Abstract
To study the efficacy and possible mechanisms of radial extracorporeal shock wave (rESW) with different frequencies for the treatment of acute skeletal muscle injury in rabbits, 48 rabbits of acute injured biceps femoris were randomly divided into 4 groups. Except for the control group, the other groups were treated by rESW with 5 Hz, 10 Hz and 15 Hz, respectively. The injury symptom index scores (ISISs) in the rESW group were significantly lower than those in the control group, with the lowest in the 10 Hz rESW group. Histomorphological features demonstrated a decrease in mononuclear cells and an increase in new myocytes across all groups, with the rESW group showing the most significant changes. The concentrations of PGE2 and IL-1β were significantly lower in all rESW groups by ELISA compared to the control group. Additionally, the 10 Hz group had lower concentrations than the 5 Hz and 15 Hz group. Compared with the control group, MyoD of the rESW groups was significantly increased, and the expression level of the 10 Hz group was higher than that of the other groups. In conclusion, rESW with 5 Hz, 10 Hz and 15 Hz take certain curative effects on acute biceps femoris injury in rabbits, and the 10 Hz rESW takes advantage over 5 Hz and 15 Hz rESW.
Similar content being viewed by others
Introduction
Acute skeletal muscle injury is the most common injury in exercise and sports competitions. Acute skeletal muscle injury can not only cause severe pain, muscle spasms and stiffness, but induce motor dysfunction to do harm to normal work and life if the appropriate interventions are not taken in a timely manner.
Compared with other common therapies, including non-steroidal analgesics, traditional Chinese medicine acupuncture, ultrasound therapy, etc1,2,3, extracorporeal shock wave therapy has been widely used in skeletal muscle pain because of its advantages of non-invasive, effective and fewer complications4,5. However, scholars had different views on the application of rESW in pain caused by acute skeletal muscle injury. Opponents argued that extracorporeal shock wave treatment might further aggravate the bleeding, exudation and inflammatory reaction of damaged tissues, while supporters held that this therapy was helpful for treating acute skeletal muscle injury6,7. The lack of standardized treatment parameters, involving impact intensity, frequency and quantity, committed to the different research results. Therefore, the selection of ideal treatment parameters was important for improving the efficacy of rESW. At present, the research on the treatment parameter of acute skeletal muscle injury by rESW mainly focused on energy flow density and pressure8,9. However, impact frequency, another important parameter, was seldom studied as a separate influencing factor, meanwhile, the effect and related mechanism of rESW on acute skeletal muscle injury were rarely reported. The hypothesis of this study aimed to reveal the therapeutic parameters of shock waves in acute musculoskeletal pain, providing an experimental basis for clinical treatment.
The present study explored the therapeutic effects of different frequencies of rESW in rabbits with acute biceps femoris injury by morphological and histological evaluation. In addition, enzyme-linked immunosorbent assay (ELISA), quantitative real-time polymerase chain reaction (qRT-PCR) and western blotting were also used to assess the expression of inflammatory factors and myoblast proteins in the injured tissues.
Results
A successful model was established
In this study, the muscle injury model was made by repeatedly striking the rabbit biceps femoris with a self-made fixed apparatus. The detailed operation steps are described in the section of the “method”. In order to verify the success of the model, the biceps femoris on the affected side was chosen and compared with the healthy side for histomorphology 12 h after modeling. The appearance of skin on the healthy side was intact. The HE staining results showed that normal muscle cells were uniformly sized and polygonal, with blue nuclei in the sarcomere and pink uniformly distributed cytoplasm around the nuclei. The muscle cells in the whole section were intact without infiltration of inflammatory cells in Fig. 1a, b. In the affected side, massive ecchymoses were seen on the skin, and tissue-damaged signs such as muscle cell swelling, fragmentation and inflammatory cell infiltration were seen by HE staining in Fig. 1c, d. These differences between two sides suggest that the model was successful. Rabbits were raised normally and drank freely during the whole experiment. 2 rabbits died from the improper anesthetic dosage and were promptly replaced with spare New Zealand rabbits under the same conditions.
Comparison of injury syndrome index scores (ISISs)
The day after shock wave therapy was regarded as day 1, denoted by D1. Morphological and behavioral evaluation was performed by the injury syndrome index score, including the appearance of damaged tissues and the activity of rabbits. On the whole, there were significant differences in the ISISs between different groups and at different time points within the same group, and there was a cross effect between groups and time (see Table 1). The pairwise comparison showed that the relationship between different time points was D1 > D2 > D4 > D7 (p < 0.0001), indicating that the ISISs in each group showed a continuous downward trend over time, and the ISIS at D7 decreased to the lowest; The relationship between the change rate of the ISISs in each time period was 10 Hz > 5 Hz = 15 Hz > control group (10 Hz > 5 Hz = 15 Hz (p = 0.0128), 5 Hz = 15 Hz > control group(p = 0.0008)), indicating that on the 2nd, 4th and 7th day after modeling, the scores of the three shock wave groups were significantly lower than those of the control group, and the scores of the 10 Hz group were the most significantly lower, while the scores of the 5 Hz and 15 Hz groups were not significantly different (see Fig. 2).
Histological analysis
In all groups, monocytes were decreased gradually, and myocytes were increased first and then decreased, with the proliferation most obvious on the fourth day. (see Fig. 3a).
(a) Schematic diagram of histopathological changes in the damaged biceps femoris on days 2, 4 and 7 after modeling in different groups [note: yellow arrow monocyte, black arrow myocyte, scale = 100 μm (1:200)]. The monocytes were indicated by the yellow arrow, the new myocytes were indicated by the black arrow. Scale bar = 100 μm, magnification ×200. (b) The number of monocytes. (compared with the day 2, *p < 0.05; compared with day 4, #p < 0.05; compared with control group, &p < 0.05; compared with 10 Hz group, Δp < 0.05). (c) The number of myocytes. (compared with day 2,*p < 0.05; compared with day 4, #p < 0.05; compared with control group, &p < 0.05; compared with 10 Hz group, Δp < 0.05).
On the 2nd, 4th and 7th days after modeling, compared with the control group at the same time point, monocytes in the three rESW groups were significantly reduced (F = 265.761, p < 0.0001), and myocytes were significantly increased (F = 84.869, p < 0.0001). The number of monocytes in the 10 Hz rESW group was significantly lower than that in the other two rESW groups at the same time point, while the number of myocytes was significantly higher than that in the other two rESW groups (p < 0.0001). There were no significant differences in the number of monocytes and myocytes between the 5 Hz group and the 15 Hz group. (see Fig. 3b, c).
ELISA and Western blot (WB) of the PGE2 and IL-1β
PGE2 and IL-1β by ELISA and WB in each group showed a gradual downward trend (PGE2 by ELISA: F = 401.062, p < 0.0001; IL-1β by ELISA: F = 145.655, p < 0.0001; PGE2 by WB: F = 87.297, p < 0.0001; IL-1β by WB: F = 143.282, p < 0.0001). On the 2nd, 4th and 7th days after modeling, the expressions of PGE2 and IL-1β in the three rESW groups were increased significantly compared with those in the control group at the same time point (PGE2 by ELISA: F = 136.250, p < 0.0001; IL-1β by ELISA: F = 87.219, p < 0.0001; PGE2 by WB: F = 33.090, p < 0.0001; IL-1β by WB: F = 30.647, p < 0.0001). The concentrations in 10 Hz group were significantly lower than those in 5 Hz and 15 Hz groups (PGE2 and IL-1β by ELISA:p < 0.0001; PGE2 by WB:10 Hz vs 5 Hz, p = 0.022, 10 Hz vs 15 Hz, p = 0.042; IL-1β by WB: 10 Hz vs 5 Hz, p = 0.002, 10 Hz vs 15 Hz, p = 0.002). As shown in Fig. 4 (a-f), there was no significant difference in PGE2 and IL-1β between the 5 Hz group and the 15 Hz group. (see Fig. 4). Details of the original gels and blots (see Supplementary Information 1, 3–6, 10–12).
Expression of MyoD by Western blot and qRT-PCR
The expression of MyoD in the injured area of New Zealand rabbits in each group first increased and then decreased, with the highest expression level on the 4th day (MyoD by WB: F = 70.832 , p < 0.0001; MyoD by qRT-PCR: F = 1725.064, p < 0.0001). On the 2nd, 4th and 7th days after modeling, compared with the control group at the same time point, the expression levels of MyoD in the three rESW groups were increased significantly (MyoD by WB: F = 46.423, p < 0.0001; MyoD by qRT-PCR: F = 177.305, p < 0.0001), and the expression level of MyoD in the 10 Hz group was higher than that in the 5 Hz and 15 Hz groups (MyoD by WB and qRT-PCR: p < 0.0001). There was no significant difference in the expression level of MyoD between the 5 Hz group and the 15 Hz group. (see Fig. 5a–c). Details of the original gels and blots (see Supplementary Information 2, 7–9).
Discussion
This study showed that the inflammatory response of acute skeletal muscle injury was reduced and muscle regeneration was enhanced in both groups after different frequencies of rESWT at follow-up compared to baseline. Moreover, the frequency of 10 Hz is the most prominent.
Factors affecting the prognosis of acute skeletal muscle injury are mainly related to the infiltration of inflammatory factors, and the muscle regeneration capacity. Some studies showed that shock waves might have biological effects of reducing inflammation and promoting regeneration6,10. However, the application of shock waves in acute skeletal muscle injury was limited due to the controversial parameter settings.
So far, there have been many animal models of acute skeletal muscle injury, which were mainly different in animal species and injury forms. In this study, the model of acute biceps femoris muscle injury was established according to the report of Chongsatientam, A11, which was modified and completed by a manufactured striking device. Compared with other acute skeletal muscle injury models, this model was easy to be operated, and was consistent with the muscle injury mechanism that occurred during exercise. Different from some other experiments, in which rats were selected as research objects, New Zealand rabbits were selected in the present experiment because of their moderate size, which was conducive to the implementation of shock waves and closer to the real situation of clinical treatment.
Studies showed that the inflammatory factors such as PGE2 and IL-1β began to increase after acute skeletal muscle injury, reached a peak at 24–48 h after injury, and then gradually decreased 12. On the other hand, muscle satellite cells were activated immediately after injury and began to express MyoD, which peaked on the 4th day after injury and then gradually declined13. Therefore, in this experiment, the 2nd, 4th, and 7th days after modeling were selected as three time points to observe the histomorphology and the expression of PGE2, IL-1β, and MyoD. In the biceps femoris muscle injury model, the peak of inflammatory factors occurred 24 to 48 h after modeling. In order to explore the effect of early intervention of rESW on inflammatory factors, rESW treatment was implemented on the first day after modeling according to the study of Zissler10.
Visual observation of the appearance of the injured area showed that compared with the control group, the three shock wave groups could significantly improve the characterization of acute skeletal muscle injury, especially in improving muscle swelling and subcutaneous congestion. Histomorphological results under a light microscope indicated that rESW could significantly reduce the infiltration of monocytes, improve muscle cell swelling, and promote the proliferation of myocytes. This result was consistent with that of Langendorf et al.6, who used a rat model of acute incision injury of the biceps femoris.
PGE2 is a kind of substance with strong physiological activity, which can modulate pain information in the central and peripheral nervous systems14, reduce the body's pain threshold, activate peripheral pain receptors, and mediate the transmission of nociceptive information15. Therefore, the reduced level of PGE2 in the local injury area can accelerate the resolution of inflammation and relieve pain symptoms16. IL-1β is mainly derived from monocytes and macrophages and it is a cytokine closely related to various inflammatory responses17. The local IL-1β content is extremely low without injury. Therefore, IL-1β is an important indicator that can reflect the process of acute soft tissue injury18. Some animal and human studies have confirmed that after shock wave therapy for musculoskeletal pain, specific inflammatory factors, including PGE2 and IL-1 β, are significantly reduced19,20,21. In this experiment, we also found that the three shock wave groups could significantly inhibit the expression of inflammatory factors PGE2 and IL-1β compared with those in the control group. Especially in the 10 Hz group, the levels of PGE2 and IL-1β were decreased more significantly than those in the other two shock wave groups at the same time point.
MyoD is a transcription factor during muscle cell regeneration that activates quiescent muscle satellite cells22. Studies showed that the expression of MyoD in damaged muscles was rapidly up-regulated after injury, while MyoD was not expressed in uninjured muscles23. In this study, the expression of MyoD in each group was increased first and then decreased over time, which was consistent with Tian et al.13. Recent studies reminded us that the application of rESW significantly promoted the regeneration and repair of the acute skeletal muscle injury in rats, and up-regulated the expression of MyoD in the injured skeletal muscle10. In this experiment, it was also observed that the expression of MyoD in the three shock wave groups was higher than that in the control group at the same time point. Therefore, rESW promoted the proliferation and differentiation of muscle satellite cells by up-regulating the expression of MyoD. In addition, we observed that the expression of MyoD in 10 Hz group was significantly higher than those in the other two rESW groups. This alteration indicated that 10 Hz rESW had better repair ability.
In this experiment, three rESW groups could effectively eliminate inflammation and promote skeletal muscle regeneration and repair, especially the 10 Hz group had the best effect. This may be related to the physical effect of shock waves. Alkhamaali et al.24 found that when the impact frequency increased continuously, the mechanical shear stress acting on the tissue would gradually increase, which meant that the therapeutic effect would be improved. However, for acutely injured tissues, continuously increasing energy would lead to secondary damage. Duryea et al.25 also found that the cavitation effect of shock waves caused the bubbles in the tissue to burst into a large number of small bubbles, which was the process of playing its biological effect. However, if the remaining bubbles did not have enough time to dissolve, they would gather together and hinder the energy diffusion to the surrounding area, and this phenomenon was more obvious under the impact of high-speed frequency. Wang et al.26 also found that when the impact frequency exceeded 10 Hz, the residual bubbles did not have enough time to dissolve before the next pulse, which would lead to the reduction of therapeutic efficacy. However, Selnur held the opposite view, stating that there was no significant difference in treatment efficacy between different frequencies, which might be attributed to that selective destruction of the nonmyelinated peripheral sensitive fibers in the focal area of treatment at high energy levels might help prolong the analgesic time to counteract the impact of shock wave frequency27. Above, our results showed that 10 Hz rESW treatment could generate enough energy to optimize mechanical and cavitation effects and achieve better curative effects. This study mainly discussed the results through the physical properties of shock waves, while the underlying mechanisms of action had not been fully elucidated. In future research, exploring how rESW produces biological effects at the molecular level may be an appropriate direction.
Inflammation is a double-edged sword for wound recovery, as it can activate the repair system in the body to accelerate wound healing. However, persistent high-intensity inflammation is also an unfavorable factor for wound healing. The results of this experiment indicate that rESW effectively controls inflammation through its unique physical effects, avoiding the sustained destruction of inflammatory factors. At the same time, rESW also promotes tissue regeneration and accelerates wound healing by regulating regeneration factors.
There are some limitations in this study. Only three treatment frequencies were analyzed in this study, which just roughly reflected the relationship between frequency and efficacy. Furthermore, the activity scores we used in this experiment were relatively rough, only based on observing the walking behavior of the modeled rabbits, without providing more detailed activity scores for evaluating functional recovery. Besides, some anti-inflammatory factors may also play some role in the treatment process, which needs further experimental demonstration.
Conclusion
rESW with 5 Hz, 10 Hz and 15 Hz has certain efficacy in the treatment of acute skeletal muscle injury in rabbits, and the 10 Hz provides the best efficacy. rESW may play a therapeutic role by improving the histopathological prognosis, inhibiting the expression of inflammatory factors PGE2 and IL-1β and up-regulating the expression level of MyoD in the injured area.
Method
Animals
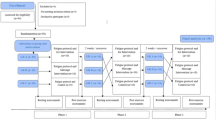
The minimum sample size calculation was determined using the resource equation approach28, assuming that the error range of the acceptable degrees of freedom (DF) in the analysis of variance was between 10 and 20 (DF = 10–20). The results of this study were repeated measures of analysis of variance data from multiple groups, and animals needed to be euthanized for each measurement. Therefore, the total sample size = [DF/(number of groups × number of measurements) + 1] × (Number of groups × number of measurements), and the final result was 24–48. This study selected 48 New Zealand rabbits as the smallest sample size. Forty-eight New Zealand male rabbits (4 months, 2.0 \(\pm\) 0.2 kg) with successful modeling were randomly by computer programs divided into four groups: control group, 5 Hz rESW group, 10 Hz rESW group and 15 Hz rESW group (n = 12/group). The animals were housed at 25℃ under a 12-h light–dark cycle with free access to food and water. A week of acclimation was allowed prior to the start of the experiment.
All rabbits were used to shape the acute biceps femoris injury model (see Fig. 6a–c). Rabbits were anesthetized by intraperitoneal injection of 20% urethane (2 g/kg), which was attributed to our experience in preliminary experiments that frequent high-altitude fell and impacted during modeling could cause severe pain, and rabbits could avoid painful tics at an anesthesia concentration of 2 g/kg). The anesthetized rabbits were placed on the control table and the skin hair of their right biceps femoris was removed by an appropriate amount of depilatory agent. The hair removal area was about 4 cm × 5 cm. A small wooden block was placed on the inside of the biceps femoris muscle, and a 500 g weight was dropped freely from a 60 cm guide cylinder to hit the predetermined impact point. Repeated 40 times, and the diameter of the damaged surface was about 2.0 cm. Massive ecchymosis could be seen immediately after the completion of the blow and there was obvious swelling at the hit part. The signs of injury shown in the pathological tissue section after the modeling indicated that the modeling was successful. Those with local skin damage or fractures were removed. After the modeling, no treatment would be done, and it would be kept for 1 day in a normal environment.
rESW treatment
For the shock wave treatment, an unfocused pneumatic ballistic device was used (STORZ MP100, Switzerland). The biceps femoris muscles of New Zealand rabbits are developed and close to human muscle tissue. According to relevant literature, the “R15” treatment probe (referring to a radiation probe with a diameter of 15 mm, a processing depth of 40 mm, and a maximum energy flow density of 0.38 mJ/mm2) was used, and other treatment parameters except for frequency were determined to be (2.0 bar, 2000 pulses) 29. On the first day after successful modeling, three rESW groups with different frequencies received 2000 shock treatments, with treatment durations of 400 s, 200 s, and 133 s for each group, and all treatments were completed by the same technician. Before treatment, 20% urethane (1.5 g/kg) was injected intraperitoneally. We found that multiple injections of anesthesia at this concentration (2.0 g/kg) could lead to longer recovery times and even death in rabbits. Therefore, we chose an anesthesia concentration of 1.5 g/kg for relatively low-energy shock wave therapy to meet experimental needs and improve anesthesia safety. It is worth mentioning that different anesthetics only differ in pharmacokinetics and do not affect the therapeutic effect. However, when using different anesthetics, it is necessary to choose the appropriate concentration of anesthetic according to the animal's condition to avoid increasing mortality. Then the injured area was fully exposed, and the appropriate amount of medical ultrasonic coupler (Shanghai Shenfeng Medical Care Co., LTD.) was added. The treatment probe was perpendicular to the central skin of the damaged area, and a small range of movement was performed to completely cover the damaged area (Fig. 6d). After treatment, it was put back into the cage and fed normally. The feeding and living conditions of each group were completely consistent.
Injury symptom index score (ISIS)
The ISIS indicator consists of four parts, including congestion, color, swelling and activity. Each section has a score of 0–2 points, so the total score is 0–8 points. According to the scoring criteria (see Table 2), subcutaneous congestion, muscle color, swelling and lower limb activity of New Zealand rabbits after modeling were observed on the 1st, 2nd, 4th and 7th days after modeling (The day of successful model building is recorded as day 0). All the scores were performed at a fixed time (8:00–9:00 a.m.). The scores were scored by the same person who was blind for the experiment.
Obtain specimens
The specimens of each group were taken on the 2nd, 4th and 7th days after modeling. The New Zealand rabbits were killed by intraperitoneal injection of 20% urethane (4 g/kg), and then the right biceps femoris was quickly cut out. In each group, muscle tissue(10 mm × 10 mm × 5 mm) was taken from the injury center and stored in the muscle tissue fixation solution, and the tissue morphology was observed by HE staining; Muscle tissues of the same size were placed in centrifuge tubes and stored in liquid nitrogen for ELISA, Western blot and PCR detection.
Hematoxylin–eosin staining (HE)
After treated rabbits were sacrificed, the injury tissues were rapidly removed and fixed in 4% formaldehyde solution for 48 h, followed by decalcifying in 10% EDTA (pH 7.4) solution for 3 weeks. The tissues were then dehydrated with the Donatello hydroextractor (DIAPATH, Italy) and embedded with paraffin. Biceps femoris of the lateral were vertically cut into 4 μm thick sections on an RM2016 microtome (Leica, China) and stained with hematoxylin and eosin. The morphological and structural changes of the tissues were observed under a light microscope at 200 magnification and pictures were collected. The monocytes and new myocytes were marked and counted by two independent researchers who were blind for the experiment.
Enzyme-linked immunosorbent assay (ELISA)
Resected damaged biceps femoris samples were frozen in liquid nitrogen. Added PBS(pH 7.4) and then pulverized or homogenized thoroughly, and centrifuged for 20 min (2000–3000 RPM). The supernatants were collected and the concentrations of PGE2 and IL-1β were measured using ELISA kits (BioTeK, China) according to the manufacturer's instructions.
Western blot (WB)
100 mg of samples from each group were cut into pieces in phosphate-buffered saline (PBS) solution, and then homogenized by adding appropriate protein lysate and protease inhibitors. The samples were centrifuged at 12,000 RPM at 4 ℃ for 10 min. The supernatant was taken for protein quantification and boiled at 100 ℃. Using standard procedures, total proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. The primary antibodies including rabbit polyclonal anti-IL-1β (AF5103), anti-PTGER2 (BS-4196R), anti-MYOD1 (8943-1-ap), and anti-GAPDH (GB12002, 1:2000, Wuhan Xavier Biotechnology Co., LTD, China) were diluted and coincubated with the nitrocellulose membranes according to the manufacturer’s instructions. The secondary antibodies included HRP-Goat Anti-Rabbit (GB23303) and HRP-Goat Anti-Mouse (GB25301). Detection and gray value analysis were performed with photographic film (Servicebio, China) and Alpha Innotech (Shanghai, China). Blots were stripped and incubated with a monoclonal antibody directed against GAPDH, which was used as a loading control to normalize PGE2, IL-1β and MyoD protein expression levels.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated with Trizol reagent (Servicebio, China) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 1 mL total RNA using an RT First Strand cDNA Synthesis Kit (Servicebio, China) following the operation manual. PCR was performed using 2× SYBR Green qPCR Master Mix (Servicebio, China) in a fluorescence quantitative PCR instrument (Bio-rad, China) under the following conditions: 90 ℃ for 10 min (initial denaturation), then 40 cycles at 95 ℃ for 15 s, and 60 ℃ for 30 s, followed by a dissociation program at 95 ℃ for 15 s, 60 ℃ for 30 s, and 95 ℃ for 15 s. Expression levels of target genes were calculated using the 2−ΔΔct values. Primer sequences were as follows: MYOD: forward GTAAGAGGAAGTC AGTGTCCAT and reverse CCTCGTTCACTTTCTTGAGCC; GAPDH: forward TGAAGGTCGGAGTGAACGGAT and reverse CGTTCTCAGCCTTGACCGTG.
Statistical analysis
The analyses were conducted using SPSS software version 26.0, and α = 0.05 was set for statistical significance. All data were expressed as mean ± standard deviation (\(\overline{x }\pm S\)). The results of ISIS of all groups were analyzed using two-way repeated measures analysis of variance (ANOVA) and Bonferroni post hoc tests for pairwise multiple comparisons. The data of monocytes and myocytes in HE staining, the concentrations of PGE2 and IL-1β, and the expression of MyoD were analyzed by two-way ANOVA followed by Bonferroni’s multiple comparisons test.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Shu, B., Yang, Z., Li, X. & Zhang, L. Q. Effect of different intensity pulsed ultrasound on the restoration of rat skeletal muscle contusion. Cell Biochem. Biophys. 62, 329–336 (2012).
Morelli, K. M., Brown, L. B. & Warren, G. L. Effect of NSAIDs on recovery from acute skeletal muscle injury: A systematic review and meta-analysis. Am. J. Sports Med. 46, 224–233 (2018).
Walsh, R., Kinsella, S. & McEvoy, J. The effects of dry needling and radial extracorporeal shockwave therapy on latent trigger point sensitivity in the quadriceps: A randomised control pilot study. J. Bodyw. Mov. Ther. 23, 82–88 (2019).
Maffulli, N. CORR Insights®: Extracorporeal shock wave therapy improves nontraumatic knee contracture in a rat model. Clin. Orthop. Relat. Res. 481, 835–836 (2023).
Wu, W. et al. Exposure of zebra mussels to radial extracorporeal shock waves: Implications for treatment of fracture nonunions. J. Orthop. Surg. Res. 16, 707 (2021).
Langendorf, E. K. et al. Exposure to radial extracorporeal shockwaves induces muscle regeneration after muscle injury in a surgical rat model. J. Orthop. Res. 38, 1386–1397 (2020).
Morgan, J., Hamm, M., Schmitz, C. & Brem, M. H. Return to play after treating acute muscle injuries in elite football players with radial extracorporeal shock wave therapy. J. Orthop. Surg. Res. 16, 708 (2021).
Hansen, L. K. et al. The effect of low intensity shockwave treatment (Li-SWT) on human myoblasts and mouse skeletal muscle. BMC Musculoskelet. Disord. 18, 557 (2017).
Wang, B. et al. Low-intensity extracorporeal shock wave therapy promotes myogenesis through PERK/ATF4 pathway. Neurourol. Urodyn. 37, 699–707 (2018).
Zissler, A. et al. Extracorporeal shock wave therapy accelerates regeneration after acute skeletal muscle injury. Am. J. Sports Med. 45, 676–684 (2017).
Chongsatientam, A. & Yimlamai, T. Therapeutic pulsed ultrasound promotes revascularization and functional recovery of rat skeletal muscle after contusion injury. Ultrasound Med. Biol. 42, 2938–2949 (2016).
Nicholas, J. et al. Time course of chemokine expression and leukocyte infiltration after acute skeletal muscle injury in mice. Innate Immun. 21, 266–274 (2015).
Tian, Z. L. et al. Detection of satellite cells during skeletal muscle wound healing in rats: Time-dependent expressions of Pax7 and MyoD in relation to wound age. Int. J. Legal Med. 130, 163–172 (2016).
Aoki, T. & Narumiya, S. Prostaglandins and chronic inflammation. Trends Pharmacol. Sci. 33, 304–311 (2012).
Ma, W., St-Jacques, B. & Duarte, P. C. Targeting pain mediators induced by injured nerve-derived COX2 and PGE2 to treat neuropathic pain. Expert Opin. Ther. Targets 16, 527–540 (2012).
Barrie, A. et al. Prostaglandin E2 and IL-23 plus IL-1β differentially regulate the Th1/Th17 immune response of human CD161(+) CD4(+) memory T cells. Clin. Transl. Sci. 4, 268–273 (2011).
Hamon, Y. et al. Interleukin-1beta secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood 90, 2911–2915 (1997).
Yang, Y. et al. Tormentic acid inhibits IL-1β-induced inflammatory response in human osteoarthritic chondrocytes. Inflammation 39, 1151–1159 (2016).
Wu, T., Wang, D., Zhang, X., Li, J. & Yuan, B. Comparison of pain relief and limb function improvement after extracorporeal shock wave therapy and thermomagnetic therapy in the treatment of low back pain. Pak. J. Med. Sci. 39, 268–273 (2023).
Huang, M. et al. Single-dose radial extracorporeal shock wave therapy modulates inflammation during meniscal tear healing in the avascular zone. Am. J. Sports Med. 52, 710–720 (2024).
Chen, J. W. et al. Inflammatory mediators are potential biomarkers for extracorporeal shockwave therapy in horses. Equine Vet. J. 52, 449–454 (2020).
Lassar, A. B. Finding MyoD and lessons learned along the way. Semin. Cell Dev. Biol. 72, 3–9 (2017).
de Morrée, A. et al. Staufen1 inhibits MyoD translation to actively maintain muscle stem cell quiescence. Proc. Natl. Acad. Sci. USA 114, E8996–E9005 (2017).
Alkhamaali, Z. K., Crocombe, A. D., Solan, M. C. & Cirovic, S. Finite element modelling of radial shock wave therapy for chronic plantar fasciitis. Comput. Methods Biomech. Biomed. Eng. 19, 1069–1078 (2016).
Duryea, A. P., Cain, C. A., Roberts, W. W. & Hall, T. L. Removal of residual cavitation nuclei to enhance histotripsy fractionation of soft tissue. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 62, 2068–2078 (2015).
Wang, T. Y., Xu, Z., Hall, T. L., Fowlkes, J. B. & Cain, C. A. An efficient treatment strategy for histotripsy by removing cavitation memory. Ultrasound Med. Biol. 38, 753–766 (2012).
Narin, S., Unver, B., Demirkıran, N. D. & Erduran, M. Comparison of radial extracorporeal shock wave therapy in plantar fasciitis treatment using two different frequencies. Cureus 12, e8284 (2020).
Arifin, W. N. & Zahiruddin, W. M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. 24, 101–105 (2017).
Xu, C. Y. et al. Effects of shock wave on compound muscle action potential and muscle histomorphology in rabbits. Chin. J. Rehabil. Med. 33(9), 1019–1023 (2018).
Acknowledgements
The contributing authors have no conflicts of interest corresponding with this manuscript. This research has been supported by the guiding project of the Fujian Provincial Department of Science and Technology (Grant number: 2021Y0049) and Startup Fund for scientific research, Fujian Medical University (Grant number: 2019QH1180).
Author information
Authors and Affiliations
Contributions
S.W. and S.H. performed the experiments, analyzed and interpreted the data, and wrote the manuscript, and they contributed equally to this work. X.X. helped with data collection and interpretation and contributed to critical manuscript revision. S.W. and R.L. conceived the study, obtained funding, and critically revised the manuscript. R.L. offered lab instruments and participated in data analysis and the revised version. All the authors have read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
All animal experiments were approved by the ethical committee of the Fujian Medical University (No. IACUC FJMU 2022-0598). All animal operation and procedures were conducted according to the approved guidelines and were in accordance with the International Guide for the Care and Use of Laboratory Animals and ARRIVE’s guidelines.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, S., Huang, S., Xu, X. et al. Effects of radial extracorporeal shock wave with different frequencies on acute skeletal muscle injury in rabbits. Sci Rep 14, 21276 (2024). https://doi.org/10.1038/s41598-024-72371-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72371-6