Abstract
In March 2023, our pediatric intensive care unit (PICU) retrospectively examined six cases of pediatric necrotizing tracheobronchitis (NTB), focusing on co-infections with influenza A virus (IAV) and Staphylococcus aureus (S. aureus). This study aimed to elucidate NTB’s clinical characteristics, diagnostics, and therapeutic approaches. Diagnostics included symptom assessment, microbiological testing that confirmed all patients were positive for IAV H1N1 with a predominant S. aureus co-infection, and bronchoscopy. The patients predominantly exhibited fever, cough, and dyspnea. Laboratory analysis revealed decreased lymphocyte counts and elevated infection markers like C-reactive protein and procalcitonin. Chest computed tomography (CT) scans detected tracheobronchial obstructions in half of the cases, while bronchoscopy showed severe mucosal congestion, edema, necrosis, and purulent-hemorrhagic exudates. Treatments encompassed comprehensive strategies like oxygen therapy, intubation, bronchoscopic interventions, thoracentesis, oseltamivir, and a regimen of antibiotics. Our findings suggested potential correlations between clinical markers, notably lymphocyte count and procalcitonin, and clinical interventions such as the number of rescues and intensive care unit (ICU) duration. This research highlights the importance of early detection and the role of bronchoscopy and specific markers in assessing NTB, advocating for continued research in larger cohorts to better understand its clinical trajectory and refine treatment approaches for this challenging pediatric disease.
Similar content being viewed by others
Introduction
Influenza A virus (IAV) continues to be a major concern in global health due to its potential for pandemics. While most IAV infections culminate in mild respiratory manifestations such as fever, cough, and sore throat, a select few evolve into grave complications, including bacterial pneumonia, myocarditis, and encephalitis. Among these, necrotizing tracheobronchitis (NTB) stands out as an infrequent but potentially fatal complication1.
NTB is characterized by tracheobronchial mucosal damage that can lead to necrosis and pseudomembrane formation, with both infectious and non-infectious origins. The sloughing of necrotic tissue and the pseudomembrane may induce acute airway obstruction, constituting a significant life hazard2,3,4. Clinically, NTB bears resemblances to pseudomembranous tracheobronchitis (PMTB)2. Due to its relative rarity, NTB is often reported in literature as individual case studies. Although Aspergillus is frequently identified as an etiological agent5,6, occasional reports have linked IAV7, Staphylococcus aureus (S. aureus)8, Mycoplasma pneumoniae (M. pneumoniae)3,9, and other pathogens10,11,12. Non-infective triggers notably involve neonatal tracheal intubation13, with sporadic associations to radiation therapy and rheumatoid arthritis14,15,16. To our knowledge, roughly ten NTB cases have been ascribed to concomitant IAV and S. aureus infections17,18,19,20,21,22,23,24, with half resulting in mortality. Among these, pediatric patients account for four instances17,19,24, of which three were fatal. While a significant proportion of NTB cases can be attributed to immunodeficiency9, those co-infected with IAV and S. aureus typically manifest robust immune responses. Understanding these varied causes of NTB, especially in conjunction with IAV and S. aureus infections, is crucial.
In light of the rarity and marked severity of NTB, combined with its intricate clinical implications, we amassed data from six pediatric NTB cases treated in our Pediatric Intensive Care Unit (PICU) in March 2023, each stemming from co-infections of IAV and S. aureus. With this investigation, we aspire to elucidate the clinical trajectories, findings from diagnostics, employed therapeutic regimens, and the eventual outcomes of these patients. Our ultimate goal is to bolster clinical insight, explore potential risk contributors, and highlight essential interventions that might contribute to more favorable patient outcomes.
Methods
Patients and data collection
We conducted a retrospective analysis of a cohort of six patients diagnosed with NTB who were admitted to our PICU in March 2023. Our data collection included their clinical presentations, salient findings from pathogen detection, infection markers, chest imaging, and bronchoscopic evaluations. Notably, bronchoscopy served dual purposes: it provided both diagnostic insights and therapeutic avenues. Upon clinical evaluation of airway obstruction in the PICU, bedside bronchoscopy was collaboratively performed by a multidisciplinary team, including bronchoscopists, otolaryngologists, intensive care physicians, endoscopy nurses, and critical care nurses. Under sedation, the bronchoscope was inserted through either the nasal cavity or the endotracheal tube. In cases where tracheal lumen obstruction was identified, biopsy forceps, foreign body forceps, and foreign body retrieval baskets were utilized repeatedly to remove the obstructing material. Following the removal of obstructions, saline lavage and suction were performed to clear the airway. Pathogen detection was conducted using a combination of reverse transcription polymerase chain reaction (RT-PCR) and metagenomic next-generation sequencing (mNGS) on pharyngeal swabs, sputum, and bronchoalveolar lavage fluid (BALF). RT-PCR was specifically employed to target viral ribonucleic acid (RNA), including that of IAV. mNGS facilitated a comprehensive analysis of the microbial community, enabling the identification of a broad spectrum of viral, bacterial, and fungal pathogens. Additionally, gram staining was performed on sputum and BALF samples to differentiate between gram-positive and gram-negative bacteria. These samples were cultured under aerobic and anaerobic conditions to isolate bacterial pathogens and assess their antibiotic sensitivities using standard microbiological techniques, such as susceptibility testing. Moreover, we meticulously documented the therapeutic strategies employed. These strategies spanned procedural measures, such as endotracheal intubation and closed thoracic drainage, as well as a spectrum of pharmacological treatments. We diligently recorded outcome data to gauge the efficacy and ramifications of these interventions.
Diagnostic criteria
While standardized diagnostic criteria for NTB are currently lacking, our clinical diagnoses drew heavily from the prevailing literature. This literature places emphasis on clinical presentations, microbiological findings, and endoscopic results16. Notably, some experts regard bronchoscopy as the gold standard for NTB diagnosis4. A diagnosis is corroborated when bronchoscopy unveils tracheobronchial mucosal necrosis and pseudomembrane formation. Furthermore, should a biopsy of the tracheobronchial mucosa display fibrinoid necrosis with concomitant inflammatory cell infiltration, the diagnostic assertion is further strengthened.
Statistical analysis
In our statistical evaluation, the Shapiro–Wilk test was first employed to assess the normality of data distribution. Given the constrained sample size and potential outliers, we presented descriptive statistics as medians (M), accompanied by the first (Q1) and third (Q3) quartiles, represented as M (Q1-Q3). To identify differences in infection markers before and after treatment, we utilized the Wilcoxon signed-rank test, with p-values adjusted for multiple comparisons using the false discovery rate (FDR). The paired Vargha-Delaney A values further aided in discerning the magnitude of group differences. Because some data did not follow a normal distribution, we systematically employed the Spearman rank correlation test for correlation analyses. For approximating confidence intervals for the Spearman coefficient, we used Fisher’s Z transformation25.Our regression analysis began with univariate linear regression due to the constraints of the dataset. Potential nonlinearities led us to consider polynomial regression. When faced with outliers or high leverage points, we resorted to robust linear regression. For the count variable ‘number of rescues (NumRescues)’, our initial analysis using negative binomial regression resulted in statistically non-significant coefficients. Given this result, we addressed zero counts by incrementing them by one, then applied a logarithmic transformation, and subsequently used linear regression. To ensure the robustness of the regression model, we verified major assumptions through residual plots, quantile–quantile (QQ) plots, and various statistical tests, including the Shapiro–Wilk, Jarque–Bera, Durbin–Watson, and Levene’s test. A two-tailed p-value less than 0.05 indicated statistical significance. All analytical procedures were performed in a Python 3.8 environment, leveraging libraries such as pandas (1.2.3), numpy (1.19.2), scipy (1.6.1), statsmodels (0.12.2), matplotlib (3.3.4), and seaborn (0.11.1).
Ethical approval
This study was approved by the Medical Ethics Committee of Children’s Hospital, Zhejiang University School of Medicine, documented under Approval Number 2023-IRB-0241-P-01. All methods were carried out in accordance with the Declaration of Helsinki and other relevant guidelines and regulations. Written informed consent was obtained from the parents of the children included in this study for participation and publication of identifiable details and accompanying images. Copies of the written consents are available for review by the Editor of this journal.
Results
Patient characteristics
Among the six patients, the median age was 9Y2M (6Y1M-9Y7M). The cohort included four males and two females, with males comprising two-thirds. The median body weight (BW) was 28.0 kg (20.8–31.1 kg). All patients were previously healthy, with no reported history of immunodeficiency disorders or recurrent infections. Detailed clinical data for each patient are provided in Table 1.
Pre-admission status
Patients were admitted a median of 2.5 days (2.0–3.8 days) post symptom onset. Two had previously been hospitalized at different institutions for durations of 2 and 4 days, respectively. From the onset of illness, fever was observed in all patients. Furthermore, in addition to this universal symptom, two patients predominantly displayed coughs, three experienced shortness of breath, and one described chest pain. All patients denied any history of foreign body aspiration. Clinical data for each patient are detailed in Table 1.
Clinical status upon admission
Vital signs were recorded as follows: the median temperature (T) was 38.4 °C (38.1–38.5 °C); the median pulse rate (PR) was 155 bpm (131–169 bpm); the median respiratory rate (RR) was 47 bpm (43–48 bpm); and blood pressure (BP) measurements showed a median of 109/76 mmHg (systolic: 104–110 mmHg, diastolic: 71–78 mmHg). The peripheral oxygen saturation (SpO2) was determined to be 96% (95–97%). Upon their arrival, all patients required oxygen support: one utilized a nasal cannula, four made use of face masks, and one was intubated. During physical examinations, tachypnea and visible retractions were evident in all patients. For one patient, breath sounds were distinctly reduced on the right side, three presented with moist rales, while the remaining two showed no signs of either dry or moist rales. Comprehensive immunological assessments included measurements of immunoglobulins and complement components, with values within normal ranges: IgG 8.41 g/L (7.79–10.89 g/L), IgA 1.39 g/L (1.12–1.67 g/L), IgM 0.80 g/L (0.57–0.97 g/L), C3 1.13 g/L (0.97–1.28 g/L), C4 0.33 g/L (0.24–0.44 g/L), and IgE 110.9 IU/mL (23.2–282.0 IU/mL). Cellular profiles revealed variations, with CD19 at 37.80% (36.00–39.00%), CD3 at 36.89% (34.50–42.72%), CD4 at 17.77% (17.30–20.33%), CD8 at 13.77% (10.35–18.60%), and CD3−CD16+CD56+ at 6.60% (5.75 − 17.00%). Despite these variations, no evidence of underlying immunodeficiencies was found.
Evaluation of infection markers
During hospitalization, we assessed several infection markers in patients, including white blood cell count (WBC), lymphocyte count (LC), C-reactive protein (CRP), procalcitonin (PCT), interleukin-6 (IL-6), and interleukin-10 (IL-10). Here, “Init” refers to initial values upon admission; “Max” to peak values; “Min” to the lowest recorded values; and “Final” to values from the last examination before discharge. Initial infection markers upon admission are detailed in Table 1.
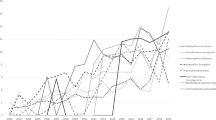
For WBC, initial analyses revealed significant differences between InitWBC and MinWBC, as well as between MinWBC and MaxWBC (p < 0.05, Fig. 1a). However, these distinctions were not significant after FDR adjustment. Effect size analysis showed an A value of 1.00 between InitWBC and MinWBC and an A value of 0 for the comparison of MinWBC and MaxWBC. Concerning LC, all comparisons exhibited significant differences (p < 0.05, Fig. 1b), except for the MaxLC versus FinalLC comparison. Post FDR correction, the difference between InitLC and MinLC was no longer significant, but other comparisons maintained their significance (p < 0.05). Effect size yielded an A value of 0.92 for InitLC versus MinLC and an A value of 0 for both comparisons involving InitLC and MinLC against MaxLC and FinalLC.
Dynamics of in-hospital infection markers and their correlations. (a) Variations in WBC. No significant differences were observed after FDR adjustment. (b) Fluctuations in LC with marked significant differences (*p < 0.05). (c) Changes in CRP levels (*p < 0.05). (d) Alterations in PCT levels (*p < 0.05). (e) IL-6 levels during hospital stay. No significant differences were observed after FDR adjustment. (f) IL-10 levels, with significant differences marked (*p < 0.05). (g) Spearman correlation between InitLC and other initial infection markers (**p < 0.01). (h) Spearman correlation of MinLC with peak and trough infection marker values (*p < 0.05).
For CRP, significant differences emerged between InitCRP and FinalCRP, and between MaxCRP and FinalCRP (corrected p < 0.05, A = 1.00, Fig. 1c). PCT showcased significant variances between InitPCT and FinalPCT, and between MaxPCT and FinalPCT (corrected p < 0.05, A = 1.00, Fig. 1d). With IL-6, a significant initial difference was noted between MaxIL-6 and FinalIL-6, which turned non-significant after FDR correction. Importantly, the effect size, as denoted by the A value, was 1.00 (Fig. 1e). For IL-10, marked differences were detected between InitIL-10 and FinalIL-10, and between MaxIL-10 and FinalIL-10 (corrected p < 0.05, A = 1.00, Fig. 1f).
Correlation analyses underscored a strong negative correlation between InitLC and InitIL-6 (ρ = − 0.94, p = 0.005, Fig. 1g). MinLC negatively correlated with both MaxIL-6 and MaxIL-10 (ρ = − 0.89, p = 0.019, Fig. 1h). Additionally, a substantial positive correlation existed between MaxIL-6 and MaxIL-10 (ρ = 0.89, p = 0.019).
Microbiological findings
Of the six patients evaluated, four tested positive for IAV H1N1 within the first 2 days of admission using pharyngeal swab, sputum, or BALF analyses via either RT-PCR or mNGS. Additionally, mNGS evaluations of BALFs diagnosed the other two patients with IAV H1N1 on the fourth day post-admission.
Upon admission, Gram-positive cocci (G+ cocci) were detected in the sputum smear or BALF smear of two patients. Between the third and sixth days post-admission, five patients tested positive for S. aureus through sputum culture, BALF culture, or mNGS. Among them, four were confirmed to have Methicillin-sensitive S. aureus (MSSA), and one was identified with Methicillin-resistant S. aureus (MRSA).
Additionally, M. pneumoniae and Haemophilus influenzae (H. influenzae) were identified in the BALF of one patient through mNGS. In another patient, although elevated M. pneumoniae IgM antibodies were observed, M. pneumoniae nucleic acids were absent in respiratory specimens. However, adenovirus nucleic acids were positively identified in the BALF via polymerase chain reaction (PCR) analysis. Detailed microbiological distributions can be found in Table 2.
Radiological findings
Within the first three days after the emergence of symptoms, chest computed tomography (CT) imaging for the six patients demonstrated marked infectious changes within the lung parenchyma. The majority presented with lung consolidation and pleural effusion. Notably, three patients had evidence of tracheobronchial obstruction, resulting in bronchial narrowing and atelectasis (Fig. 2a). Detailed information can be found in Table 2.
Evolution of chest CT findings across disease progression. (a) Early-stage chest CT reveals a foreign object within the trachea; (b) Mid-course chest CT displays signs of pneumonia and pneumothorax; (c) Convalescent-stage chest CT shows improvement in both pneumonia and pneumothorax, with the presence of a pulmonary cavity.
As the days advanced, especially between days 5 and 15, the radiological manifestations of pneumonia intensified across all patients. A majority, four out of six, presented with atelectasis and lung consolidation. Concurrently, conditions such as pneumothorax or subcutaneous emphysema emerged in five patients, with pleural effusion also being a prominent finding in the same number of individuals (Fig. 2b).
In the later stages of the disease, predominantly after two weeks, all patients demonstrated an improvement in pulmonary inflammatory changes compared to earlier stages. Instances of pneumothorax, subcutaneous emphysema, and pleural effusion also started to resolve. However, one patient developed a new pulmonary cavity, and another exhibited a more pronounced local bronchiectasis compared to earlier imaging findings (Fig. 2c).
Bronchoscopic observations and procedures
Upon hospitalization, bronchoscopic evaluations were conducted for all patients. The primary examination displayed marked congestion, edema, and necrotic erosion across the tracheal and bronchial mucosa. A delicate white pseudomembrane, prone to bleeding upon contact, was discerned. The airways were predominantly filled with purulent-hemorrhagic secretions. Notably, the trachea of three patients was obstructed by abundant purulent-hemorrhagic materials. Using bronchoscopic instruments, substantial pseudomembranous fragments and cast-like sputum were extracted. Saline lavage was then administered to cleanse the mucoid and purulent secretions. Additionally, mucosal erosions and necrosis were apparent in the pharynx of three patients (Fig. 3a). Detailed bronchoscopic findings are provided in Table 2.
Bronchoscopic evaluation of airway mucosal damage and recovery. (a) Initial bronchoscopy showing erosion of the pharyngeal mucosa, necrotic formations within the trachea, and the presence of a pseudomembrane in the left main bronchus. (b) Intermediate stage bronchoscopy revealing continued congestion and edema in the pharyngeal mucosa, mucosal erosion at the tracheal carina, and a mucus plug observed at the left second carina. (c) Recovery stage bronchoscopy indicating improved mucosal erosion in the glottal region, persistent congestion at the tracheal carina, and a notable reduction in the edema of the left upper bronchial mucosa.
A subsequent assessment after 5–7 days revealed persistent mucosal congestion and edema in the cohort. Some demonstrated recovery in areas that were previously eroded or necrotic. The airways mainly housed yellow viscous or purulent secretions, and a sputum plug was identified in one patient (Fig. 3b).
During the convalescence period, bronchoscopic evaluations showcased patent airways across all patients. Although mucosal congestion persisted, the earlier observed necrotic regions and erosions exhibited marked healing. The airways predominantly held yellow or white viscous secretions. In some instances, mucoid plugs were detected, which were subsequently lavaged (Fig. 3c).
Histopathological examinations of bronchial biopsies
During the bronchoscopic procedure, biopsies were obtained from three distinct patients. Subsequent histopathological evaluations consistently revealed fibrinoid necrosis in bronchial tissues, accompanied by exudative changes and significant inflammatory cell infiltration (refer to Fig. 4). Detailed histopathological findings are provided in Table 2.
Treatment
Upon admission, all six patients required supplemental oxygen. Two exhibited milder symptoms and did not necessitate tracheal intubation. One patient was emergently intubated outside the hospital setting, and the remaining three were intubated within the first 1–2 days after admission. During their hospital stay, each of the four intubated patients underwent 1–3 emergency interventions. These included six instances related to airway obstructions due to endogenous foreign bodies, two due to pneumothorax, and one episode of septic shock resulting from a severe infection.
All patients underwent bronchoscopic examinations, lavage, and foreign body removal within a median timeframe of 1.0 (1.0–1.8) days following admission. Following these procedures, five patients received thoracentesis and closed thoracic drainage. Notably, drainage persisted for 40 days in one patient due to an unresolved pneumothorax.
Upon diagnosing IAV infection, oseltamivir was immediately prescribed to all patients. Given the potential risk of severe bacterial co-infections, broad-spectrum antibiotics were initiated early in the treatment course. Additionally, either vancomycin or linezolid was employed to address Gram-positive bacterial infections. Two patients, diagnosed with concurrent M. pneumoniae infection, were additionally treated with azithromycin. All patients received methylprednisolone to counteract inflammation, with four also undergoing intravenous immunoglobulin (IVIG) therapy.
A detailed summary of critical clinical procedures, their timings, durations, and frequencies post-onset, is provided in Fig. 5. Specific treatment interventions for each patient are detailed in Table 3.
Clinical intervention timelines and treatment durations post-disease onset. (a) Median days post-onset for various clinical interventions, including days from onset to admission (DOTA), time to intubation (IntubationTime), initial rescue time (InitRescueTime), initial bronchoscopy time (InitBronchTime), initial thoracentesis time (InitThoracentesisTime), and initial closed thoracic drainage time (InitCTDrainageTime). The error bars indicate the interquartile range (IQR).(b) A box plot illustrating the duration (in days) of various clinical stages and procedures: Duration of Hospital Stay (DurHospStay), duration of intensive care unit (ICU) Stay (DurICUStay), oxygen therapy duration (OxygenTherapyDur), duration of mechanical ventilation (MechVentDur), and duration of closed thoracic drainage (DurCTDrainage).(c) Median frequencies of specific clinical procedures post-onset: NumRescues, number of bronchoscopies (NumBronchoscopies), number of thoracentesis procedures (NumThoracentesis), and number of closed thoracic drainage procedures (NumCTDrainage). The error bars represent the IQR.
Clinical outcomes
Among the six patients, one encountered an airway obstruction attributed to an endogenous foreign body. This patient required multiple cardiopulmonary resuscitation interventions, subsequently developing neurological impairment. Upon achieving clinical stability, the individual was transferred to a rehabilitation facility. Conversely, the remaining five patients experienced a successful discharge, exhibiting a favorable post-treatment recovery. Detailed outcomes for each patient are provided in Table 3.
Correlation and regression analysis
In our comprehensive analysis, we explored correlations among various biophysical markers and clinical parameters, with a particular focus on emergency interventions, mechanical ventilation duration, and hospital stay duration. The Spearman correlation revealed significant associations of InitPCT, MinLC, NumThoracentesis, and PR with NumRescues. However, these associations were not confirmed when assessed using negative binomial regression. After log-transforming NumRescues and applying linear regression, a notable association emerged between MinLC and the transformed NumRescues (β = − 3.31, p = 0.012, R2 = 0.829, Fig. 6a).
Correlation and regression analyses of biophysical markers, clinical interventions and patient outcome measures. (a) Linear regression of MinLC vs. log-transformed NumRescues. (b) Univariate regressions of PR, InitPCT, and NumRescues with MechVentDur. (c) Univariate regressions of InitPCT, NumBronchoscopies, NumRescues, and MechVentDur with DurICUStay. (d) Linear regression of DurCTDrainage vs. OxygenTherapyDur. (e) Quadratic polynomial and robust regressions of MaxWBC vs. DurHospStay.
Significant linear relationships were discerned between PR, InitPCT, NumRescues, and MechVentDur. The respective coefficients for MechVentDur were as follows: PR (β = 0.11, p = 0.013, R2 = 0.818), InitPCT (β = 0.25, p = 0.038, R2 = 0.698), and NumRescues (β = 2.63, p = 0.013, R2 = 0.822), visualized in Fig. 6b. Associations between InitPCT, NumBronchoscopies, NumRescues, and MechVentDur with DurICUStay were also observed. The linear regression coefficients for these associations included: InitPCT (β = 0.28, p = 0.003, R2 = 0.912), NumBronchoscopies (β = 1.41, p = 0.016, R2 = 0.803), NumRescues (β = 2.58, p = 0.016, R2 = 0.802), and MechVentDur (β = 0.86, p = 0.025, R2 = 0.755), depicted in Fig. 6c. A significant correlation between DurCTDrainage and OxygenTherapyDur was also evident (β = 0.87, p < 0.001, R2 = 0.998, Fig. 6d).
While MaxWBC showed a positive correlation with DurHospStay (ρ = 0.94, p = 0.005), it was not statistically significant in the linear regression (R2 = 0.599, p = 0.071). A quadratic polynomial regression, however, indicated a nuanced nonlinear relationship (R2 = 0.893, p = 0.035). Data diagnostics pinpointed two high-leverage points, which were supported by a robust linear regression presenting significant quadratic terms (constant β₀ = 20.92, p = 0.006; linear term β₁ = -1.21, p = 0.110; quadratic term β₂ = 0.05, p = 0.006, Fig. 6e). Based on the parameters derived from the robust linear regression with higher precision coefficients (not shown), an inflection point at a MaxWBC value of 13.16 × 109/L was mathematically determined, signifying a critical threshold where the relationship between MaxWBC and DurHospStay alters.
In assessing our regression models, the majority of the assumptions were upheld. The Shapiro–Wilk test identified deviations in residuals’ normality for the models linking NumRescues to MechVentDur and NumBronchoscopies to DurICUStay. Nevertheless, subsequent validations using the Jarque–Bera test and graphical analyses confirmed the overall normality of residuals. These observed discrepancies may be attributed to our constrained sample size.
Discussion
During the 2023 influenza season, we examined six pediatric NTB cases linked to IAV and S. aureus. These children primarily manifested fever, cough, and dyspnea, concurrent with a reduction in LC and elevations in CRP, PCT, IL-6, and IL-10. Influenza was promptly diagnosed through RT-PCR or mNGS of respiratory samples, while bacterial involvement was confirmed via smears, cultures, and mNGS. Initial chest CT may reveal potential airway obstructions, with bronchoscopy confirming tracheal mucosal necrosis. The rapidity of disease progression and significant risk of airway obstruction underscore the imperative of early intervention. Essential treatments encompassed oxygen therapy, mechanical ventilation, bronchoscopic procedures, thoracic drainage, and timely initiation of antivirals, antibiotics, and corticosteroids. Moreover, we observed potential correlations between initial PR and PCT with mechanical ventilation duration. The nadir LC appeared to be inversely associated with the frequency of emergency interventions, and peak WBC exhibited a non-linear relationship with the length of hospitalization, initially negative, then becoming positive. Other vital clinical parameters, including the frequency of emergency interventions, number of bronchoscopies, and duration of closed thoracic drainage, seemed to be associated with variations in mechanical ventilation, ICU stays, and oxygen therapy durations.
The pathogenesis of this condition remains incompletely understood, but it is potentially associated with the synergistic interplay between IAV and S. aureus. IAV predominantly replicates within the airway and alveolar epithelial cells, leading to cellular damage and a compromise of barrier integrity, which in turn elicits extensive immune cell infiltration. Owing to the degradation of this epithelial barrier and altered immune defenses, secondary bacterial infections become more prevalent, with Streptococcus pneumoniae and S. aureus standing out as primary culprits26. Notably, strains of S. aureus, especially those producing Panton–Valentine leukocidin (PVL), exhibit a specific affinity for the exposed basement membrane following epithelial shedding. The released PVL targets and disrupts newly recruited polymorphonuclear leukocytes, resulting in the liberation of inflammatory mediators. In the context of an IAV infection, the phagocytic capacity of alveolar macrophages diminishes, permitting unhindered bacterial proliferation. This combined action of IAV and S. aureus results in severe tracheobronchial and pulmonary lesions, manifesting as NTB and necrotizing pneumonia23,27.
Recognizing early clinical manifestations of NTB is pivotal for accurate diagnosis and timely intervention. In our cohort, the six observed patients predominantly fell within the school-aged bracket. While the literature primarily reports pediatric NTB cases between ages 7 and 9 years17,19, it also highlights occurrences in younger children, particularly between 1 and 3 years24. Our observations confirm this trend and extend to cases in children aged 1Y11M and 5Y2M. This suggests that, although NTB primarily affects school-aged children, it can also present in younger age groups. However, our study’s limited sample size may restrict the generalizability of these findings to the broader NTB population. NTB co-infected by IAV and S. aureus initially presents with influenza-like symptoms, occasionally accompanied by dyspnea and chest pain. Some patients may also experience hoarseness if the larynx is involved. As it evolves, airway obstruction becomes prevalent, mirroring most case narratives17,19,24. Early in the disease trajectory, we detected a pronounced elevation in infection-related markers, namely CRP, PCT, IL-6, and IL-10. These findings are consistent with previous research indicating the utility of these biomarkers in diagnosing bacterial infections, particularly those involving the bloodstream28. Notably, elevated levels of IL-6 and IL-10 have been identified as reliable indicators of disease severity in pediatric patients with sepsis in ICU29. The early detection of these elevated markers in our patients suggests the presence of a severe bacterial infection. Although WBC levels may appear normal or slightly reduced, there’s a marked decline in LC, corroborating previous studies30. Expedient etiological identification is of the essence. Respiratory assays, encompassing influenza antigens, RT-PCR, and mNGS, bolster early IAV discernment. Even though pinpointing bacterial origins early on poses challenges, smear tests swiftly discern G+ cocci, and mNGS heightens diagnostic precision. Bacterial culture, nonetheless, remains instrumental for tailoring antibiotic regimens. In our cases, initial chest CTs for three patients revealed tracheal and bronchial opacities suggestive of foreign bodies. Yet, a majority in existing literature display merely pulmonary inflammatory alterations in early scans, with a scant few indicating bronchial wall thickening or foreign shadows18,24. This hints at the circumscribed diagnostic potential of early chest CTs. A conclusive diagnosis predominantly hinges on bronchoscopy, a consensus echoed across numerous case reports17,19,24. Conclusively, while NTB’s onset might mimic severe influenza, a notable surge in infectious markers often suggests an ensuing bacterial superinfection. Accurate early etiological discernment, especially if bronchial anomalies are evident on initial CTs, warrants immediate bronchoscopic evaluation and robust intervention, preempting potential airway obstructions.
Exploring factors linked to disease severity can offer insights, though interpretations must be approached with caution due to our study’s small sample size. We investigated correlations between initial clinical presentations, laboratory results, essential clinical interventions and the severity spectrum of the disease. Our analysis observed a notable negative association between MinLC and NumRescues, suggesting that lower LC levels may necessitate more frequent medical interventions. This association, supported by log-transformed linear regression, underscores the need for cautious interpretation due to the small sample size. A study indicates that severe influenza infections correlate with significant reductions in LC30. Aligning with these findings, recent studies have shown that reductions in specific lymphocyte subsets, notably T cells and Natural Killer cells (NK cells), correlate with increased disease severity in respiratory infections like coronavirus disease 2019 (COVID-19) and influenza, highlighting their role in prognosis and treatment planning31,32. Furthermore, influenza infections have been shown to suppress Th17 and γδ T cell functions and potentially inhibit NK cell responses, reducing the production of tumor necrosis factor (TNF) and consequently increasing bacterial burden33. This is consistent with the cellular immune findings in our six patients, all of whom showed decreased proportions of T cells and NK cells upon admission. This result aligns with a recent case of an adult with influenza B and S. aureus co-infection leading to NTB34. Additionally, we observed significant negative correlations between InitLC and InitIL-6, as well as between MinLC and MaxIL-6 and MaxIL-10, suggesting intricate interactions between decreased LC and increased cytokine release. Research on COVID-19 has suggested that early CD3+ T cell counts and IL-6 levels may serve as predictive markers for severe disease progression in viral infections35,36, raising the possibility that similar markers could be relevant in our study of influenza infections, warranting further investigation. These preliminary findings underscore the necessity for larger, more definitive studies to confirm these potential associations and further elucidate the role of lymphocytes and cytokines in the progression of severe respiratory infections.
In evaluating NTB severity, we explored associations with MechVentDur and DurICUStay, finding a notable positive correlation between InitPCT and both variables. Furthermore, a potent positive relationship between MechVentDur and DurICUStay hinted at potential collinearity, which warrants careful scrutiny. Elevated initial PCT levels might serve as an early indication of an impending bacterial infection37,38, which could necessitate extended durations of mechanical ventilation and ICU stays. A significant relationship was observed between PR and MechVentDur. Given the variability of PR across different pediatric age groups, and acknowledging that the patient ages in our study ranged predominantly from 6Y1M to 9Y7M, PR at admission might tentatively correlate with initial disease severity. Clinically, severe infections in children are commonly associated with increased heart rates39,40,41. Additionally, a nuanced nonlinear relationship was revealed between MaxWBC and the duration of hospital stay: an initial shortening in stay duration with an increase in MaxWBC, followed by an elongation upon exceeding the 13.16 × 109/L threshold. This pattern could reflect the sequence of an initial IAV infection with normalized or reduced WBC, followed by a subsequent S. aureus infection increasing the WBC count. Prior studies suggest that higher WBC counts may be associated with increased severity of infections such as COVID-19 and sepsis, indicating their potential value in predicting patient outcomes42,43. These observations reveal the possible role of WBC counts in monitoring and managing severe infectious diseases.
From an interventional standpoint, notable associations were observed between NumRescues and both MechVentDur and DurICUStay, between NumBronchoscopies and DurICUStay, and between DurCTDrainage and OxygenTherapyDur. Increased rescue events may indicate heightened disease severity, potentially correlating with longer durations of mechanical ventilation and ICU stays. The frequency of bronchoscopies might act as an indicator of the extent of airway mucosal damage; significant damage may necessitate more frequent bronchoscopic interventions to mitigate respiratory obstructions, potentially leading to prolonged ICU stays. Notably, in the six patients discussed, pneumothorax was the primary reason for continuous closed thoracic drainage. Given that oxygen therapy is a primary intervention for pneumothorax44, its synchronization with DurCTDrainage is consistent with established clinical guidelines. Our study contributes to understanding how these clinical interventions may influence the course of the disease.
Our investigation included six cases of NTB. Within this group, G+ cocci were observed on a smear in one patient, but culturing for S. aureus was unsuccessful; two additional cases were marked by concurrent pathogen infections. These variations in etiology underscore the importance of meticulous clinical evaluation, which influences the interpretation of infectious markers and the strategic planning of treatment regimens. The study’s retrospective design inherently carries limitations such as the absence of a randomized control group and the potential for confounding factors. The rarity of NTB further constrained our sample size. While correlation and regression analyses were utilized to examine significant biomarkers, therapeutic interventions, and treatment progressions, the small cohort size limited the application of complex multivariate regression, thereby restraining a comprehensive analysis of the relationships between variables. Our statistical results suggest associations between specific biomarkers and disease progression, yet they should be interpreted with caution and not be construed as confirmation of causality. Future research efforts should aim to increase sample sizes to enhance the understanding of NTB and its clinical markers, thereby better informing clinical practice.
Conclusion
In conclusion, this study explores the clinical manifestations, diagnostic methods, and treatment measures of pediatric NTB caused by co-infections of IAV and S. aureus. Our findings underscore the importance of early detection, through advanced diagnostic modalities and timely intervention to mitigate severe complications of NTB. Factors such as diminished LC and elevated infection markers were examined as potential indicators of disease severity. Although the limited sample size warrants cautious interpretation, our study provides a foundation for understanding the clinical course of NTB. Continued investigations with larger cohorts are essential to further elucidate this condition and optimize therapeutic strategies for pediatric populations.
Data availability
Data will be made available from the corresponding author on reasonable request.
References
Paules, C. & Subbarao, K. Influenza. Lancet 390, 697–708 (2017).
Kawakami, N. et al. Pseudomembranous tracheobronchitis with severe tracheal stenosis and masked bronchial obstruction. J. Emerg. Med. 60, e39–e44 (2021).
Lei, W. et al. Pseudomembranous necrotizing laryngotracheobronchitis due to Mycoplasma pneumoniae: A case report and literature review. BMC Infect. Dis. 22, 183 (2022).
Wen, S.-H. et al. Pseudomembranous laryngotracheobronchitis due to coinfection with human bocavirus 1 and Mycoplasma pneumoniae: A case report. Transl. Pediatr. 10, 673–678 (2021).
Fernández-Ruiz, M. et al. Aspergillus tracheobronchitis: Report of 8 cases and review of the literature. Medicine 91, 261 (2012).
Cho, J. S. et al. Pseudomembranous aspergillus tracheobronchitis: Case report of a rare manifestation of airway invasive aspergillosis. Taehan Yongsang Uihakhoe Chi 83, 737–743 (2022).
Chang, J. et al. Necrotizing tracheobronchitis causing airway obstruction complicated by pandemic 2009 H1N1 influenza. Medicine 99, e18647 (2020).
Nakatsumi, H. et al. Methicillin-resistant Staphylococcus aureus necrotizing bronchitis after radiotherapy in combination with axitinib. Intern. Med. 61, 2931–2934 (2022).
Lee, Y. H., Seo, H., Cha, S. I., Kim, C. H. & Lee, J. A case of pseudomembranous tracheitis caused by Mycoplasma pneumoniae in an immunocompetent patient. Ann. Transl. Med. 7, 205 (2019).
Froessl, L. J. & Abdeen, Y. Pseudomembranous tracheobronchitis due to mycobacterium tuberculosis. Cureus 13, e17173 (2021).
Strauss, R. et al. Pseudomembranous tracheobronchitis due to Bacillus cereus. Clin. Infect. Dis. 33, E39-41 (2001).
Khan, M. S., Przebinda, A. S., Claros-Sorto, J. & Porter, A. Pseudomembranous tracheobronchitis: A rare presentation of: Pseudomonas aeruginosa: Infection. J. Bronchol. Interv. Pulmonol. 23, 319 (2016).
Gaugler, C. et al. Neonatal necrotizing tracheobronchitis: Three case reports. J. Perinatol. 24, 259–260 (2004).
Yang, F. & Lu, X. Acute obstructive fibrinous laryngotracheobronchitis induced by severe glyphosate surfactant intoxication: A case report. World J. Emerg. Med. 11, 125–126 (2020).
Choi, H. S. Radiation-induced pseudomembranous tracheobronchitis: A report of two cases. Thorac. Cancer 13, 2390–2393 (2022).
Kajikawa, S., Noda, K. & Nozaki, Y. Necrotizing tracheobronchitis associated with rheumatoid arthritis. Respir. Med. Case Rep. 20, 31–33 (2016).
Fang, X. & Cao, L. A case of necrotic tracheobronchitis caused by severe H1N1 combined with Staphylococcus aureus infection. Zhonghua Er Ke Za Zhi 57, 229–231 (2019).
Park, S. S. et al. A case of severe pseudomembranous tracheobronchitis complicated by co-infection of influenza A (H1N1) and Staphylococcus aureus in an immunocompetent patient. Tuberc. Respir. Dis. 78, 366 (2015).
Sharp, J. K., Hereth, J. & Fasanello, J. Bronchoscopic findings in a child with pandemic novel H1N1 influenza A and methicillin-resistant Staphylococcus aureus. Pediatr. Pulmonol. 46, 92–95 (2011).
Tsokos, M., Zöllner, B. & Feucht, H.-H. Fatal influenza A infection with Staphylococcus aureus superinfection in a 49 year-old woman presenting as sudden death. Int. J. Legal Med. 119, 40–43 (2005).
Takahashi, S. & Nakamura, M. Necrotizing tracheobronchitis caused by influenza and Staphylococcus aureus co-infection. Infection 46, 737–739 (2018).
Yamazaki, Y., Hirai, K. & Honda, T. Pseudomembranous tracheobronchitis caused by methicillin-resistant Staphylococcus aureus. Scand. J. Infect. Dis. 34, 211–213 (2002).
Gabrilovich, M. I., Huff, M. D., McMillen, S. M. & Quinter, C. Severe necrotizing tracheobronchitis from Panton–Valentine leukocidin–positive MRSA pneumonia complicating influenza A-H1N1-09. J. Bronchol. Interv. Pulmomol. 24, 63–66 (2017).
Wu, X., Wu, L. & Chen, Z. The important role of endoscopy in management of pediatric pseudomembranous necrotizing tracheitis. Front. Pediatr. 8, 360 (2020).
Bishara, A. J. & Hittner, J. B. Testing the significance of a correlation with nonnormal data: Comparison of Pearson, Spearman, transformation, and resampling approaches. Psychol. Methods 17, 399–417 (2012).
Paget, C. & Trottein, F. Mechanisms of bacterial superinfection post-influenza: A role for unconventional T cells. Front. Immunol. 10, 336 (2019).
Morgan, M. Staphylococcus aureus, Panton–Valentine leukocidin, and necrotising pneumonia. BMJ 331, 793–794 (2005).
Yang, X. et al. PCT, IL-6, and IL-10 facilitate early diagnosis and pathogen classifications in bloodstream infection. Ann. Clin. Microbial. Antimicrob. 22, 103 (2023).
Zhang, Y., Li, B. & Ning, B. Evaluating IL-6 and IL-10 as rapid diagnostic tools for gram-negative bacteria and as disease severity predictors in pediatric sepsis patients in the intensive care unit. Front. Immunol. 13, 1043968 (2022).
Cheng, Y. et al. Dynamic changes of lymphocyte counts in adult patients with severe pandemic H1N1 influenza A. J. Infect. Public Health 12, 878–883 (2019).
Qian, F. et al. Specific dynamic variations in the peripheral blood lymphocyte subsets in COVID-19 and severe influenza A patients: A retrospective observational study. BMC Infect. Dis. 20, 1–11 (2020).
Ma, L. et al. Early peripheral blood lymphocyte subsets and cytokines in predicting the severity of influenza B virus pneumonia in children. Front. Cell. Infect. Microbiol. 13, 1173362 (2023).
Robinson, K. M., Kolls, J. K. & Alcorn, J. F. The immunology of influenza virus-associated bacterial pneumonia. Curr. Opin. Immunol. 34, 59 (2015).
Wang, S., Yang, J., Sun, W. & Tao, Y. Severe necrotizing tracheobronchitis caused by influenza B and methicillin-resistant Staphylococcus aureus co-infection in an immunocompetent patient. Ann. Clin. Microbiol. Antimicrob. 23, 55 (2024).
Pan, P. et al. Characteristics of lymphocyte subsets and cytokine profiles of patients with COVID-19. Virol. J. 19, 57 (2022).
Luporini, R. L. et al. IL-6 and IL-10 are associated with disease severity and higher comorbidity in adults with COVID-19. Cytokine 143, 155507 (2021).
Hamade, B. & Huang, D. T. Procalcitonin: Where are we now?. Crit. Care Clin. 36, 23–40 (2020).
Richards, O. et al. Procalcitonin increase is associated with the development of critical care-acquired infections in COVID-19 ARDS. Antibiotics 10, 1425 (2021).
Guarracino, F., Bertini, P. & Pinsky, M. R. Cardiovascular determinants of resuscitation from sepsis and septic shock. Crit. Care 23, 118 (2019).
Wheeler, D. S., Jeffries, H. E., Zimmerman, J. J., Wong, H. R. & Carcillo, J. A. Sepsis in the pediatric cardiac intensive care unit. World J. Pediatr. Congenit. Heart Surg. 2, 393–399 (2011).
Koch, E., Lovett, S., Nghiem, T., Riggs, R. A. & Rech, M. A. Shock index in the emergency department: Utility and limitations. Open Access Emerg. Med. 11, 179–199 (2019).
Fang, W.-F. et al. Incorporation of dynamic segmented neutrophil-to-monocyte ratio with leukocyte count for sepsis risk stratification. Sci. Rep. 9, 19756 (2019).
Zhu, B. et al. Correlation between white blood cell count at admission and mortality in COVID-19 patients: A retrospective study. BMC Infect. Dis. 21, 574 (2021).
Currie, G. P., Alluri, R., Christie, G. L. & Legge, J. S. Pneumothorax: An update. Postgrad. Med. J. 83, 461–465 (2007).
Funding
This work was supported by the National Key Research and Development Program of China (Grant Numbers: 2021YFC2701800, 2021YFC2701801), and the Medical Health Science and Technology Project of the Zhejiang Provincial Health Commission (Grant Number: 2022PY067).
Author information
Authors and Affiliations
Contributions
Study conception and design: ZH Yang, CC Hu, CM Zhang. Acquisition of data: CC Hu, N Zhang, D Xu. Analysis and interpretation of data: CC Hu, ZJ Chen, J Yu. Drafting of the manuscript: CC Hu. Critical revision: ZH Yang, CM Zhang. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, C., Zhang, N., Xu, D. et al. Clinical presentations and diagnostic approaches of pediatric necrotizing tracheobronchitis with influenza A virus and Staphylococcus aureus co-infections. Sci Rep 14, 20880 (2024). https://doi.org/10.1038/s41598-024-71867-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71867-5