, J.-H. Lee, and J.-K. Huh designed the research; Y.L. Park, J.-H. Lee, and Y.H. Ryu collected and analyzed the data. J.-H. Lee and J.-K. Huh revised the manuscript and edited the manuscript; All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the institutional review board of Yonsei University Gangnam Severance Hospital approved this retrospective study (IRB No. 3-2023-0320), and waiver of written informed consent for this retrospective study. This study was also conducted according to the principles of the Declaration of Helsinki for research on humans.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, JY., Lee, C., Park, Y.L. et al. Diagnostic criteria for temporomandibular joint osteoarthritis using standardized uptake value in single-photon emission computed tomography–computed tomography. Sci Rep 14, 31569 (2024). https://doi.org/10.1038/s41598-024-71639-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71639-1