Abstract
Generalizing defensive responses to new stimuli resembling learned threats is an adaptive process within an ever-changing environment. However, evaluation mechanisms excessively biased toward generalization (i.e., overgeneralization) may underlie anxiety-related symptoms. In the context of obesity, fear memory and fear generalization processes have never been investigated. In this study, participants with obesity and healthy participants as controls underwent a single-cue auditory fear conditioning paradigm and recognition memory tasks. We analyzed the autonomic reactions evoked by threat-predictive and new stimuli, as well as the recognition performance towards the same cues. We found that participants with obesity displayed similar autonomic defensive responses to a learned fearful stimulus, but enhanced reactions to new stimuli, when compared with the controls. We detected no significant differences between groups in recognition abilities. Our results provided the first evidence that obesity may widen fear generalization patterns. This alteration may encourage future research in investigating the link between emotional dysregulation and clinical anxiety-related symptoms in obesity.
Similar content being viewed by others
Introduction
The phenomenological underpinning of anxiety is the primary emotion of fear, in which we experience changes in our internal state that have the aim of preparing our body to react to danger1. This mechanism is crucial to guarantee our survival. Nevertheless, this process should be easily reversed when the danger vanishes. However, in some circumstances, some individuals respond actively (i.e., higher sweating, tachycardia, breathlessness) not only when the threat has disappeared, but also in the case of other events that are not threatening in principle. Dunsmoor and Paz (2015) pointed out that evaluation mechanisms excessively biased toward fear generalization (i.e., fear-related responses to new stimuli resembling learned threats) may underlie anxiety disorders2. Overgeneralization of defensive behaviors is common in different mental health disorders, including specific phobia, obsessive–compulsive disorder, panic disorder, generalized anxiety disorder, and post-traumatic stress disorder3. Indeed, overgeneralization has been proposed as a pathogenic marker that characterizes clinical anxiety crosswise2.
To the best of our knowledge, it has never been tested in the context of human obesity. Recent evidence suggests altered emotional recognition and regulation in obesity4,5. Moreover, from a psychopathological perspective, the prevalence of anxiety symptoms in obesity is higher than in the healthy-weight population6,7. Anxiety represents a risk predictor for developing obesity8, while an elevated body mass index (BMI) predicts chronicity of anxiety9. Trait anxiety has been recognized as one of the possible and important vulnerability factors in emotional eating among some individuals affected by obesity10. Indeed, some individuals who experience high levels of anxiety symptoms may be engaged in emotional overeating11, reinforcing the tendency towards obesity12. Moreover, anxiety symptoms may be amplified by obesity in relation to the degree of metabolic dysfunctions9,13. However, it should be noticed that the dynamics between obesity and anxiety are complex and not fully understood, requiring novel approaches from different (neuro)scientific perspectives. In this sense, because of all the previous evidence linking anxiety and obesity as well as anxiety in several psychopathological conditions and fear generalization2, we hypothesized the presence of fear overgeneralization in obesity. Thus, could individuals with obesity show fear overgeneralization?
Here, we aimed to fill out this gap in the literature, providing a first answer to this question through an experimental approach. We enrolled a group of participants affected by severe obesity and we compared them with a group of healthy individuals to a well-established fear conditioning paradigm14,15,16. In this paradigm, participants learn to associate a neutral conditioned stimulus (CS; such as a tone) with an aversive unconditioned stimulus (US; often a mild electrical shock or a loud sound), generating a fearful reaction. This learning is testified by the fact that the next presentation of the CS alone elicits a conditioned response (CR), which is generally an increased sympathetic autonomic reaction (e.g., increased skin conductance response, SCR), even hours after learning. Importantly, new stimuli (NSs) that were never associated with the US but that resemble the CS, also elicit significant SCRs, while new dissimilar stimuli do not induce such responses (fear generalization2).
We used two main experimental outcomes to test fear generalization17,18. The first one was the SCR, which represents the measure relative to the physiological reactions generated by the occurrence of learned stimuli recognized as threatening; because of its nature, we referred to this outcome as the autonomic one. The second outcome was the participant’s ability to identify the stimulus paired with the US during the learning phase (i.e., the CS); then, we refer to this outcome as recognition performance. The adoption of both outcomes seems necessary since they are mediated by different neural circuits17,19,20. Moreover, anxiety symptoms are typically defined by subjective reports21.
In this work, we tested the hypothesis that fear generalization in obesity may be altered as observed in other clinical conditions3. Hence, in within-group corrected multiple comparisons, we hypothesized that the autonomic reactions triggered by the NSs would be similarly strong to those elicited by the CS in our participants with obesity (i.e., generalization). In contrast, we expected that CS-evoked autonomic reactions would be stronger than those elicited by the NSs in the healthy group (i.e., specificity). In between-group corrected multiple comparisons, we hypothesized that the autonomic responses to the NSs would be stronger in the group of participants with obesity than in the healthy group. We explored these hypotheses also taking into account interindividual differences in terms of anxiety symptoms, which were measured through self-report questionnaires.
Results
Participants
In this paradigm (Fig. 1), we discarded 11 participants (5 participants with obesity and 6 healthy participants) because no SCRs were detected in the test. Overall, 16 participants with obesity and 16 healthy participants were enrolled. In Table 1, demographic details and scores on the psychological questionnaires are reported.
Schematic diagram depicting the experimental outline. In the first session (day 1), participants with obesity (n = 16) and healthy participants (n = 16) underwent a preconditioning phase in which they were exposed to neutral pictures and a neutral tone (800 Hz). Then, they underwent a single-cue fear conditioning in which the neutral tone (conditioned stimulus, CS, 800 Hz) was paired with multisensory cues composed of fearful pictures and auditory scream samples (unconditioned stimuli, USs). During the whole session, SCRs were recorded. In the second session (day 2), subjects were re-exposed to the neutral pictures, and then they underwent an autonomic reactions test during which they were presented with the CS and two new stimuli (NS1, 400 Hz and NS2, 1200 Hz) while being recorded in their SCRs. Then, participants underwent a 2AFC recognition task during which they were presented with tone pairs each composed of the CS and one of the two NSs, and they were asked to recognize the CS providing a confidence level for each choice. Participants then underwent a 2AFC perceptual discrimination test, in which they had to judge whether the two tones in each pair (CS and/or NSs) were “the same tone” or “different tones”. Last, participants rated the fear feelings evoked by the USs.
One participant with obesity (corresponding to 6.5% of the sample) reported a BMI between the range of 35.0–39.9, corresponding to Class 2 (moderate-risk) obesity while the majority of the group (93.75%) reported a BMI over 40, corresponding to class 3 (high-risk) obesity. In the healthy control group, 37.5% of participants reported a BMI between the range of 18.5–25.0 corresponding to normal weight, while the remaining part (62.5%) reported a BMI between the range of 25.0–29.9 (i.e., overweight). Notably, since BMI only considers body weight and height and does not take into account overall body composition, including body fat, muscular individuals may be classified as overweight, especially men.
Considering the results relative to the psychological assessment, participants with obesity and healthy participants showed comparable scores in the state subscale of the State-Trait Anxiety Inventory Form Y22,23 (STAI-Y1) during the first experimental session (unpaired t test, t(30) = 1.659, P = 0.108, ηp2 = 0.084) as well as during the second experimental session (t(30) = 0.967, P = 0.341, ηp2 = 0.030). They also reported similar scores in the trait subscale of the STAI-Y (STAI-Y2) (t(30) = 0.682, P = 0.500, ηp2 = 0.015). Given the higher prevalence of anxiety in obesity6,7, the results that we obtained in our sample were unexpected.
When we computed the scores relative to the STAI-Y, we observed that no participants reported a score over the clinical threshold (40, according to Spielberger and colleagues, 1983) in the state scale (STAI-Y1) measured each day of the experimental sessions. When we focused on the trait scale, 37.5% of the participants with obesity and 31.25% of healthy participants reported a score over the clinical threshold (40, according to Spielberger and colleagues, 1983), suggesting that these participants generally experience higher symptoms of anxiety in their lives. Notably, the percentage of participants with a score over the clinical threshold was similar between the two groups (χ2(1) = 0.13; P = 0.7).
Preconditioning
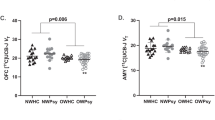
No significant between-group differences emerged in both visual (t(30) = 0.969, P = 0.340, ηp2 = 0.030) and auditory (t(30) = 0.618, P = 0.542, ηp2 = 0.013) stimuli (Fig. 2A,B), suggesting that the electrodermal responsivity was similar between conditions when exposed to non-emotional events.
Autonomic reactions during pre-conditioning, conditioning, and pre-test phases. (A) Dot plot representing the mean SCRs elicited by the neutral pictures during the first session in the two conditions. Electrodermal reactions were similar between the two groups. (B) Mean SCRs evoked by the 800 Hz tone in the pre-conditioning were comparable between the two groups. (C) Participants’ reactions to the USs in the conditioning phase were similar in the two groups. (D) The two groups similarly responded to the neutral pictures during the pre-test phase in the second session. All data are mean and SEM. Student’s unpaired t test (A, B, C, D).
Conditioning
No between-group differences in the SCRs to the USs (t(30) = 1.528, P = 0.137, ηp2 = 0.072) were observed, revealing comparable autonomic reactions toward unconditioned fearful triggers (Fig. 2C).
Pre-test
No between-group differences in SCR levels (t(30) = 1.500, P = 0.144, ηp2 = 0.070) emerged when the neutral pictures were tested again (Fig. 2D).
Test session. Autonomic outcome
During the test session, CS-evoked autonomic reactions were significantly stronger than those elicited by the same 800 Hz-tone before learning the CS-USs association (i.e. preconditioning) in the healthy participants (one-sample t test against 0: t(15) = 7.995, P < 0.001, ηp2 = 0.810) and the participants with obesity (one-sample t test against 0: t(15) = 4.102, P < 0.001, ηp2 = 0.529), thus indicating successful fear acquisition in both groups.
The main effect of Group (F(1,30) = 2.423, P = 0.130, ηp2 = 0.075) was not significant. Since this result is potentially not in line with our hypotheses, we computed the Bayes factor using the software JASP24 to classify the strength of evidence25,26,27. This computation was performed to test whether the non-significant result supports a null hypothesis over a theory, or whether the data are just insensitive27. The Bayes factor computed confirmed strong evidence in favor of a not significant main effect of Group (BF10 = 0.035)28. The main effect of Tone (F(2,60) = 4.336, P = 0.017, ηp2 = 0.126) was significant. Interestingly, we observed a significant Group × Tone interaction (F(2,60) = 6.783, P = 0.002, ηp2 = 0.184). Simple main effects analysis revealed that the CS elicited similar SCRs between groups (P = 0.794), meaning that participants with obesity did not show altered defensive responses to a learned threat relative to healthy participants. Critically, autonomic reactions to new stimuli exhibited divergent patterns in the two groups, since SCR levels of participants with obesity were higher than those of healthy participants to both the NS1 (P = 0.023) and the NS2 (P = 0.029). This evidence suggested enhanced autonomic reactions to new cues similar to a learned threatening stimulus in obesity. We then moved to explore the fear tunings of both groups by comparing within-subject responses to the CS and the NSs. In the group of participants with obesity, CS-related SCRs were comparable to those elicited by the NS1 (P = 1.000) and the NS2 (P = 0.259), which were in turn similar (P = 0.409), pointing to a generalized pattern of defensive responses. On the contrary, the group of healthy participants showed significantly stronger SCRs to the CS than the NS1 (P = 0.001) and nearly significantly stronger than the NS2 (P = 0.051), whereas the two NSs did not differ from each other (P = 0.258), indicating a more specific profile of defensive responses (Fig. 3).
Autonomic reactions during the test session. Healthy participants showed a specific pattern of defensive responses: the CS evoked significantly higher SCRs than the NS1 and almost significantly higher than the NS2. Instead, participants with obesity showed a generalized pattern of defensive reactions since SCRs were similarly strong to the CS and the NSs. CS-evoked responses were similar between groups but participants with obesity reacted stronger to both the NSs relative to the healthy participants. *P < 0.05, **P < 0.01. All data are mean and SEM. The level “0” corresponds to the pre-conditioning mean SCR response to the CS. 2 × 3 mixed ANOVA followed by Bonferroni-adjusted post hoc comparisons.
This finding suggested fear generalization to new stimuli in participants with obesity.
Since we found a significant Group × Tone interaction in the mixed ANOVA model, we performed additional analyses to explore whether anxiety levels affected the between-group differences in autonomic responses that we found in the main model. To this purpose, we re-performed the analysis three separate times by adding trait anxiety (STAI-Y 2) scores, state anxiety (STAI-Y 1) scores during session 1, and state anxiety (STAI-Y 1) scores during session 2 as covariates (mixed ANCOVA). In these models, trait anxiety (F(1,29) = 0.849, P = 0.365, ηp2 = 0.028), state anxiety during session 1 (F(1,29) = 0.065, P = 0.801, ηp2 = 0.002), and state anxiety during session 2 (F(1,29) = 1.179, P = 0.286, ηp2 = 0.039) were not significant, thus indicating that the between-group differences in autonomic reactions were not related to anxiety levels in our sample.
We also computed the correlations between the autonomic reactions during the test session and the self-report measures of state anxiety (STAI-Y 1) and trait anxiety (STAI-Y 2). No significant relationships were found in the overall sample (n = 32) between the reactions to all the tones (CS, NS1, and NS2) and all the self-report scales, nor in the group of healthy participants (n = 16) and the group of participants with obesity (n = 16) between all the tones and all the self-report scales (see Table 2).
These findings pointed out that, in our sample of participants, the defensive responses to the learned threat and overgeneralization of autonomic reactions were not related to self-reported trait/state anxiety levels.
Test session. Recognition and perceptual outcome
Overall, all participants correctly identified the CS amongst the NSs with an accuracy score above the 50% chance level (participants with obesity: t(15) = 4.878, P < 0.001, ηp2 = 0.613; healthy participants: t(15) = 6.429, P < 0.001, ηp2 = 0.734). No significant difference between the two groups in correct/incorrect responses (t(30) = 0.104, P = 0.918, ηp2 < 0.001) was observed in recognition performance (Fig. 4A). The Bayes factor resulted in anecdotal evidence in favor of no difference between groups (BF10 = 0.338). In the analysis of confidence ratings, participants who exhibited a perfect (100%) recognition of the CS generated missing cells for the respective confidence for errors, resulting in a decreased number of data (n = 11 participants with obesity and n = 11 controls). The groups reported similar confidence levels when making both correct (t(30) = 0.567, P = 0.575, ηp2 = 0.011, BF10 = 0.380) and incorrect choices (t(20) = 0.292, P = 0.773, ηp2 = 0.004, BF10 = 0.397) (Fig. 4B).
Recognition performance and appraisal of fear feelings during the test session. (A) Recognition patterns were similar between the two groups. (B) Confidence levels were comparable between the two groups, both for correct responses and for recognition errors. (C) Fear ratings of the USs were similar in the two groups. All data are mean and SEM. Student’s unpaired t test (A, B, C).
Hence, in our participants, no alteration in recognizing a learned threat amongst new similar stimuli was observed.
Since we found no between-group differences in the recognition task, we did not repeat the analyses by adding anxiety levels as covariates.
No differences in correctness (t(30) = 1.000, P = 0.325, ηp2 = 0.032, BF10 = not computed because the variance was equal to 0 in the control group) and confidence ratings (t(30) = 0.340, P = 0.736, ηp2 = 0.004, BF10 = 0.352) emerged between groups when we tested the ability in discriminating the CS from the NSs. Indeed, both groups discriminated the CS from the NSs with an elevated precision (participants with obesity: 0.991 ± 0.009 SEM; controls: 1.000 ± 0.000 SEM) and with comparably high confidence (participants with obesity: 9.607 ± 0.162 SEM; controls: 9.670 ± 0.091 SEM).
Finally, mean fear ratings were similar between groups (t(30) = 0.122, P = 0.904, ηp2 < 0.001, BF10 = 0.338) (Fig. 4C).
Overall, these findings suggested no difference between our groups in the ability to discriminate between the different stimuli used in our experiment, nor in the appraisal of fear feelings.
Also in these cases, we did not perform further analyses with anxiety levels as covariates, since no between-group differences emerged in these tasks.
Discussion
This study aimed to provide the first evidence about fear generalization in obesity. Our results pointed out that participants with obesity displayed a widened autonomic fear generalization to new stimuli when compared with healthy participants. In other words, they tended to overgeneralize defensive responses, as reported in other clinical conditions29,30,31,32,33,34. Notably, no alteration was found in terms of the fear learning process, since the two groups showed comparable defensive responses in the case of the learned fearful stimulus.
This evidence about altered fear generalization in our participants with obesity emerged even though they reported similar levels of state and trait anxiety to our healthy participants, and state-trait anxiety levels did not affect these differences between groups nor were related to autonomic reactions to the cues. Considering the high prevalence of anxiety symptoms in obesity6,7,8, this result of no difference in the scores reported by the two groups in the psychological questionnaire investigating anxiety symptoms, and the similar percentage of participants with a score above the clinical threshold in the two groups, were unexpected. However, it should be considered that this psychological feature was not used as an inclusion/exclusion criterion in our study. This result may be read both in the direction of the inter-variability of psychological traits in clinical populations and as a result of a selection bias due to the availability of low-anxious participants with obesity to take part in the study. Moreover, it was also mentioned that, in the literature, methodological differences in the measurement of anxiety may be observed; thus, a self-report questionnaire might have been a sub-optimal modality to detect clinical anxiety in this population. In this sense, the adoption of behavioral measures, as proposed in this study, may enhance our knowledge about emotional processing in clinical populations.
Why do individuals with obesity show fear overgeneralization? As mentioned in the Introduction, there is evidence about the role of anxiety symptoms in the life-span emotional experience in individuals with obesity35, which may be linked to the alteration of fear learning mechanisms. Crucially, the results relative to the relationship between the experimental outcome and the self-reported level of anxiety symptoms did not support entirely this hypothesis, open to possible other lines of discussion. One further possibility may stem from memory generalization mechanisms, which may be altered in individuals with obesity. To our knowledge, only one study36 so far explored generalization, and it was conducted in the appetitive learning domain (with food as a reward in a conditioning protocol). The study reported overgeneralization in female individuals with obesity, but only in those showing pronounced trait anxiety. We underline that overeating is one (but not the only one) mechanism related to obesity, thus the results provided by van den Akker and colleagues (2019) should be generalized to obesity with caution. However, considering the different types of stimuli adopted by van den Akker and colleagues (2019) (food) and in this study (emotional), we may suggest overall altered memory generalization mechanisms in obesity.
Another possible speculative interpretation of our results may point toward the cerebral underpinnings of the studied process. In detail, a common neural substrate underlying obesity- and fear-related mechanisms may be detected in the prefrontal cortex (PFC). Several studiessee37 enlightened a substantial contribution of structural and functional prefrontal alterations in predicting food overconsumption. For example, reduced functional coupling between dorsolateral (dlPFC) and ventromedial (vmPFC) prefrontal cortices—two regions involved in emotional down-regulation38‒and their diminished grey matter volumes39 have been linked to dietary self-regulatory impairments. On the other hand, the activity of dlPFC and inferior frontal gyrus (IFG) is enhanced during active inhibition of food craving40,41. In parallel, vmPFC is crucially implicated in regulating fear generalization42 and disruption of vmPFC threat processing has been associated with overgeneralization in generalized anxiety disorder43. In a speculative way, one might hypothesize that a shared neural network might underlie fear generalization across anxiety disorders and obesity.
At odds with the autonomic findings and with previous data showing reduced recognition memory in individuals with obesity44, we did not observe any difference between our groups in the ability to identify the learned fearful stimulus, nor in confidence levels for their (correct and incorrect) recognition. This is in line with previous findings demonstrating that autonomic reactions to new stimuli and the recognition of the same cues may diverge17, and with the evidence that in the population with obesity, emotional recognition may deviate from subjective reports45. Indeed, it might be also taken into account that declarative and non-declarative memory processes are mediated by different neural systems19,20, specifically a complex neural network encompassing the amygdala46 and sensory cortices 47,48,49,50 for non-declarative memories, and the hippocampal and medial temporal lobe networks51 for declarative memories. This evidence highlights the importance of collecting both autonomic and cognitive measures when assessing memory processes in the context of clinical diseases, including obesity.
Finally, we would offer an insight into the chance of a parallelism between human and animal research in the framework of fear generalization in obesity. Although this topic has never been investigated in humans, some—even controversial—evidence about fear conditioning can be traced in the literature on rodent models. By overfeeding rats with a palatable high fat/high sugar diet, enhanced defensive responses to a threatening cue together with reduced defensive responses to an aversive context were observed52, as well as enhanced anxiety-like behavioral patterns and impairment of fear inhibition53. However, rats fed with an obesogenic diet during their adolescence showed decreased fear learning as well as impaired fear extinction54. Moreover, diet-induced obese mice exhibited impairment of both hippocampus-dependent contextual and amygdala-dependent cued fear responses55. Finally, no effects of a saturated fats/sugar-rich and high-sugar diet were detected on the expression of cued and contextual fear56. Future studies may investigate fear processing parallelly in both humans and animals. It is worth mentioning that the experimental paradigm used in this research has a higher translational value57.
Some limitations can be observed in this study. The first limitation is the reduced sample size. Thus, to increase the interpretability of our results, we have computed and reported the Bayes factor in the Results section. It should be noticed that we included only (cis-gender) male participants in our samples, because of gender differences in obesity-related phenotype58,59, psychological functioning60, and emotional processing61,62,63. This means that our results should be read with caution in the context of obesity; nevertheless, to extend our findings, fear generalization should be tested in female participants with obesity. It should also be observed that the number of excluded participants due to the complete absence of SCRs during the autonomic reactions test was higher than in our previous studies (25.58% of the sample in this study, while 6.01%16 and 2.84%17 in previous studies). One possible explanation may be traced to the type of USs used here. Indeed, differently from Manassero and colleagues16,17,18, we used female screams co-presented with still images from horror movies instead of the more traditional (and, likely, more effective in inducing robust fear conditioning) electrical shocks. We made this experimental choice since electrical shocks should be avoided in clinical contexts because of ethical constraints21. A final limitation of this study is the lack of a control CS not paired to the USs (i.e., a CS-) which makes it more challenging to reveal the specificity of the conditioning and the overgeneralization. As in our previous study16, we chose to adopt single-cue conditioning ‒instead of a differential conditioning procedure‒ because it more ecologically reflects real-life fear-related experiences14,15,64,65,66,67.
This study, which may be considered the first in the field, would be generative of future studies. For example, they should address whether the widened generalization of defensive responses in individuals with obesity may be related to the individual expression of anxiety symptoms, especially as a psychological trait. As reported before, we would compare individuals with higher expressions of anxiety symptoms and individuals with lower expressions in the fear conditioning paradigm. Moreover, it should be investigated whether and how the widened generalization of defensive responses may affect eating behaviors and contribute to the maintenance of obesity. Indeed, overgeneralization that characterizes individuals with obesity may reflect a higher proneness to be emotionally triggered by environmental stimuli, which activate alarm reactions and fear feelings thereby addressing or reinforcing food-based coping strategies.
Collectively, our findings provide a first glimpse into obesity-related fear overgeneralization and open a new path of investigation to test multiple and clinically relevant hypotheses within a translational perspective. Even though preliminary, our evidence would suggest considering fear generalization as a psychological target in rehabilitative interventions in the context of obesity.
Methods
This study was approved by the ethical committees of the Istituto Auxologico Italiano, IRCCS (ID: 21X101), and the University of Turin (Prot. N. 0338128), following the guidelines of the European Convention on Human Rights and Biomedicine. Subjects participated voluntarily; they gave informed written consent and were naïve to the rationale of the experiment. Participants could withdraw from their participation at any time. They received no compensation for their participation.
Participants
Only male participants were enrolled since females and males differ in emotional experience and expression63,68. Moreover, gender-specific differences in body morphology, particularly fat distribution in obesity, are well recognized59,69,70. Individuals with expertise in music or musical training were excluded since musicians outperform in pitch discrimination71.
Individuals with a body mass index (BMI) greater than 30 kg/m2, an index of obesity72, were recruited at the involved hospital during a diagnostic hospitalization. BMI was expressed as body mass (kg)/height (m)2, where weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, using standard methods. All participants were nonsmokers and free from gastrointestinal, cardiovascular, psychiatric, neurological, or metabolic disorders or any concurrent medical condition not related to obesity, following the clinical definition of ‘Metabolically Healthy Obesity’73.
Healthy participants were included as controls. They were enrolled out of the hospital. The previous inclusion/exclusion criteria were used, except for the BMI which had to be in a range of 18.5–29.9.
Psychological assessment
One psychological self-report questionnaire which is extensively used in both research and clinical contexts was administered. Specifically, we adopted the Italian version of the State-Trait Anxiety Inventory Form Y (STAI-Y)22,23 to assess the current state of anxiety through items that measure subjective feelings of apprehension, tension, nervousness, worry, and activation/arousal of the autonomic nervous system (state scale). Moreover, the questionnaire evaluates relatively stable aspects of anxiety proneness, including general states of calmness, confidence, and security (trait scale). The questionnaire consists of 40 items, 20 items for each scale. In terms of reliability, it was reported α = 0.90 for the trait scale, and α = 0.93 for the state scale; test–retest reliability ranged from 0.73 to 0.86 and from 0.16 to 0.62 for scores on the trait and state scales22. Specifically, the trait scale was completed only once, before the experiment; instead, the state scale was completed twice, at the beginning of each experimental session.
Experimental stimuli
Auditory stimuli were pure sine wave tones with oscillation frequencies of 800 Hz (CS), 400 Hz (NS1), and 1200 Hz (NS2), lasting 6 s with onset/offset ramps of 5 ms. Tones were digitally generated using Audacity 2.1.2 (Audacity® freeware). Unconditioned stimuli (USs) consisted of 12 different female scream samples lasting 3 s, which were co-presented with 12 different still images from horror movies. All experimental scenarios were controlled by Presentation® 21.1 (NeuroBehavioral Systems, Berkeley, CA; https://www.neurobs.com/). All auditory stimuli were binaurally delivered through headphone speakers (Direct Sound EX29) at 50 dB intensity. Visual stimuli were presented on a 22-inch Samsung monitor (resolution: 1600 × 1200 pixels; refresh rate: 60 Hz), against a uniform black background, and were displaced in the center of gaze along the horizontal meridian. The viewing distance was held constant at 100 cm. During the task, participants were comfortably seated in front of the monitor, with their arms in resting position on their legs.
Preconditioning
Two tasks were performed before the conditioning procedure to quantify the electrodermal reactivity at the baseline.
Visual stimuli. Four pictures from the Set of Fear Inducing Pictures (SFIP74): these pictures (i.e., neutral_296; neutral_568; neutral_572; neutral_581) were selected since they do not have any emotional valence or salience. The task started with a white central fixation cross, which was also presented during each inter-trial interval (ITI). After each ITI, which randomly ranged between 21 and 27 s, each picture appeared for 6 s. During the entire task, participants were asked to maintain the fixation (on the white central fixation cross or the picture). SCRs were recorded throughout this phase. This task was repeated also before the beginning of the testing phase, in the second experimental session. The order of the administration of the four pictures was random between participants and between repetitions.
Auditory stimuli. We presented the CS (800 Hz) four times, with an ITI randomly ranging between 21 and 27 s. The white central fixation cross was displayed on the screen, and participants were instructed to fix it at each CS onset and to hold central fixation for the whole duration of the trial. SCRs were recorded. At the end of the task, participants were asked to confirm whether the tones were easily audible and comfortable.
Conditioning
This task was administered immediately after the preconditioning phase. Participants underwent a single-cue auditory fear conditioning as done by Manassero and colleagues (2024) since it more ecologically mimics real-life fear-related events14,15,64,65,66,67.
It consisted of the presentation of 15 trials of the conditioned stimulus (CS, 800 Hz), with an ITI randomly ranging between 21 and 27 s. For 12 trials (80% reinforcement rate), the CS co-terminated with the US: after 3 s of fixation, US images appeared on the screen and US sounds overlapped the CS tone. For the remaining 3 trials, the CS was presented alone. The sequence of CS and CS + US was randomly generated for each participant. Participants were instructed to fix the central cross at each CS onset and for the entire trial. No feedback about CS-US contingency was furnished.
Autonomic reactions test
After a resting period (1 min), which served to stabilize the electrodermal activity and allow its level to decline to baseline, participants underwent this task to test the memory of the CS-US association and the autonomic reactions to NSs. 12 trials of auditory stimuli were presented, i.e. 4 trials of the CS (800 Hz), 4 trials of the NS1 (400 Hz), and 4 trials of the NS2 (1200 Hz), and the sequence was completely randomized for each participant. The ITI had a duration randomly ranging between 21 and 27 s. During the entire task, the central fixation cross was shown, and participants were instructed to fix it. SCRs were recorded.
Two-alternative forced-choice (2AFC) recognition test
This task tested participants’ ability to recognize the CS when presented within the NSs. 16 tone-pairs, each composed of the CS and one of the two NSs (NS1 or NS2) were presented in random sequence: 4 × CS vs NS1, 4 × NS1 vs CS, 4 × CS vs NS2, 4 × NS2 vs CS. On each trial, the interval between the two stimuli was 1000 ms. After each pair offset, an ITI randomly ranging between 21 and 27 s occurred. During the entire task, the fixation cross was shown on the screen. Participants were explained that in each couple of sounds, there was a tone that they had heard on the first session (i.e., the day before) and a new tone. Participants verbally reported which one (the first or the second) was the tone heard in the first session, paired with the US (i.e., the CS). Participants verbally provided a confidence rating about each own response, on a scale from 0 (completely unsure) to 10 (completely sure). No feedback was provided.
Two-alternative forced-choice (2AFC) perceptual discrimination test
Following the recognition task, participants’ ability to discriminate the CS from the NSs was tested. 7 pairs of auditory stimuli (i.e. CS vs NS1, NS1 vs CS, CS vs NS2, NS2 vs CS, CS vs CS, NS1 vs NS1, NS2 vs NS2) with a 1000-ms intra-pair-interval were presented in a random sequence (ITI randomly ranging between 21 and 27 s). For each pair, subjects judged whether the two tones were “the same tone or different tones”, and provided a confidence rating on an analog scale from 0 (completely unsure) to 10 (completely sure). During the entire task, the fixation cross was shown on the screen. No feedback was furnished.
US fear ratings
After the perceptual task, we collected the US fear ratings to quantify the fear feelings evoked by the fearful pictures. This phase consisted of the re-presentation of the 12 US images, with a 3 s ITI in a random sequence. For each US (lasting 3 s as during the conditioning), participants provided a rating on an analog scale from 0 (absence of fear) to 10 (maximal imaginable fear) about the level of fear elicited by the cues. During the ITIs, the fixation cross was presented on the screen. No feedback was furnished.
Main experimental outcomes
Autonomic outcome
Event-related SCRs were used as an index of autonomic reactions to the stimuli. To record the autonomic signal, two Ag–AgCl non-polarizable electrodes filled with isotonic paste were attached to the index and middle fingers of the non-dominant hand by Velcro straps. The transducers were connected to the GSR100C module of the BIOPAC MP-150 system (BIOPAC Systems, Goleta, CA) and signals were recorded at a channel sampling rate of 1000 Hz. SCR waveforms were analyzed offline using AcqKnowledge 4.1 software (BIOPAC Systems, Goleta, CA; https://www.biopac.com/product/acqknowledge-software/). Each SCR was evaluated as event-related if: (i) the trough-to-peak deflection occurred 1–6 s after the stimulus onset; (ii) the duration ranged between 0.5 s and 5.0 s, and (iii) the amplitude was greater than 0.02 microsiemens (μS). Responses that did not fit these criteria were scored zero. Raw SCR data were square-root transformed to normalize the distributions75. To account for inter-individual variability, these normalized values were then scaled according to each participant’s average unconditioned response by dividing each response by the mean square-root transformed unconditioned stimulus response76,77. To obtain a quantification of each participant’s post-conditioning response level, we computed the delta score between these scaled values and each subject’s mean square-root transformed response to the 800 Hz-tone (CS) prior to the conditioning (i.e. preconditioning phase).
Recognition memory outcome
To test the ability to recognize the CS, we computed the percentages of correct and incorrect responses (where the sum was equal to 1.00) and the corresponding mean levels of confidence in the 2AFC recognition test.
Perceptual discrimination outcome
To test the ability to discriminate the auditory stimuli, we computed the percentage of correct responses and the mean level of confidence judgments in the 2AFC perceptual discrimination test.
Appraisal of fear feelings
To quantify the amount of fear elicited by USs, we gathered the individuals’ responses in the US fear ratings task. The groups’ overall mean level of fear was computed.
Statistical analyses
Since all variables (with the only exception of SCRs to the USs during the conditioning phase, showed by the group of participants with obesity) passed the D’Agostino-Pearson omnibus normality test, parametric statistics were adopted.
Psychological questionnaires
To test potential between-group differences in the STAI-Y, we performed Student’s unpaired t tests.
Preconditioning and pretest
To test any difference between groups at the baseline SCR levels to the CS and neutral visual cues, and pretest SCRs to the neutral visual cues, we used three independent Student’s unpaired t tests.
Conditioning
Through a Student’s unpaired t test, we also tested any difference between the two groups in SCRs to the USs during conditioning.
Autonomic outcome
To test whether SCRs during the autonomic reactions test were stronger than during the preconditioning, we computed Student’s one sample t tests against 0. To test the potential between-group and within-group differences in the SCRs to the CS, the NS1, and the NS2 during the test session (fear tunings: the decay of reactions’ amplitude along the gradient of cues’ perceptual distance from the CS, where the decay rate quantifies fear generalization78), we performed a 2 × 3 mixed ANOVA with Group (participants with obesity vs control participants) as between-subjects variable and Tone (NS1, CS, NS2) as within-subjects variable. Bonferroni adjustment was applied for simple main effects analyses. For the mixed ANOVA, we assessed the Sphericity assumption through Mauchly’s Test. To test the correlations between STAI-Y (1 and 2) scores and the autonomic reactions to the CS, the NS1, and the NS2, we computed Spearman rank-order correlation coefficients.
Recognition memory outcome
To test the between-group differences in the recognition performance and respective confidence ratings, we performed Student’s unpaired t tests. To test whether recognition levels (correct responses) were significantly higher than the 50% chance level for each group, we calculated Student’s one sample t tests against 0.50.
Perceptual and fear feelings’ outcomes
To test between-group differences in the perceptual discrimination and respective confidence ratings, as well as in the US fear ratings, we performed Student’s unpaired t tests.
In case of significant effect of Group or significant Group × Tone interaction in the mixed ANOVA model, or significant differences in t tests referred to the test session, we performed three further mixed ANCOVA models by adding STAI-Y (state-session 1, state-session 2, and trait) scores as covariates to test the influence of anxiety levels on the experimental outcomes.
The null hypothesis was rejected at P < 0.05 significance level. All statistical analyses were performed using SPSS Statistics 22 (IBM; https://www.ibm.com/it-it/products/spss-statistics) and Prism 9 (GraphPad; https://www.graphpad.com/), while Bayes factors were computed with JASP 0.18.3.0 (https://jasp-stats.org/).
A priori power analysis
We computed the appropriate sample size based on a power analysis performed through G*Power 3.1.9.2 (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower.html). For the main statistics, i.e. mixed ANOVA (within-between interaction) with two groups and three measurements, with the following input parameters: α equal to 0.05, power (1-β) equal to 0.85, and a hypothesized effect size (f) equal to 0.25, the estimated sample size resulted in n = 16 per experimental group.
Data availability
The data that support the findings of this study are available in Zenodo at https://doi.org/https://doi.org/10.5281/zenodo.11574270. To access the data, please contact: Federica Scarpina (federica.scarpina@unito.it).
References
LeDoux, J. E. & Pine, D. S. Using neuroscience to help understand fear and anxiety: A two-system framework. Am. J. Psych. 173, 1083–1093 (2016).
Dunsmoor, J. E. & Paz, R. Fear generalization and anxiety: Behavioral and neural mechanisms. Biol. Psych. 78, 336–343 (2015).
Dymond, S., Dunsmoor, J. E., Vervliet, B., Roche, B. & Hermans, D. Fear generalization in humans: Systematic review and implications for anxiety disorder research. Behav. Ther. 46, 561–582 (2015).
Micanti, F. et al. The relationship between emotional regulation and eating behaviour: A multidimensional analysis of obesity psychopathology. Eat. Weight Disord. 22, 105–115 (2017).
Casagrande, M., Boncompagni, I., Forte, G., Guarino, A. & Favieri, F. Emotion and overeating behavior: Effects of alexithymia and emotional regulation on overweight and obesity. Eat. Weight Disord. 25, 1333–1345 (2020).
Gariepy, G., Nitka, D. & Schmitz, N. The association between obesity and anxiety disorders in the population: A systematic review and meta-analysis. Int. J. Obes. 34, 407–419 (2010).
Amiri, S. & Behnezhad, S. Obesity and anxiety symptoms: a systematic review and meta-analysis. Neuropsychiatr. 33, 72–89 (2019).
Sharafi, S. E. et al. Prevalence of anxiety and depression in patients with overweight and obesity. Obes. Med. 17, 100169 (2020).
Fulton, S., Décarie-Spain, L., Fioramonti, X., Guiard, B. & Nakajima, S. The menace of obesity to depression and anxiety prevalence. Trends Endocrinol. Metab. 33, 18–35 (2022).
Schneider, K. L., Appelhans, B. M., Whited, M. C., Oleski, J. & Pagoto, S. L. Trait anxiety, but not trait anger, predisposes obese individuals to emotional eating. Appetite 55, 701–706 (2010).
Frayn, M. & Knäuper, B. Emotional eating and weight in adults: A review. Curr. Psychol. 37, 924–933 (2018).
Bourdier, L. et al. Are emotionally driven and addictive-like eating behaviors the missing links between psychological distress and greater body weight?. Appetite 120, 536–546 (2018).
Tang, F., Wang, G. & Lian, Y. Association between anxiety and metabolic syndrome: A systematic review and meta-analysis of epidemiological studies. Psychoneuroendocrinology 77, 112–121 (2017).
Wong, A. H. & Lovibond, P. F. Rule-based generalisation in single-cue and differential fear conditioning in humans. Biol. Psychol. 129, 111–120 (2017).
Resnik, J. & Paz, R. Fear generalization in the primate amygdala. Nat. Neurosci. 18, 188–190 (2015).
Manassero, E. et al. Medial anterior prefrontal cortex stimulation down-regulates implicit reactions to threats and prevents the return of fear. eLife 13, e85951 (2024).
Manassero, E., Mana, L., Concina, G., Renna, A. & Sacchetti, B. Implicit and explicit systems differently predict possible dangers. Sci. Rep. 9, 13367 (2019).
Manassero, E., Giordano, A., Raimondo, E., Cicolin, A. & Sacchetti, B. Sleep deprivation during memory consolidation, but not before memory retrieval, widens threat generalization to new stimuli. Front. Neurosci. 16, 902925 (2022).
Squire, L. R. Memory systems of the brain: A brief history and current perspective. Neurobiol. Lear. Mem. 82, 171–177 (2004).
LaBar, K. S. & Cabeza, R. Cognitive neuroscience of emotional memory. Nat. Rev. Neurosci. 7, 54–64 (2006).
Lau, J. Y. et al. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. J. Am. Acad. Child Adolesc. Psychiatry. 47, 94–102 (2008).
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R., & Jacobs, G. A. Manual for the State-Trait Anxiety Inventory, Ed. Palo Alto, Palo Alto (1983).
Pedrabissi, L. & Santinello, M. Nuova versione italiana dello STAI–Forma Y, Organizzazioni Speciali, Firenze (1989).
JASP Team, 2024. JASP [Computer software]. Version 0.18.3. https ://jasp-stats.org/.
Jeffreys, H. Theory of Probability 1st edn. (Oxford University Press, 1939).
Kass, R. E. & Raftery, A. E. Bayes factors. J. Am. Stat. Assoc. 90, 773–795 (1995).
Dienes, Z. Using Bayes to get the most out of non-significant results. Front. Psychol. 5, 1–17 (2014).
Lee, M. D. & Wagenmakers, E. J. Bayesian Cognitive Modeling: A Practical Course (Cambridge University Press, 2014).
Lissek, S. et al. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behav. Res. Ther. 43, 1391–1424 (2005).
Lissek, S. et al. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am. J. Psychiatry 167, 47–55 (2010).
Lissek, S. et al. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol. Psychiatry 75, 909–915 (2014).
Kaczkurkin, A. N. & Lissek, S. Generalization of conditioned fear and obsessive-compulsive traits. J. Psychol. Psychother. 7, 3 (2013).
Morey, R. A. et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl. Psychiatry 5, e700 (2015).
Kaczkurkin, A. N. et al. Neural substrates of overgeneralized conditioned fear in PTSD. Am. J. Psych. 174, 125–134 (2017).
Morris, M. J., Beilharz, J. E., Maniam, J., Reichelt, A. C. & Westbrook, R. F. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci. Biobehav. Rev. 58, 36–45 (2015).
van den Akker, K., Schyns, G., Breuer, S., van den Broek, M. & Jansen, A. Acquisition and generalization of appetitive responding in obese and healthy weight females. Behav. Res. Ther. 123, 103500 (2019).
Lowe, C. J., Reichelt, A. C. & Hall, P. A. The prefrontal cortex and obesity: A health neuroscience perspective. Trends Cogn. Sci. 23, 349–361 (2019).
Hare, T. A., Camerer, C. F. & Rangel, A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324, 646–648 (2009).
Schmidt, L. et al. Neuroanatomy of the vmPFC and dlPFC predicts individual differences in cognitive regulation during dietary self-control across regulation strategies. J. Neurosci. 38, 5799–5806 (2018).
Kober, H. et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc. Natl. Acad. Sci. U.S.A. 107, 14811–14816 (2010).
Siep, N. et al. Fighting food temptations: The modulating effects of short-term cognitive reappraisal, suppression and upregulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage 60, 213–220 (2012).
Spalding, K. N. The role of the medial prefrontal cortex in the generalization of conditioned fear. Neuropsychology 32, 1–17 (2018).
Cha, J. et al. Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: Implications for generalized anxiety disorder. J. Neurosci. 34, 4043–4053 (2014).
Gunstad, J., Paul, R. H., Cohen, R. A., Tate, D. F. & Gordon, E. Obesity is associated with memory deficits in young and middle-aged adults. Eat. Weight Disord. 11, e15–e19 (2006).
Scarpina, F. et al. Implicit facial emotion recognition of fear and anger in obesity. Eat. Weight Disord. 26, 1243–1251 (2021).
Phelps, E. A. & LeDoux, J. E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48, 175–187 (2005).
Sacco, T. & Sacchetti, B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science 329, 649–656 (2010).
Grosso, A. et al. The higher order auditory cortex is involved in the assignment of affective value to sensory stimuli. Nat. Comm. 6, 8886 (2015).
Cambiaghi, M. et al. Higher-order sensory cortex drives basolateral amygdala activity during the recall of remote, but not recently learned fearful memories. J. Neurosci. 36, 1647–1659 (2016).
Concina, G., Renna, A., Grosso, A. & Sacchetti, B. The auditory cortex and the emotional valence of sounds. Neurosci. Biobehav. Rev. 98, 256–264 (2019).
Mayes, A., Montaldi, D. & Migo, E. Associative memory and the medial temporal lobes. Trends Cogn. Sci. 11, 126–135 (2007).
Reichelt, A. C., Maniam, J., Westbrook, R. F. & Morris, M. J. Dietary-induced obesity disrupts trace fear conditioning and decreases hippocampal reelin expression. Brain Behav. Immun. 43, 68–75 (2015).
Baker, K. D. & Reichelt, A. C. Impaired fear extinction retention and increased anxiety-like behaviours induced by limited daily access to a high-fat/high-sugar diet in male rats: Implications for diet-induced prefrontal cortex dysregulation. Neurobiol. Learn. Mem. 136, 127–138 (2016).
Vega-Torres, J. D. et al. Exposure to an obesogenic diet during adolescence leads to abnormal maturation of neural and behavioral substrates underpinning fear and anxiety. Brain Behav. Immun. 70, 96–117 (2018).
Yamada-Goto, N. et al. Impairment of fear-conditioning responses and changes of brain neurotrophic factors in diet-induced obese mice. J. Neuroendocrinol. 24, 1120–1125 (2012).
Ferreira, A., Castro, J. P., Andrade, J. P., Madeira, M. D. & Cardoso, A. Cafeteria-diet effects on cognitive functions, anxiety, fear response and neurogenesis in the juvenile rat. Neurobiol. Learn. Mem. 155, 197–207 (2018).
Erhardt, A. & Spoormaker, V. I. Translational approaches to anxiety: Focus on genetics, fear extinction and brain imaging. Curr. Psychiatry Rep. 15, 417 (2013).
Legato, M. J. Gender-specific aspects of obesity. Int. J. Fertil. Womens Med. 42, 184–197 (1997).
Kanter, R. & Caballero, B. Global gender disparities in obesity: A review. Adv. Nutr. 3, 491–498 (2012).
Hyde, J. S. Gender similarities and differences. Annu. Rev. Psychol. 65, 373–398 (2014).
Brody, L. R. & Hall, J. A. Gender and emotion in context. Handb. Emot. 3, 395–408 (2008).
McClure, E. B. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychol. Bull. 126, 424–453 (2000).
Abbruzzese, L., Magnani, N., Robertson, I. H. & Mancuso, M. Age and gender differences in emotion recognition. Front. Psychol. 10, 2371 (2019).
Grosso, A., Santoni, G., Manassero, E., Renna, A. & Sacchetti, B. A neuronal basis for fear discrimination in the lateral amygdala. Nat. Comm. 9, 1214 (2018).
Concina, G., Cambiaghi, M., Renna, A. & Sacchetti, B. Coherent activity between the prelimbic and auditory cortex in the slow-gamma band underlies fear discrimination. J. Neurosci. 38, 8313–8328 (2018).
Concina, G. et al. Expression of IGF-2 receptor in the auditory cortex improves the precision of recent fear memories and maintains detailed remote fear memories over time. Cereb. Cortex. 31, 5381–5395 (2021).
Concina, G., Renna, A., Milano, L. & Sacchetti, B. Prior fear learning enables the rapid assimilation of new fear memories directly into cortical networks. PLoS Biol. 20, e3001789 (2022).
Brody, L. R. & Hall, J. A. Gender and emotion. In Handbook of emotions (eds Lewis, M. & Haviland, J. M.) 447–460 (Guilford Press, 1993).
Blaak, E. Gender differences in fat metabolism. Curr. Opin. Clin. Nutr. Metab. Care 4, 499–502 (2001).
Haslam, D. Gender-specific aspects of obesity. J. Men’s Health Gend. 2, 179–185 (2005).
Kishon-Rabin, L., Amir, O., Vexler, Y. & Zaltz, Y. Pitch discrimination: Are professional musicians better than non-musicians?. J. Basic Clin. Physiol. Pharmacol. 12, 125–144 (2001).
World Health Organization. Obesity and overweight, Fact Sheet Number 311. World Health Organization: Geneva, Switzerland (2006).
Blüher, M. Metabolically healthy obesity. Endocr. Rev. 41, bnaa004 (2020).
Michałowski, J. M. et al. The set of fear inducing pictures (SFIP): Development and validation in fearful and nonfearful individuals. Behav. Res. Methods 49, 1407–1419 (2017).
Lykken, D. T. & Venables, P. H. Direct measurement of skin conductance: A proposal for standardization. Psychophysiology 8, 656–672 (1971).
Schiller, D. et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463, 49–53 (2010).
Battaglia, S., Garofalo, S. & di Pellegrino, G. Context-dependent extinction of threat memories: Influences of healthy aging. Sci. Rep. 8, 12592 (2018).
Onat, S. & Büchel, C. The neuronal basis of fear generalization in humans. Nat. Neurosci. 18, 1811–1818 (2015).
Acknowledgements
We thank all the participants for their contribution to this study. We also thank Melania Lattuada, Veronica Cintori, Ester Fusaro, Annalisa Parodi, and Maria Clarissa Chantal Caraig for their help in the experimental procedures and for their continuous support and advice.
Funding
This work was supported by the “Translational Research Project” of the Department of Neurosciences, University of Turin 2020, by the “Compagnia di San Paolo, Progetto d’Ateneo”, University of Turin 2017 (CSTO167503), by the Grant “Progetti di ricerca di Rilevante Interesse Nazionale (PRIN)” 2017 (Project n. 20178NNRCR_002) from the Italian Ministry of University and Research (MIUR), Fondazione Cariverona 2020, and Banca D’Italia 2023. This study is part of the MIUR Project “Dipartimenti di Eccellenza” 2018–2022 of the Rita Levi-Montalcini Department of Neurosciences (University of Turin). This research was partially funded by the Italian Ministry of University and Research (MIUR) under the National Recovery and Resilience Plan (PNRR), NODES.SPOKE 5: INDUSTRY FOR HEALTH AND SILVER ECONOMY. Moreover, this research was partially funded by the Italian Ministry of Health (Ricerca Corrente). The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
EM: Conceptualization, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review and editing. FS: Conceptualization, Funding acquisition, Methodology, Writing—review and editing. ST: Investigation. GC: Formal analysis. MS: Resources, Supervision. AP: Formal analysis. AM: Funding acquisition, Project administration, Resources, Supervision. BS: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Manassero, E., Scarpina, F., Tagini, S. et al. Overgeneralization of autonomic defensive reactions in obesity. Sci Rep 14, 23562 (2024). https://doi.org/10.1038/s41598-024-72439-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72439-3