Abstract
The goal of our research is to elucidate and better assess placental function in rats with preeclampsia through an innovative application of ultrasound-based radiomics. Using a rat model induced with L-NAME, we carefully investigated placental dysfunction via microstructural analysis and immunoprotein level assessment. Employing the Boruta feature selection method on ultrasound images facilitated the identification of crucial features, consequently enabling the development of a robust model for classifying placental dysfunction. Our study included 12 pregnant rats, and thorough placental evaluations were conducted on 160 fetal rats. Distinct alterations in placental microstructure and angiogenic factor expression were evident in rats with preeclampsia. Leveraging high-throughput mining of quantitative image features, we extracted 558 radiomic features, which were subsequently used to construct an impressive evaluation model with an area under the receiver operating curve (AUC) of 0.95. This model also exhibited a remarkable sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of 88.7%, 91.5%, 90.2%, 90.4%, and 90.0%, respectively. Our findings highlight the ability of ultrasound-based radiomics to detect abnormal placental features, demonstrating its potential for evaluating both normative and impaired placental function with high precision and reliability.
Similar content being viewed by others
Introduction
Hypertensive disorders during pregnancy are among the leading causes of maternal and perinatal mortality worldwide1. In particular, preeclampsia (PE), which affects approximately 3–5% of all pregnancies, is responsible for an estimated 42,000 maternal deaths each year2,3. Preeclampsia is an enigmatic multisystemic disorder specific to pregnancy with diverse presentations4; moreover, childbirth is the only route to a cure. Offspring of women with pre-eclampsia have lower birth weight and higher blood pressure throughout childhood and young adulthood compared to those not exposed to the condition5. Despite the significant health burden associated with preeclampsia, identifying a simple and noninvasive method to assess the risk of adverse pregnancy outcomes following preeclampsia remains a challenge2. Closely attending clinical warning signs during pregnancy and improving prenatal examination, and early diagnostic methods are crucial in the diagnosis and treatment of preeclampsia.
It is well known that preeclampsia often presents with impaired remodeling of spiral arterioles, which ultimately results in impaired ability the maternal–fetal interface to optimally establish nutrient exchange, characterized by high capacitance and low resistance6,7. Recent research has identified preeclampsia as one of the "great obstetrical syndromes" that involves multiple and sometimes overlapping pathologic processes activating a common pathway comprising of endothelial cell activation, intravascular inflammation, and syncytiotrophoblast stress, ultimately resulting in the recognizable clinical features of these disorders8,9. Maternal vascular inflammatory syndrome is believed to be triggered by abnormal levels of soluble fms-like tyrosine kinase-1 (sFlt-1) and placental growth factor (PlGF), often referred to as angiogenic imbalance10. Research11,12 have confirmed that the ratio of antiangiogenic factor sFlt-1 to proangiogenic factor PIGF can be used as an indicator to assess placental dysfunction in preeclampsia patients. These angiogenic factors are secreted into the maternal circulation by placentas that have become dysfunctional due to cellular oxidation or endoplasmic reticulum stress caused by placental ischemia–reperfusion injury13,14,15. The result of this placental insufficiency-based angiogenic imbalance is hypertension, proteinuria, and other maternal multiorgan damage. Placental insufficiency may precede the onset of clinical manifestations, but is closely associated with disease symptoms16. Therefore, with the placenta being an essential but poorly understood organ in the development of preeclampsia, a noninvasive imaging method that can evaluate the functional status or impairment of the placenta to identify signs of imminent pathology, may assist in early clinical intervention and ameliorate the effects of the disease later in pregnancy.
Ultrasound is an important method for prenatal screening that can detect abnormal structural changes in the placenta noninvasively and in real time. Sebire et al17 reported that prenatal ultrasound can identify a range of features related to placental pathology. However, the visual recognition of features through the naked eye is very limited and highly subjective. In recent years, with the advancement of artificial intelligence technology (AI) tools for, the importance of using AI to assist physicians in diagnosis and treatment has gradually been recognized. Similarly, radiomics is a complex image analysis technique designed to improve the usability of data available to clinicians through advanced mathematical analysis18. Specifically, radiomics allows high-throughput extraction of the spatial distribution of signal intensities and interrelationships between pixels, and quantification of texture information using AI-based analyses18,19. Lili Zhu et al20 demonstrated the feasibility of machine learning models based on the clinical and radiomic features of 2-D ultrasound images to determine the posttransplant renal function (pTRF). Additionally, Moro et al.21 have developed ultrasound-based radiomic models that suggested that radiomics can better ability to discriminate between high-risk endometrial cancer and other endometrial cancers. These studies highlight the potential for novel uses of ultrasound radiomics to improve clinical decision making and in precision medicine. However, little research has been conducted in applying such methods to quantify the risk to the placenta and to objectively assess its function22.
Both rat and human placentas are blood sinus chorionic placentas, consisting of complex dendritic microvilli structures23. Moreover, animal models are highly effective tools for investigating the pathogenesis of preeclampsia as well as potential treatment methods24. The majority of animal studies have focused on rats and mice, with these models providing insight into a diverse range of mechanisms underlying the development of preeclampsia, including trophoblast dysfunction, impaired placental vascularization, and hypercirculatory placental factors25,26. Given this context, the aim of this study was to quantify the placental structure of the rat placenta through a combination of ultrasound radiomics tools, and ultimately to develop a reliable model for the assessment of placental function in preeclamptic rats, thus validating the potential of ultrasound radiomics techniques in identifying abnormal placental structures as well as poor functional status.
Methods
Animals and drug treatment
Sprague Dawley female rats (8 weeks old, weighing approximately 200–240 g) were purchased from the Vital River Laboratory Animal Technology. All rats were housed in a relatively consistent environment at room temperature (18–20 °C) and normal humidity (50–60%), with food and water available ad libitum, and were reared and bred in individually ventilated cages. After one week of acclimatization, the female rats were mated with sexually mature males (2:1), and the first day of gestation (gestation day, GD) was defined as the day when the vaginal plug was found. All pregnant rats were anesthetized by intraperitoneal injection of sodium pentobarbital (30 mg/kg). Rats were euthanized intravenously at the 19 days of gestation (GD19), fetal rats were subsequently evaluated by ultrasound; then, relevant specimens were collected for preservation. The experimental protocol was approved by the Institutional Animal Care and Use Committee (Ethical approval was obtained from the hospital institution Ethical Review Board, No. 2021–20; Date of approval, January 27, 2021). The animal experiments were performed were in accordance with the National Institutes of Health Guidelines for Use and Care of Animals (National Institutes of Health Publication No. 85–23). The authors complied with the ARRIVE guidelines.
The pregnant rats were randomly divided into two groups: control group (sham operation, n = 6), which received a 0.9% intraperitoneal injection of normal saline daily from GD10 to GD18; and the L-NAME group (n = 6), which received an intraperitoneal injection of 125 mg/kg/day L-NAME (NG-Nitro-L-arginine Methyl Ester, L-NAME, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) daily from GD10 to GD18 intraperitoneally. The blood pressure of the rats was measured using a BP-2000 Animal Noninvasive Blood Pressure Analysis System27. The systolic BP (SBP) reported by the system represented the average of at least six valid BP measurements, featuring a normal wave pattern. The SBP of the rats in the two groups was detected at progestation (GD0), GD1 and GD18. Each pregnant rat was placed in a metabolic cage at GD0, GD1, or GD18, for the collection of 24h urine samples (see the supplemental details).
Histopathological examination
The specimen of the placenta was collected and then observed in its entirety, paying particular attention to its general appearance and basic morphology. After adequate fixation (4% paraformaldehyde), the tissue was dehydrated in gradient ethanol solution and immersed in wax according to a standard protocol. Subsequently, the wax blocks were carefully sliced (thickness 4 μm) and placed on slides. After dewaxing, hematoxylin–eosin staining was performed. The overall morphology and microstructure of the placenta were evaluated under a light microscope.
Western blotting
Western blotting was conducted according to the specifications of the antibody manufacturer. Briefly, 20 μg aliquots of lysates were subjected to SDS-PAGE. The resulting protein bands were then transferred onto a PVDF membrane. Antibodies against sFlt-1 and PIGF, along with a Phototope-HRP western blot kit, were used for the western blotting assay.
Evaluation of placental function
The placentas were categorized into two distinct subgroups: the normal function group and the dysfunction group. A smooth pregnancy course, devoid of L-NAME drug intervention, served as a prerequisite for inclusion in the normal function category. Conversely, successful induction of preeclampsia was necessary for classification into the dysfunction group. Any of the following items indicated inclusion in dysfunction group: abnormal placental pathological structure, aberrant placental tissue expression, or deviant fetal rat development. Notably, animals in the control group that exhibited any of the three aforementioned anomalies were systematically excluded from analysis.
The procedures of ultrasound-based radiomics
-
(i)
Placental ultrasound image acquisition
On the 19th day of gestation in pregnant rats, the pregnant rats were fixed in a supine after anesthesia, and their abdomen was disinfected after hair removal. Then ultrasonic examination was performed using an instrument (EPIQ Elite W, Intera, Philips, The Netherlands) with the linear array probe eL18-4 (the center frequency was 9.0 MHz). A sonographer with ≥ 5 years of experience in obstetrics and gynecology ultrasound imaging performed all of the examinations. Four complete images were randomly acquired for each fetal rat placenta. The placenta area was not blocked by fetal rat limbs or other body parts that produce an acoustic shadow, and tried to place the placenta area in the near field area to obtain a complete and accurate image of the placenta. The data were reviewed by a senior physician of 10 years and those with substandard or incomplete images were excluded. The operators and auditors involved in data collection were blinded to the groups. There were no annotations of arrows or any measured values on the images, and the images were collected and stored in DICOM format (.DCM).
-
(ii)
Placenta delineation
The regions of interest (ROIs) on the placental images were delineated using ITK-SNAP software (3.2.0-RC1) by automatically drawing boundaries between the ROI and background. The masks of the segmented placenta were reviewed and manually adjusted by a radiologist experienced in obstetric imaging. A detailed illustration of the steps of semiautomatic placenta segmentation is provided in Fig. 1.
-
(iii)
Feature extraction
The feature catalog was divided into six main categories, containing a total of 93 features. Then, texture extraction of the 93 features was carried out on 6 components respectively, and 558 features were obtained (please refer to the supplemental materials for details). All features are numbered sequentially (1–558) so that each feature is associated with a feature serial number (FSN).
-
(iv)
Features analysis
Multifactor analysis was carried out to remove redundant features, preventing overfitting of the model. The Boruta method was used for feature selection, and a random forest algorithm was used to measure the feature importance, ultimately reducing the dimensionality of the input model.
-
(v)
Model construction
After feature selection, the model was constructed with the training set as the inputs. Through experimental comparison, the extreme random tree algorithm28 was selected as the classifier. Using Bayesian search theory and tenfold cross-validation as the standard, iterative optimization was carried out, and the optimal phenotypic model was ultimately established and trained.
Statistical analyses
IBM SPSS Statistics 20.0 (SPSS, Inc., New York) was used for statistical analysis in this study. Continuous variables were statistically analyzed with independent t tests or nonparametric rank sum tests. Categorical variables were analyzed with the chi-square test. Redundant features were removed based on the Pearson correlation coefficient, and the Boruta feature selection method was utilized to reduce the feature dimension of the input model. This was done to select useful features for the model. For bilateral tests, P < 0.05 was considered to indicate statistical significance.The microscopic observation and image analysis of placental pathology were conducted under the guidance of two pathologists.
Results
The gestational outcomes for the rats are shown in Fig. 2. The control group included 87 fetal rats, with an average of 15 offspring per pregnant rat, while the L-NAME group included 73 fetal rats, with an average of 12 offspring per pregnant rat. The L-NAME group had a lower birth rate than the control group (Fig. 2ab). Additionally, the mean placental weight of the L-NAME group (1.54 ± 0.61) was significantly lower than that of the control group (2.12 ± 1.01) (P < 0.05) (Fig. 2f); however, the average weight of fetal rats in the L-NAME group did not differ significantly from that of the control group. This may be because preeclamptic fetal rats, although generally thinner than controls, often have generalized edema and occasionally show malformations (Fig. 2cd).
Comparison of fetal rat and placental development. (a) Overall embryonic development of rats in the control group; (b) Overall embryonic development of rats in the L-NAME group, which showed a higher mortality rate among fetal rats and even absorption of ‘fetus’; (c) The fetal rat of control groups. (d) The fetal rat of L-NAME group. (e) The comparison of fetal weight between the control group and the L-NAME group; (f) The comparison of placental weight between the control group and the L-NAME group; (g) The comparison of fetal placental weight ratio between the control group and the L-NAME group, The fetal placental weight ratio is indicative of placental functionality.
Pathological changes in the placenta
Compared with the control group, the L-NAME group exhibited obvious placental pathological changes. Upon observation of the general placental appearance, the placentas in the L-NAME group exhibited a duller color and smaller size. Microscopic examination revealed that in the control group, the cell structure of the labyrinth area was neatly arranged and orderly, with uniform intercellular space and a dense spongy layer structure (Fig. 3A). In contrast, the labyrinthine cells in the L-NAME group had a disordered structure, uneven intercellular space, greatly increase of red blood cells in the maternal blood space (Fig. 3B), leading to a large number of red blood cells confined within the connective tissue intervals between the maternal blood spaces, especially in the peripheral placental region, while the control group showed only a moderate amount of erythrocyte aggregation (Fig. 3C). Fibrous deposits and infiltration of macrophages and lymphocytes were more often observed in the tissue interspace. Additionally, the L-NAME group had a thicker, more loosely structured sponge layer with significantly increased cellular vacuolation (Fig. 3D).
Pathological morphology between control group and L-NAME group. (A) (C) The microscopic structure of the control group; (B) (D) The microscopic structure of the L-NAME group; The control and the L-NAME group showed a significant structural difference in the rat labyrinth zone. (A) (B) Magnification: 400×; (C) (D) Magnification: 200×.
Protein expression levels in placental tissue
Researches27,28 have confirmed that the ratio of antiangiogenic factor sFIt-1 to proangiogenic factor PIGF could be used as an indicator of assess placental dysfunction in preeclampsia. The expression levels of angiogenic factors in rat placental tissues were also quantified by protein immunoblotting (Fig. 4). Quantitative detection of protein levels revealed a significantly greater expression level of sFlt-1 and lower expression level of PlGF in the L-NAME group than in the control group (P < 0.05). The ratio of sFlt-1/PlGF was also significantly different between the two groups (P < 0.05). The results of this replication experiment also demonstrated the same outcome. The original blots and the replicates were presented in supplementary information file.
Protein expression levels in placental tissue between control group and L-NAME group. (A) Immunoblot analysis of the control and L-NAME groups of the strip charts; (B) Placental relative protein expression levels of sFIt-1 in the control and L-NAME groups; (C) Placental relative protein expression levels of PlGF in the control and L-NAME groups; (D) Placental relative protein expression levels of sFlt-1/PlGF in the control and L-NAME groups. *P < 0.05.
Analysis of placental texture characteristics
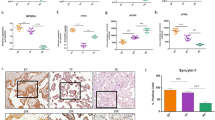
The Uniform Manifold Approximation and Projection (UMAP) visualization method29 was utilized to reduce the dimensionality of high-dimensional features, thus enabling the visualization of feature sets. The original data analyzed for each placenta were converted into feature vectors, allowing further feature sets to be generated. The feature sets of all features (Fig. 5a, blue negative and red positive), the 431 features obtained after single-factor analysis (Fig. 5b), and the 68 features selected after feature selection (Fig. 5c) were plotted. The negative samples included in the clusters of positive samples decreased after feature selection, and the classification trend could be discerned.
(a), (b), (c) Visualization of the distribution of ultrasound image features in rat placenta samples; (d), (e) Single-factor analysis of the radiomics features of the normal and dysfunction groups; (f) Visualization of features contributing to decision making in the rat placental function assessment model. SHAP: Shapley Additive explanations, for each sample model produces a predictive value, Shap value is the value assigned to each feature in the sample; FSN: Feature serial number.
To demonstrate the feasibility of radiomics features in placental function analysis, univariate analysis was performed for each characteristic in the normal (293, 84% in the control group) and dysfunction groups (265, 91% in the L-NAME group), and box plots were created (Fig. 5de). As depicted in the figure, the mean values of the original image in the dysfunction group were greater, with the placental echo showing uneven strength in the ultrasound image. The gray variance feature values were greater in the dysfunction group than in the normal group, reflecting differences in ultrasonic echoes attributable to variations in tissue density between the two groups, leading to significant differences in ultrasonic image density or fineness. An area under the curve (AUC) of approximately 0.7 was achieved when these features were evaluated separately for placental function.
The Shap method was applied to visualize the features' contribution to model decision-making30. The 20 features that significantly influenced model decision-making are plotted in the Fig. 5f. The contribution to model decision-making with the test set is shown on the abscissa, and feature numbers are shown on the ordinate. The variation in eigenvalues could substantially affect the classification model’s prediction outcomes.
Evaluation of model diagnostic effectiveness
The data were randomly divided into a training set and an independent test set at a ratio of 446:112, and the tenfold cross-validation method was used to evaluate the model. The confusion matrix and receiver operating characteristic (ROC) curve of the optimization model are shown in Fig. 6. The AUC of the final functional evaluation model was 0.95, and the sensitivity, specificity, accuracy, positive predictive value and negative predictive value were 88.7%, 91.5%, 90.2%, 90.4% and 90.0%, respectively (Table 1).
Discussion
In this study, we investigated the abnormal changes that occur in the placenta using a preeclamptic rat model. We combined this with radiomics to extract and analyze ultrasound image features of the rat placenta, and developed a radiomics model to identify placental insufficiency in rats. By doing so, we confirmed the feasibility of ultrasound radiomics for assessing placental function.
The ultrasound echo signal is determined by the details of tissue microstructure (including the tissue composition, fiber size, cell density, and cell size), pulse frequency, etc., which affect the backscatter of ultrasound waves23. Therefore, the microstructure of tissues and organs is an important basis of ultrasound imaging. The rat placenta, like the human placenta, is a functional organ that carries maternal and fetal gases and nutrients, containing small units responsible for accomplishing functional tasks31. Although placental ultrasound data measurements and visual observations are limited, the use of radiomics tools allows the identification of subtle but significantly different deviations from normal. In a previous study, we constructed a GMNet model that could sensitively identify abnormal changes in the placental microstructure in pregnancies affected by hypertension disorders of pregnancy (HDP)32. Krishan also discovered the ability to identify changes in the features of placental ultrasound images prior to the clinical presentation of HDP through AI techniques33. However, what clinicians require is not only a sensitive identification of placental changes, but also timely detection and real-time assessment of placental insufficiency to allow for early prenatal treatment and to avoid negative outcomes. Antenatal assessment of the potential risk posed by placental abnormalities to the mother and fetus is a challenging task. Even with postnatal histological examination of the placenta, only established diseases can be diagnosed34. Compounding these difficulties is the typical presence of only one fetus at a time and the extended gestation period in humans, making it challenging to integrate and control longitudinal data on natural abnormal variants. As a result, a noninvasive approach for the prenatal assessment of the functional status of the placenta is urgently needed to reduce the risk of adverse pregnancy outcomes. Here, we successfully developed a radiomic feature-based for evaluating the placental function of preeclampsia rats that is highly effective (AUC: 0.95) in distinguishing microscopic details in placental ultrasound images through radiomic features. Ultrasound placental analysis using machine learning is a promising technique that paves the way for further research in this field.
In recent years, there has been a growing interest in understanding and researching the placenta. Projects such as the Human Placenta Project were developed to meet the need for better real-time assessment of human placental structure and function35. The Placental Atlas Tool was also developed to create a shared platform for placental data research36. Additionally, previous studies have demonstrated a strong association between the onset and progression of preeclampsia and placental dysfunction during early pregnancy37,38,39. The placenta in pregnancies affected by preeclampsia undergoes a constantly changing and unhealthy metabolic state throughout gestation40. In particular, vascular endothelial dysfunction due to placental ischemia–reperfusion and hypoxic injury may be an important causal factor in the development of preeclampsia10,41,42. An altered angiogenic biomarker (sFlt-1/PlGF ratio) can indicate placental dysfunction, and its use in clinical practice has the potential to significantly reduce the burden of morbidity and mortality associated with placental dysfunction43. Our study revealed the existence of placental dysfunction in preeclamptic rats, as evidenced by significant alterations in angiogenic biomarkers (sFlt-1/PlGF ratio), further emphasizing the importance of studying and understanding placental function in humans.
There are several limitations to this study. The availability of placental tissue is limited by the number of experimental animals; the sample size should be expanded in future studies to improve the effectiveness of the model. In addition, radiomics features can only represent some aspects of images, but deep learning can extract more complex information from images; therefore, the application of AI techniques such as deep learning may yield more desirable model results.
Conclusion
In conclusion, an in-depth understanding of placental characteristics and the development of advanced prenatal placental function monitoring techniques could guide targeted treatment strategies that preserve placental function as well as improve pregnancy management. Our rat placental function assessment model based on ultrasound radiomics technology demonstrated exceptional effectiveness, confirming the feasibility of utilizing radiomics techniques to evaluate placental function and offering new avenues for exploring human placenta-related diseases.
Data availability
Data is provided within the manuscript or supplementary information files.
Change history
16 December 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-81772-6
Abbreviations
- PE:
-
Preeclampsia
- sFlt-1:
-
Soluble fms-like tyrosine kinase-1
- PlGF:
-
Placental growth factor
- L-NAME:
-
NG-Nitro-L-arginine Methyl Ester
- GD:
-
Gestation day
- ROI:
-
Region of interest
- AUC:
-
Area under the curve
- SHAP:
-
Shapley additive explanations
References
Steegers, E. A., von Dadelszen, P., Duvekot, J. J. & Pijnenborg, R. Pre-eclampsia. Lancet 376, 631–644. https://doi.org/10.1016/s0140-6736(10)60279-6 (2010).
ACOG Practice Bulletin No. 202. Gestational hypertension and preeclampsia. Obstet. Gynecol. 133, 1. https://doi.org/10.1097/aog.0000000000003018 (2019).
Chappell, L. C., Cluver, C. A., Kingdom, J. & Tong, S. Pre-eclampsia. Lancet 398, 341–354. https://doi.org/10.1016/s0140-6736(20)32335-7 (2021).
Erez, O. et al. Preeclampsia and eclampsia: The conceptual evolution of a syndrome. Am. J. Obstet. Gynecol. 226, S786-s803. https://doi.org/10.1016/j.ajog.2021.12.001 (2022).
Hoodbhoy, Z. et al. The impact of maternal preeclampsia and hyperglycemia on the cardiovascular health of the offspring: A systematic review and meta-analysis. Am. J. Perinatol. 40, 363–374. https://doi.org/10.1055/s-0041-1728823 (2023).
Phipps, E. A., Thadhani, R., Benzing, T. & Karumanchi, S. A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 15, 275–289. https://doi.org/10.1038/s41581-019-0119-6 (2019).
Wright, E. et al. Maternal vascular malperfusion and adverse perinatal outcomes in low-risk nulliparous women. Obstet. Gynecol. 130, 1112–1120. https://doi.org/10.1097/aog.0000000000002264 (2017).
Jung, E. et al. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 226, S844-s866. https://doi.org/10.1016/j.ajog.2021.11.1356 (2022).
Brosens, I., Pijnenborg, R., Vercruysse, L. & Romero, R. The, “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 204, 193–201. https://doi.org/10.1016/j.ajog.2010.08.009 (2011).
Opichka, M. A., Rappelt, M. W., Gutterman, D. D., Grobe, J. L. & McIntosh, J. J. Vascular dysfunction in preeclampsia. Cells https://doi.org/10.3390/cells10113055 (2021).
Falco, M. L., Sivanathan, J., Laoreti, A., Thilaganathan, B. & Khalil, A. Placental histopathology associated with pre-eclampsia: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 50, 295–301. https://doi.org/10.1002/uog.17494 (2017).
Hayward, R. M., Foster, E. & Tseng, Z. H. Maternal and fetal outcomes of admission for delivery in women with congenital heart disease. JAMA Cardiol. 2, 664–671. https://doi.org/10.1001/jamacardio.2017.0283 (2017).
Melchiorre, K., Giorgione, V. & Thilaganathan, B. The placenta and preeclampsia: Villain or victim?. Am. J. Obstet. Gynecol. 226, S954-s962. https://doi.org/10.1016/j.ajog.2020.10.024 (2022).
Cain, M. A. et al. Pregnancy as a window to future health: Maternal placental syndromes and short-term cardiovascular outcomes. Am. J. Obstet. Gynecol. 215(484), e481-484.e414. https://doi.org/10.1016/j.ajog.2016.05.047 (2016).
Sebire, N. J. & Sepulveda, W. Correlation of placental pathology with prenatal ultrasound findings. J. Clin. Pathol. 61, 1276–1284. https://doi.org/10.1136/jcp.2008.055251 (2008).
van Timmeren, J. E., Cester, D., Tanadini-Lang, S., Alkadhi, H. & Baessler, B. Radiomics in medical imaging-“how-to” guide and critical reflection. Insights Imaging 11, 91. https://doi.org/10.1186/s13244-020-00887-2 (2020).
Guiot, J. et al. A review in radiomics: Making personalized medicine a reality via routine imaging. Med. Res. Rev. 42, 426–440. https://doi.org/10.1002/med.21846 (2022).
Zhu, L. et al. Machine learning-based ultrasound radiomics for evaluating the function of transplanted kidneys. Ultrasound Med. Biol. 48, 1441–1452. https://doi.org/10.1016/j.ultrasmedbio.2022.03.007 (2022).
Moro, F. et al. Developing and validating ultrasound-based radiomics models for predicting high-risk endometrial cancer. Ultrasound Obstet. Gynecol. 60, 256–268. https://doi.org/10.1002/uog.24805 (2022).
Lambin, P. et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 14, 749–762. https://doi.org/10.1038/nrclinonc.2017.141 (2017).
Charest, P. L. et al. Histomorphologic analysis of the late-term rat fetus and placenta. Toxicol. Pathol. 46, 158–168. https://doi.org/10.1177/0192623318755135 (2018).
Chau, K., Welsh, M., Makris, A. & Hennessy, A. Progress in preeclampsia: The contribution of animal models. J. Hum. Hypertens 36, 705–710. https://doi.org/10.1038/s41371-021-00637-x (2022).
Tomimatsu, T. et al. Preeclampsia: Maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int. J. Mol. Sci. 20, 1. https://doi.org/10.3390/ijms20174246 (2019).
Huang, J. et al. Mangiferin ameliorates placental oxidative stress and activates PI3K/Akt/mTOR pathway in mouse model of preeclampsia. Arch. Pharm. Res. 43, 233–241. https://doi.org/10.1007/s12272-020-01220-7 (2020).
Lipták, B., Kaprinay, B. & Gáspárová, Z. A rat-friendly modification of the non-invasive tail-cuff to record blood pressure. Lab. Anim. (N. Y.) 46, 251–253. https://doi.org/10.1038/laban.1272 (2017).
Akusok, A., Eirola, E., Bjork, K. M. & Lendasse, A. In International Conference on Extreme Learning Machine (ELM). 181–185 (2019).
Herraiz, I. et al. Angiogenesis-related biomarkers (sFlt-1/PLGF) in the prediction and diagnosis of placental dysfunction: An approach for clinical integration. Int. J. Mol. Sci. 16, 19009–19026. https://doi.org/10.3390/ijms160819009 (2015).
Heazell, A. E., Whitworth, M., Duley, L. & Thornton, J. G. Use of biochemical tests of placental function for improving pregnancy outcome. Cochrane Database Syst. Rev. 2015, Cd011202. https://doi.org/10.1002/14651858.CD011202.pub2 (2015).
Leon-Medina, J. X., Pares, N., Anaya, M., Tibaduiza, D. A. & Pozo, F. Data classification methodology for electronic noses using uniform manifold approximation and projection and extreme learning machine. Mathematics https://doi.org/10.3390/math10010029 (2022).
Lundberg, S. M. et al. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2, 56–67. https://doi.org/10.1038/s42256-019-0138-9 (2020).
Furukawa, S., Tsuji, N. & Sugiyama, A. Morphology and physiology of rat placenta for toxicological evaluation. J. Toxicol. Pathol. 32, 1–17. https://doi.org/10.1293/tox.2018-0042 (2019).
Sun, H., Jiao, J., Ren, Y., Guo, Y. & Wang, Y. Multimodal fusion model for classifying placenta ultrasound imaging in pregnancies with hypertension disorders. Pregnancy Hypertens 31, 46–53. https://doi.org/10.1016/j.preghy.2022.12.003 (2023).
Gupta, K. et al. Ultrasound placental image texture analysis using artificial intelligence to predict hypertension in pregnancy. J. Matern. Fetal. Neonatal. Med. 35, 5587–5594. https://doi.org/10.1080/14767058.2021.1887847 (2022).
Gonen, N. et al. Placental histopathology and pregnancy outcomes in “early” vs. “late” placental abruption. Reprod. Sci. 28, 351–360. https://doi.org/10.1007/s43032-020-00287-3 (2021).
Guttmacher, A. E., Maddox, Y. T. & Spong, C. Y. The human placenta project: Placental structure, development, and function in real time. Placenta 35, 303–304. https://doi.org/10.1016/j.placenta.2014.02.012 (2014).
Ilekis, J. V. et al. The Placental Atlas Tool (PAT): A collaborative research and discovery platform for the placental research community. Placenta 80, 42–48. https://doi.org/10.1016/j.placenta.2019.03.016 (2019).
Palei, A. C., Granger, J. P. & Spradley, F. T. Placental ischemia says “NO” to proper NOS-mediated control of vascular tone and blood pressure in preeclampsia. Int. J. Mol. Sci. https://doi.org/10.3390/ijms222011261 (2021).
Hu, X. & Zhang, L. Uteroplacental circulation in normal pregnancy and preeclampsia: Functional adaptation and maladaptation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22168622 (2021).
Skeith, L., Blondon, M. & Áinle, F. N. Understanding and preventing placenta-mediated pregnancy complications. Hamostaseologie 40, 356–363. https://doi.org/10.1055/a-1184-8388 (2020).
Hu, M., Li, J., Baker, P. N. & Tong, C. Revisiting preeclampsia: A metabolic disorder of the placenta. Febs J 289, 336–354. https://doi.org/10.1111/febs.15745 (2022).
Stepan, H. et al. Clinical utility of sFlt-1 and PlGF in screening, prediction, diagnosis and monitoring of pre-eclampsia and fetal growth restriction. Ultrasound Obstet. Gynecol. 61, 168–180. https://doi.org/10.1002/uog.26032 (2023).
Feng, X. et al. New views on endothelial dysfunction in gestational hypertension and potential therapy targets. Drug. Discov. Today 26, 1420–1436. https://doi.org/10.1016/j.drudis.2021.03.001 (2021).
Stepan, H., Hund, M. & Andraczek, T. Combining biomarkers to predict pregnancy complications and redefine preeclampsia: The angiogenic-placental syndrome. Hypertension 75, 918–926. https://doi.org/10.1161/hypertensionaha.119.13763 (2020).
Acknowledgements
We sincerely appreciated the contribution of laboratory animals to scientific research.
Funding
Funding was provided by the Shanghai Municipal Health Commission Scientific Research Project Program (No. 202240083), the Natural Science Foundation of Shandong Province (No. ZR2024QH216), the Medical and Health Science and Technology Project of Shandong Province (No. 202309020311) and the Postdoctoral Research Project from Affiliated Hospital of Jining Medical University (No. JYFY362641).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by S.Hs.; Preparation, data collection and analysis were performed by Z.C., W.Yc., W.Sc.; J.J., W.Yy. analyzed and interpreted the data; G.Y., R.Yy. and B.B. assessed the outcomes of the research and reviewed the paper. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Funding section in the original version of this Article was omitted. The Funding section now reads: “Funding was provided by the Shanghai Municipal Health Commission Scientific Research Project Program (No. 202240083), the Natural Science Foundation of Shandong Province (No. ZR2024QH216), the Medical and Health Science and Technology Project of Shandong Province (No. 202309020311) and the Postdoctoral Research Project from Affiliated Hospital of Jining Medical University (No. JYFY362641).”
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, H., Jiao, J., Wang, Y. et al. Ultrasound based radiomics model for assessment of placental function in pregnancies with preeclampsia. Sci Rep 14, 21123 (2024). https://doi.org/10.1038/s41598-024-72046-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72046-2