Abstract
The role of torso computed tomography (CT) in evaluating body composition has been unexplored. This study assessed the potential of low-dose torso CT from positron emission tomography (PET)/CT for analyzing body composition and its relation to muscle strength. We retrospectively recruited 384 healthy Korean adults (231 men, 153 women) who underwent torso 18F-FDG PET/CT, bioelectrical impedance analysis (BIA), and muscle strength tests (handgrip strength [HGS] and knee extension strength [KES]). CT images were segmented into three compartments: torso volumetric, abdominal volumetric, and abdominal areal. Muscle amounts from each compartment were indexed to height (m2). BIA and HGS served as reference standards, with correlation coefficients (r) calculated. Torso muscle volumetric index (TorsoMVI) had the strongest correlations with BIA-derived values (r = 0.80 for men; r = 0.73 for women), surpassing those from the abdominal compartments. TorsoMVI was also correlated significantly with HGS (r = 0.39, p < 0.01) and differentiated between normal and possible sarcopenia in men (n = 225, 5960 ± 785 cm3/m2 vs. n = 6, 5210 ± 487 cm3/m2, p = 0.02). In women, KES correlated more strongly with muscle parameters than HGS. Despite gender-specific variations, torso CT-derived parameters show promise for evaluating body composition and sarcopenia.
Similar content being viewed by others
Introduction
A growing body of evidence suggests that body composition and physical performance are essential factors in health-related parameters, not only for healthy individuals but also for patients with various medical conditions, including malignancies, metabolic diseases, and cardiovascular diseases1,2,3,4. Body composition, comprising muscle, fat, bone, and other tissues, is crucial, with muscle-related parameters being pivotal in relation to physical performance and aging5. Therefore, a comprehensive assessment of body composition, including muscle mass, is vital for better characterization and management of both healthy individuals and patients.
Several tools have been used to quantitatively analyze body composition for health screening and medical purposes. Among them, bioelectrical impedance analysis (BIA) is the most practical and robust tool owing to its accessibility6. BIA calculates the differential impedance and resistance of different body tissues to provide body composition information. BIA-derived appendicular skeletal mass (ASM) serves as a reference standard for diagnosing sarcopenia, as it strongly correlates with various physical health-related parameters, including calf circumference, grip strength, and gait speed7. However, the use of electric current in the indirect measurement raises questions about its ultimate accuracy and reproducibility8.
Other medical image-based approaches, such as dual X-ray absorptiometry (DXA), computed tomography (CT), and magnetic resonance imaging, are also used for body composition analysis. CT is considered the gold standard method to assess muscle mass and quality simultaneously and is recommended as the gold standard tool by guidelines9. Although routine CT acquisition for body composition analysis or diagnosing sarcopenia is impractical, opportunistic analysis of body composition from chest or abdomen CT has been implemented as an alternative approach2,10. This involves using Hounsfield unit (HU) or region of interest–based segmentation of CT images to directly measure the amount of muscle mass, abdominal visceral fat (AVF), and subcutaneous fat (SF), providing a reproducible method for body composition analysis. These measurements have shown a close association with physical performance, chemotherapy compliance, and prognosis in patients with various malignancies11,12. However, the current regional CT approach is limited to analyzing a single slice around the third lumbar vertebra (L3), and a more comprehensive evaluation involving as many skeletal muscles as possible is desirable, considering appendicular muscles as the standard reference for evaluating sarcopenia13. Therefore, CT images offering a wider field of view (FOV) beyond the chest or abdomen are considered a promising option.
Torso or whole-body CT images obtained by positron emission tomography (PET)/CT have emerged as potential candidates for body composition analysis. As one of the components of the PET/CT fusion system, CT supports PET through attenuation correction and anatomical correlation14. Conversely, HU-based quantitative information from CT itself has been largely overlooked when interpreting the PET/CT scans. Considering the recent trend of abdominal or chest CT-based automated areal or volumetric body composition analysis10, it is also possible to analyze much larger CT datasets from PET/CT in a single step using deep learning algorithms such as convolutional neural networks15. Consequently, torso PET/CT-based body composition analysis has been reported, providing extensive information on both muscle and fat15,16. However, before clinical adoption, it is essential to establish race, sex, or age-specific normal data incorporating entire torso muscle and fat and to validate the results by comparing them with other body composition-related parameters.
Herein, we aimed to provide torso CT-derived parameters of body composition from healthy Korean adults and compare these values with conventional body composition and muscle strength-related parameters to establish the foundation of torso PET/CT-based body composition analysis.
Results
Participant baseline characteristics
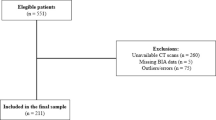
A total of 384 healthy adults (231 males, 56.5 ± 9.2 years; 153 females, 56.3 ± 9.2 years) were included in the study. Table 1 and Supplementary Table 1 present the baseline characteristics of the participants according to sex and PET/CT scanner type, respectively.
Among the participants, 20 female participants (Discovery 690, n = 7; Discovery 690 Elite, n = 9; Discovery 710, n = 3; Discovery STE 8, n = 1) and six male participants (Discovery 690, n = 1; Discovery 690 Elite, n = 4; Discovery 710, n = 1) were identified as having possible sarcopenia based on their lower hand grip strength (HGS) values, which were below the Asian Working Group for Sarcopenia 2019 (AWGS-2019) recommended cut-off (Fig. 1)13. All male participants had skeletal muscle index (SMI) values within the normal reference range. Only one female participant who underwent PET/CT imaging with Discovery STE 8 scanner had a SMI value of 5.5 kg/m2 by BIA, which was below the normal cut-off value (5.7 kg/m2) suggested by AWGS-2019 consensus. However, her HGS value (20.5 kg) was within the normal reference range (> 18 kg), thus no participant was classified as having sarcopenia.
Quantitative body composition parameters derived from torso PET/CT images
Table 1 and Supplementary Table 2 summarize the measured body composition parameters derived from CT images of PET/CT. Muscle volumetric indices from the torso compartment were approximately 10 times higher than those from the abdominal compartment, regardless of sex. Similarly, the volumetric SF value from the torso compartment exhibited approximately 8–9 times higher values compared with the abdominal compartment. However, volumetric AVF values from the torso compartment were only 2–3 times higher than those from the abdominal compartment, due to intrinsically restricted FOV of AVF, which primarily covers the abdomen. All AVF values were higher in males, while SF values were higher in females. The myosteatosis-related parameters including the volumetric or areal muscle indices with very low attenuation (VLA) or low attenuation (LA) in each compartment, representing apparent intramuscular adipose tissue and intramuscular lipid pool, which reflect poor and intermediate muscle quality, were relatively higher in women compared to men. Analysis of covariance, adjusted for baseline parameters such as CT acquisition parameters, body mass index (BMI), or HGS, revealed no significant differences in muscle or fat-related parameters among the different scanner types.
Correlation of body composition parameters measured from Torso CT and BIA
When compared with SMI by BIA, TorsoMVI had the highest correlation (men r = 0.80 [confidence interval (CI) 0.75–0.84]; women r = 0.73 [CI: 0.65–0.80]), followed by abdominal muscle areal index (AbdMAI) (men r = 0.73 [CI: 0.66–0.79]; women r = 0.72 [CI: 0.63–0.79]) and abdominal muscle volumetic index (AbdMVI) (men r = 0.66 [CI: 0.58–0.73]; women r = 0.64 [CI: 0.54–0.73]) regardless of sex (Figs. 2 and 3). In line with this trend, other volumetric parameters from the torso compartment, torso skeletal volumetric index (TorsoSMVI) and TorsoMVI-normal attenuation (NA), also showed strong correlation with SMI compared with those from the abdominal compartment.
Scatter plots showing Pearson correlation coefficients between muscle and fat parameters from torso computed tomography (CT) and bioelectrical impedance analysis (BIA) according to sex. (a) Skeletal muscle index versus torso muscle volumetric index (TorsoMVI), (b) Body fat mass versus torso fat volumetric index (TorsoFVI), (c) Skeletal muscle index versus abdominal muscle volumetric index (AbdMVI), (d) Body fat mass versus abdominal fat volumetric index (AbdFVI), (e) Skeletal muscle index versus abdominal muscle areal index (AbdMAI), (f) Body fat mass versus abdominal fat areal index (AbdFAI). Men are represented by blue dots and lines, and women by red dots and lines.
Regarding fat analysis, the correlation coefficients between CT-derived and BIA-derived parameters exhibited generally higher values than those observed in the muscle analysis (Fig. 2). Similar to the muscle correlation analysis, the torso fat volumetric index (TorsoFVI) showed the highest correlation coefficients with body fat mass of BIA (men r = 0.93 [CI: 0.91–0.95], women r = 0.93 [CI: 0.90–0.95]), followed by the abdominal fat areal index (AbdFAI) (men r = 0.90 [CI: 0.87–0.92], women r = 0.89 [CI: 0.85–0.92]), and finally the abdominal fat volumetric index (AbdFVI) (men r = 0.84 [CI: 0.80–0.87], women r = 0.88 [CI: 0.84–0.91]). In most cases, SF-related parameters from the three compartments showed slightly higher correlations with BIA-derived fat parameters (body fat mass, body fat percentage) than AVF-related parameters, regardless of sex.
Correlation of body composition parameters with muscle strength related parameters
Among the various muscle-related measures obtained from BIA or torso CT, BIA-derived SMI showed the highest correlation with HGS (men r = 0.46 [CI: 0.35–0.56]; women r = 0.33 [CI: 0.18–0.46]) and KES (males r = 0.24 [CI: 0.11–0.36]; females r = 0.43 [CI: 0.29–0.55]), compared with all the parameters obtained from CT (Fig. 4). Notably, men and women showed distinct relationships between upper and lower limb strength. The correlation coefficient of HGS was higher in men, whereas that of KES was higher in women. Among the CT-derived parameters and strength-related parameters in men, those from the torso compartment had the most significant association with HGS (TorsoMVI: r = 0.39 [CI: 0.27–0.49]), surpassing others from the abdominal compartment (AbdMVI: r = 0.25 [CI: 0.12–0.37]; AbdMAI: r = 0.26 [CI: 0.14–0.38]). However, this trend was not observed in women. Regarding HGS, the volumetric compartment of the abdomen demonstrated higher correlation compared with those from the volumetric torso and the areal abdomen compartments (AbdMVI: r = 0.26 [CI: 0.11–0.40]; TorsoMVI: r = 0.21 [CI: 0.05–0.36]; AbdMAI: r = 0.16 [CI: 0.00–0.31]). In terms of KES in women, all CT measures from the three compartments showed nearly similar values of statistically significant correlation coefficients (TorsoMVI: r = 0.35 [CI: 0.20–0.48]; AbdMVI: r = 0.34 [CI: 0.19–0.47]; AbdMAI: r = 0.36 [CI: 0.21–0.49]).
Subgroup analysis between the normal and the possible sarcopenia
Further categorization based on HGS gave rise to some distinct features between men and women. Several torso-derived volumetric parameters (TorsoMVI: 5,960 ± 785 vs. 5,210 ± 487 cm3/m2, p = 0.02; TorsoSMVI: 5,700 ± 751 vs. 5,017 ± 485 cm3/m2, p = 0.02; TorsoMVI-NA: 3,992 ± 585 vs. 3,495 ± 461 cm3/m2, p = 0.04) showed significantly different values between normal and possible sarcopenia in men (Table 2). However, none of the CT-derived parameters in women exhibited any significant differences or tendencies differentiating normal and possible sarcopenia. Age was identified as another decisive factor, but it only showed significance in men. Men with possible sarcopenia were older than those with normal muscle strength (67.0 ± 9.5 vs. 56.2 ± 9.1 years, p = 0.01). In contrast, HGS (men: 39.2 ± 6.1 vs. 25.1 ± 1.9 kg, p < 0.001; women: 23.1 ± 3.6 vs. 13.5 ± 2.6 kg, p < 0.001) and KES (men: 74.3 ± 26.1 vs. 49.8 ± 9.3 kg, p = 0.001; women: 38.8 ± 11.2 vs. 26.2 ± 8.5 kg, p < 0.001) were commonly shared parameters differentiating normal and possible sarcopenia in both sexes.
Discussion
In this study, we conducted a low-dose CT from PET/CT-based body composition analysis on middle-aged, healthy adults undergoing PET/CT for health screening, segmenting the torso CT into three distinct compartments. The results revealed that body composition analysis from the torso compartment correlated more closely with measurements from BIA and muscle strength than area or volume-based analyses derived from the abdominal compartment. Metrics obtained from abdominal compartments lacked statistical significance in distinguishing sarcopenia, suggesting that the quantity and quality of muscle indices from the torso compartment are emerging as the most dependable indicators. Similarly, fat-related indices derived from the torso exhibited the strongest correlation with BIA results.
Although the abdomen is the most common site for body composition analysis10,17,18, its correlation with BIA indices was surpassed by the torso compartment in this study. Additionally, in men, only a few muscle parameters derived from the torso could differentiate possible sarcopenia from normal conditions. Therefore, body composition analysis from the torso compartment holds greater potential than that from the abdominal one in evaluating sarcopenia and physical performance.
In clinical practice, the use of whole-body CT scan is primarily confined to patients who have suffered major trauma19. However, the potential benefits of supplemental body composition analysis from CT scans in these trauma patients are not well-documented. Given that PET/CT scans are predominantly performed in patients with malignancy20, they provide an ancillary data on body composition, which is a factor closely intertwined with patient prognosis. As more evidence emerges about the robust correlation between physical strength and CT-derived body composition, clinicians could harness this data to gauge a patient's current physical and nutritional status. Such information could provide instrumental in tailoring suitable exercise and nutritional regimens for cancer patients. Furthermore, when assessing cancer patients exhibiting cachexia, there is a potential to introduce a novel imaging biomarker related to body composition, going beyond the current cachexia indices that primarily focus on weight loss and chronic illness21.
While none of the parameters obtained from PET/CT showing superiority to those from BIA when correlated with muscle strength, CT measurements offer additional advantages over that from BIA5. First, CT allows the estimation of the trunk muscles and the classification of AVF and SF, which are impossible to assess using the BIA. Second, BIA could not estimate the quality of muscles per se, such as intra-muscular fat. Additionally, CT is free from confounding factors such as the hydration status of the body, ambient temperature, and exercise, which are known drawbacks of BIA-based analysis. Considering that body composition analysis conducted by CT is more reproducible and considered the gold standard by the guidelines, the integration of sophisticated analytical software, such as that utilized in this study, could catalyze the broader application of PET/CT for opportunistic body composition analysis15. As the FOV of torso PET/CT is limited, not covering the mid-to-distal upper/lower limbs, acquiring total body PET/CT images from vertex to foot, including all limb muscles, would yield a more holistic overview compared to both BIA and torso PET/CT22. The widespread adoption of half body or total body PET/CT scanners, which can cover the whole-body more efficiently, may facilitate the implementation of such an approach in near future23. Moreover, if PET/CT analytical tools are refined to distinctly evaluate appendicular and trunk muscles, it would provide a more accurate estimation of appendicular muscle than BIA.
Additionally, the FOV of torso PET/CT is limited to the trunk and does not cover the mid-to-distal upper and lower limbs, which contain abundant muscle and little adipose tissue. This limitation might result in higher correlation coefficients between CT- and BIA-derived parameters in fat analysis than those in muscle analysis, regardless of compartments, as most adipose tissue is present in the trunk.
Even though numerous studies have utilized PET/CT for body composition analysis, few have provided raw data, impeding direct comparison among studies. We were able to find only one study conducted by Lee et al. that reported an average value of TorsoMVI approximately 3,500 cm3/m2 from a sample of 520 South Korean older adults, characterized by male-to-female ratio of 0.5 and an average age in the mid-70s15. When adjusted for the same gender ratio, average value of TorsoMVI obtained from our participants appears to lie between 4,500 and 5,000 cm3/m2. As our participants were approximately 20 years younger than those in the study mentioned above, such differential figures from these two studies might be eligible for a preliminary data. When it comes to abdominal compartment analysis, numerous studies were available for comparison. Notably, a study conducted from our institution with healthy participants presented figures akin to ours from the abdominal areal compartment24. However, AbdMAI, AbdSMAI, and AbdMAI-NA were slightly higher than ours, whereas AbdMAI-LA and AbdMAI-VLA were somewhat lower. These differences might come from age discrepancies between the cohorts and variation in CT protocols — either with or without contrast enhancement25,26.
As all participants of this study undertook the PET/CT images with both arms raised under fixed and low dose of CT, it may be difficult to directly extrapolate into and compare with the patients whose arms were down at the time of PET/CT acquisition. Arm positioning might affect the image quality27. In addition, difference of vendor and CT protocol slightly affects the value of HU thereby hampering precise comparison between muscle-related parameters especially in quality-related one25. Therefore, acknowledging inherent difference with additional validating process among scanners might be required under various scanners.
Women possess a relatively lower ratio of lean tissue distribution in the upper body, whereas men have a higher proportion of muscle cross-sectional area in the biceps brachii than women28. Accordingly, HGS showed a higher correlation with muscle component analysis in men than KES. In contrast, KES had a higher correlation with muscle parameters than HGS in women. Although it cannot be generalized, additional research is required to determine which muscle strength is a better indicator to diagnose possible sarcopenia for each gender29. These differences in physical performance and fat distribution were probably from microscopically distinct compositions or function of muscle fiber types, as well as grossly distinct body shapes, distribution, and compositions between genders30.
Although area and volume-based parameters from the abdominal compartment was strongly correlated, most of the values derived from the areal compartment exhibited better correlation with BIA-derived muscle or fat parameters compared with those from the volumetric compartment. As few studies have been conducted regarding the comparison between single slice and multi-slices around the abdomen17,31, reason for this paradoxical result is not yet clear. In case of PET/CT, respiratory motion may affect the CT image quality on abdominal region because of shallow breathing during CT acquisition. Susceptibility of measurement error across a FOV might be another plausible hypothesis.
Our research has some limitations. First, we included a small number of healthy participants from a specific race and used data from a single vendor with a low dose CT protocol, which was insufficient to interpolate as an absolute reference value for most of the PET/CT images used in oncologic applications. In addition, further comparative study between DXA and torso or whole-body PET/CT is required to identify the pros and cons of each method beyond BIA. Finally, comparison with other physical performance–related parameters such as gait speed and chair stand test are required, as those tests are pivotal constituents for the sarcopenia screening32.
Our study indicated that CT-based volumetric parameters obtained from the torso compartment of PET/CT showed superior performance in evaluating body composition compared with those obtained from the abdominal compartment in healthy adults. Although we observed gender differences in the association with physical strength, our findings suggested that torso PET/CT holds promise as an effective option for evaluating body composition and sarcopenia.
Methods
Study participants
Participants were retrospectively selected from a pool of healthy adult participants aged 19 years and older who underwent torso 18F-FDG PET/CT, BIA, and physical fitness evaluations by muscle strength test as part of their health screening in the Health Screening and Promotion Center of our institution between 2011 and 2020. Exclusions were made for individuals presenting with notable abnormalities in their baseline blood tests (serum blood glucose level > 126 mg/dL; aspartate transaminase or alanine transferase levels > twice the upper limit of normal; estimated glomerular filtration rate < 60 ml/min/1.73m2; free T4 and thyroid stimulating hormone level > the normal range). Additionally, those who had not completed the BIA or physical fitness assessments were excluded from the study.
This study was approved by the institutional review board at Asan Medical Center (IRB No. 2022–0496), and was performed in line with the principles of the Declaration of Helsinki.
BIA
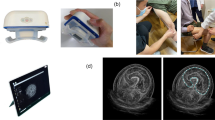
The BIA-derived body composition analysis was conducted using InBody 720 (Biospace Co., Seoul, Korea). Prior to the test, participants fasted for 8 h and removed any jewellery or wearable items, such as rings, watches, and necklaces. They were also instructed not to use a sauna or bathe for an extended period to minimize water loss. Temperature of the test room was maintained at 20–25 °C. During the test, participants stood upright, making contact with the electrodes of the analyzer through both hands and feet. To achieve consistent measurements, their arms and legs were slightly separated and extended from the body. Data collected via BIA included height, weight, BMI, body fat mass, body fat percentage, muscle mass, ASM, and body water in each of the upper and lower limbs. The SMI (kg/m2) of BIA was calculated by dividing the ASM by the square of height. Individuals with limb casts, limited mobility preventing standing, or limb amputations were not considered for this analysis.
Muscle strength–related parameters
The physical fitness evaluation was conducted by muscle strength test using the comprehensive physical fitness diagnostic equipment, Helmas 2.0 (O2 Run, Inc., Seoul, Korea). The examination environment was maintained at a temperature of 20–22 °C with a relative humidity of 60%. Participants were advised to wear comfortable, non-restrictive clothing, and the test was carried out under conditions that ensured maximum stability. The specific methodologies for each test are detailed below:
-
Upper limb strength (Hand Grip Strength, HGS): The participants are to position their second knuckle on the handle of the dynamometer, keeping their arm abducted at approximately 30 degrees while ensuring the elbow remains unbent. On the tester's signal, participant should exert maximum grip force on the dynamometer handle for 5 s. Two consecutive measurements are to be taken for each hand, and superior value from each side to be recorded. For analysis, the highest measurement from either side is used.
-
Lower limb strength (Knee Extension Strength, KES) Participants should sit ensuring their hips and back are firmly against the backrest, and their hamstrings are closely aligned. They are to place both feet between the leg strength tester pad and the bar. After securing a grip on the handle of the instrument, they should exert their maximum force by pushing both feet forward. From two measurements, the higher value is used for analysis.
PET/CT acquisition
For this study, participants were scanned using one of four randomly selected PET/CT scanners: Discovery STE 8, Discovery 690, Discovery 690 Elite, and Discovery 710 (GE Healthcare Chicago, USA). Participants were required to fast for at least 6 h before the injection of 18F-FDG at a dosage of 5.18 MBq/kg, and they were encouraged to drink more than 1 L of water. The PET/CT scan was started about 1 h after the 18F-FDG injection. The scanning procedure commenced with a non-enhanced CT image, using a scout view and a low-dose CT protocol (120 or 140 kVp, 25 mA, 0.5 s rotation, 3.75 mm slice thickness, and a pitch of 0.938–0.984). Table 3 presents the detailed acquisition and reconstruction parameters of CT. This image spanned from the base of the skull to the upper thigh region. Following the CT phase, PET imaging was acquired over 7 or 8 beds consecutively, with each bed being scanned for a duration of 2.0 to 2.5 min. All participants who underwent the PET/CT scan had their arms raised.
Segmentation and analysis of CT-derived quantitative parameters
CT images were analyzed using a commercially available software, DeepCatch (MEDICALIP, Seoul, Korea). The software automatically segmented seven body composition components using three-dimensional (3D) U-Nets: skin, bone, muscle, two type of fat (AVF, SF), internal organs, vessels, and the central nervous system.
From the segmented data, following three distinct compartments were identified within the torso CT for regional quantitation of muscle and fat (Fig. 5):
-
1.
Torso volumetric compartment: This covered the entire FOV of the torso, encompassing the trunk and bilateral proximal upper/lower limbs. Metrices derived here were torso muscle volume (TorsoMV, cm3) and torso fat volume (TorsoFV, cm3).
-
2.
Abdominal volumetric compartment: Spanning from the lower margin of the 12th rib to the upper margin of iliac crest, this section yielded the abdominal muscle volume (AbdMV, cm3) and abdominal fat volume (AbdFV, cm3).
-
3.
Abdominal areal compartment: Measurements at single-slice L3 level rendered the abdominal muscle area (AbdMA, cm2) and the abdominal fat area (AbdFA, cm2).
The total fat value was calculated as the sum of the volumes or areas of AVF and SF.
For muscle quality assessment, the CT density of muscle component within each compartment (TorsoMV, AbdMV, and AbdMA) was further divided based on HU ranges33:
-
1.
Normal attenuation (NA): HU range from + 30 to + 150 indicates healthy muscle with little intramuscular fat.
-
2.
Low attenuation (LA): HU range from −29 to + 29 reflects the intramuscular lipid pool.
-
3.
Very low attenuation (VLA): HU range from −190 to −30 represents apparent intramuscular adipose tissue.
Torso skeletal muscle volume (TorsoSMV), abdominal skeletal muscle volume (AbdSMV), and abdominal skeletal muscle area (AbdSMA) were calculated after subtraction of volume or areas of VLA from TorsoMV, AbdSMV, or AbdMA, respectively.
To adjust for body size, muscle and fat parameters (cm3 or cm2) derived from the PET/CT were normalized to volume index or area index values by dividing them by the square of each individual's height (m2).
Diagnosis of sarcopenia and possible sarcopenia
Sarcopenia was classified according to the AWGS-2019 consensus criteria. First, low muscular strength assessed by HGS (male < 28 kg; female < 18 kg) was defined as “possible sarcopenia”. Next, SMI (male < 7.0 kg/m2; female < 5.7 kg/m2) measured by BIA was used as the primary indicator for diagnosing sarcopenia13.
Statistical analysis
Descriptive statistics were presented as mean ± standard deviation and were categorized according to group distinctions such as sex, PET/CT scanner type, and muscular strength (normal or abnormal). Group comparisons were carried out using the Mann–Whitney U test or one-way ANOVA with a Tukey post hoc test. In cases where bias adjustment was required for multiple comparisons, ANCOVA with a Bonferroni correction was employed. The degree of correlation among each parameter of body composition and muscular strength was assessed using the Pearson correlation coefficient (r) with 95% confidence interval. All statistical analyses were performed using the SPSS version 23.0 (IBM SPSS Inc., Chicago, Ill., USA). A two-sided p value < 0.05 was considered statistically significant.
Data availability
All data generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
Janssen, I. Influence of sarcopenia on the development of physical disability: The Cardiovascular Health Study. J. Am. Geriatr. Soc. 54, 56–62 (2006).
Mourtzakis, M. et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 33, 997–1006 (2008).
Abe, T. et al. Influence of severe sarcopenia on cardiovascular risk factors in nonobese men. Metab. Syndr. Relat. Disord. 10, 407–412 (2012).
Kim, J. H. et al. Assessment of appendicular skeletal muscle mass by bioimpedance in older community-dwelling Korean adults. Arch. Gerontol. Geriatr. 58, 303–307 (2014).
Lee, K. et al. Recent issues on body composition imaging for sarcopenia evaluation. Korean J. Radiol. 20, 205–217 (2019).
Trevino-Aguirre, E., Lopez-Teros, T., Gutierrez-Robledo, L., Vandewoude, M. & Perez-Zepeda, M. Availability and use of dual energy X-ray absorptiometry (DXA) and bio-impedance analysis (BIA) for the evaluation of sarcopenia by Belgian and Latin American geriatricians. J. Cachexia Sarcopenia Muscle. 5, 79–81 (2014).
Cheng, K. Y. et al. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J. Cachexia Sarcopenia Muscle. 12, 2163–2173 (2021).
Coppini, L. Z., Waitzberg, D. L. & Campos, A. C. Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr. Opin. Clin. Nutr. Metab. Care 8, 329–332 (2005).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 16–31 (2019).
Weston, A. D. et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology 290, 669–679 (2019).
Rutten, I. J. et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur. J. Surg. Oncol. 43, 717–724 (2017).
Surov, A. et al. CT-defined sarcopenia predicts treatment response in primary central nervous system lymphomas. Eur. Radiol. https://doi.org/10.1007/s00330-023-09712-y (2023).
Chen, L. K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21, 300–307 (2020).
Schaefferkoetter, J., Shah, V., Hayden, C., Prior, J. O. & Zuehlsdorff, S. Deep learning for improving PET/CT attenuation correction by elastic registration of anatomical data. Eur. J. Nucl. Med. Mol. Imaging. 50, 2292–2304 (2023).
Lee, Y. S. et al. Deep neural network for automatic volumetric segmentation of whole-body CT images for body composition assessment. Clin. Nutr. 40, 5038–5046 (2021).
Choi, H. et al. Association of adipopenia at preoperative PET/CT with mortality in stage I non-small cell lung cancer. Radiology. 301, 645–653 (2021).
Waduud, M. A. et al. Volumetric versus single slice measurements of core abdominal muscle for sarcopenia. Br. J. Radiol. 92, 20180434 (2019).
Pickhardt, P. J. Value-added opportunistic CT screening: State of the art. Radiology. 303, 241–254 (2022).
Stengel, D. et al. Association of low-dose whole-body computed tomography with missed injury diagnoses and radiation exposure in patients with blunt multiple trauma. JAMA Surg. 155, 224–232 (2020).
Yu, H. et al. Expert consensus on oncological [18F]FDG total-body PET/CT imaging (version 1). Eur. Radiol. 33, 615–626 (2023).
Go, S. I. et al. Cachexia index as a potential biomarker for cancer cachexia and a prognostic indicator in diffuse large B-cell lymphoma. J. Cachexia Sarcopenia Muscle 12, 2211–2219 (2021).
Huston, S. F., Abdelmalik, A. G., Nguyen, N. C., Farghaly, H. R. & Osman, M. M. Whole-body 18F-FDG PET/CT: The need for a standardized field of view–a referring-physician aid. J. Nucl. Med. Technol. 38, 123–127 (2010).
Sachpekidis, C. et al. Application of the long axial field-of-view PET/CT with low-dose [18F]FDG in melanoma. Eur. J. Nucl. Med. Mol. Imaging 50, 1158–1167 (2023).
Kim, H. K. et al. Age-related changes in muscle quality and development of diagnostic cutoff points for myosteatosis in lumbar skeletal muscles measured by CT scan. Clin. Nutr. 40, 4022–4028 (2021).
Cropp, R. J., Seslija, P., Tso, D. & Thakur, Y. Scanner and kVp dependence of measured CT numbers in the ACR CT phantom. J. Appl. Clin. Med. Phys. 14, 4417 (2013).
Boutin, R. D., Kaptuch, J. M., Bateni, C. P., Chalfant, J. S. & Yao, L. Influence of IV contrast administration on CT measures of muscle and bone attenuation: Implications for sarcopenia and osteoporosis evaluation. Am. J. Roentgenol. 207, 1046–1054 (2016).
Brink, M. et al. Arm raising at exposure-controlled multidetector trauma CT of thoracoabdominal region: Higher image quality, lower radiation dose. Radiology 249, 661–670 (2008).
Miller, A. E. J., MacDougall, J. D., Tarnopolsky, M. A. & Sale, D. G. Gender differences in strength and muscle fiber characteristics. Eur. J. Appl. Physiol. 66, 254–262 (1993).
Kristensen, M. T., Hulsbaek, S., Faber, L. L. & Kronborg, L. Knee extension strength measures indicating probable sarcopenia is associated with health-related outcomes and a strong predictor of 1-year mortality in patients following hip fracture surgery. Geriatrics. https://doi.org/10.3390/geriatrics6010008 (2021).
Zhong, X. & Zimmers, T. A. Sex differences in cancer cachexia. Curr. Osteoporos. Rep. 18, 646–654 (2020).
Anyene, I. et al. Body composition from single versus multi-slice abdominal computed tomography: Concordance and associations with colorectal cancer survival. J. Cachexia Sarcopenia Muscle 13, 2974–2984 (2022).
Ryu, J. Y., Kim, M., Kim, K. S., Kim, S. & Won, C. W. Chair stand test as a proxy for physical performance and muscle strength in sarcopenia diagnosis: The Korean frailty and aging cohort study. Aging Clin. Exp. Res. 34, 2449–2256 (2022).
Kim, H. K. & Kim, C. H. Quality matters as much as quantity of skeletal muscle: Clinical implications of myosteatosis in cardiometabolic health. Endocrinol. Metab. 36, 1161–1174 (2021).
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI17C2410) and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (Grant Number: 2021R1A2C3009056).
Author information
Authors and Affiliations
Contributions
D.Y.L., J.S.O. and J.R.; acquisition of data: D.H.K., S.B. and H.K.; technical support: J.S.O., J.S.K., M.O., and Y.K.; management of data: D.Y.L., J.W.K., D.H.K., S.B. and H.K.; analysis and interpretation of data: D.Y.L. and J.W.K.; writing original draft of the manuscript: D.Y.L. All authors read, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
The need for informed consent was waived by the IRB.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, D.Y., Oh, J.S., Kim, J.W. et al. Comparative analysis of body composition using torso CT from PET/CT with bioelectrical impedance and muscle strength in healthy adults. Sci Rep 14, 21597 (2024). https://doi.org/10.1038/s41598-024-71878-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71878-2