Abstract
Echogenic needles improve the reflection of the ultrasound beam. The aim of the study was to compare needle performance during ultrasound-guided cannulation of the infraclavicular axillary vein with an in-plane needle approach, using echogenic needles or non-echogenic standard needles. One hundred adult patients undergoing surgical procedures that required a central venous catheter were randomized for either echogenic or non-echogenic needles. The primary outcome was access time. Secondary outcomes encompassed total procedure time, success in first attempt, number of attempts, number of skin punctures, change of site for vascular access, catheter placement, subjective experience with needle visualization and needle procedure, and adverse events. Median (IQR) [range] venous access time was 21 (15–56) [6–440] in echogenic needle group and 26 (14–91) [6–925] in the non-echogenic needle group (p = 0.40). No statistically significant differences were found in the secondary outcome measures. One patient (non-echogenic needle group) experienced pneumothorax. In three patients in each group (6%) arterial puncture occurred. Echogenic needles did not significantly improve needle control or safety when used for infraclavicular axillary vein cannulation with an in-plane needle approach. The results indicate that standard needles are appropriate for ultrasound guided subclavian vascular access in a perioperative situation.
Similar content being viewed by others
Introduction
Ultrasound guidance is frequently used as an alternative to landmark-based techniques to facilitate central venous access1,2. Particularly in patient with anatomical variations, as well as in obese populations and in bariatric surgery ultrasound has become an important tool for venous cannulation3 However, needle visualization by ultrasound can sometimes be difficult. Assessing the 2D ultrasound image does not always lead to correctly identifying the needle tip and shaft4. Unsuccessful central venous cannulation might lead to insufficient patient treatment or delay of the surgical procedures. Despite the use of ultrasound guidance, central venous catheter placement is still associated with a risk of adverse events and complications, most often related to an incorrect placement of the injection needle.
Echogenic needles are customized needles that improve the ultrasound beam’s reflection to enhance needle visualization in an ultrasound image5. Echogenic needles might improve success rates, reduce performance time, and decrease complication rates due to better needle control. While echogenic needles are commonly used for regional anesthesia techniques, they have not yet become standard for central venous access. Echogenic needles have been tested in patients for venous access in the jugular and subclavian veins6,7. However, the benefit of the cannulation technique of the infraclavicular axillary vein should be reconfirmed in new studies.
The aim of the study was to compare needle performance during ultrasound-guided cannulation of the infraclavicular axillary vein with an in-plane needle approach, using echogenic needles or non-echogenic standard needles. The primary outcome was access time. Access failure and complication rates were secondary outcomes. We hypothesized that the use of echogenic needles could reduce the time until central venous access of the infraclavicular axillary vein.
Results
One hundred patients undergoing a surgical procedure that required a central venous catheter placement gave written informed consent and were included in the study (Fig. 1). Thirty-four patients underwent the Whipple procedure, 23 patients had a liver resection, 18 patients were treated with kidney transplantations, and the remaining 25 had different types of abdominal surgeries including two vascular procedures.
Five patients were excluded from the analysis. One patient was excluded due to protocol violation (the needle became unsterile during unpacking). In four patients, the puncture site was changed to the jugular internal vein due to difficult vascular access. They were excluded from most data analysis but included in an intention-to-treat analysis of success rates. The left side was used for cannulation in 94 patients (45 echogenic, 49 non-echogenic) and five patients on the right side (four echogenic, one non-echogenic). The demographic data and patient characteristics are presented in Table 1.
Primary outcome
The median venous access time was 21 s in the echogenic needle group and 26 in the non-echogenic needle group. This difference was not statistically significant (Table 2). Subgroup analysis by anesthesiologists did not show significant differences.
Secondary outcomes
Success in first attempt was achieved in 34 patients (69%) in the echogenic needle group and 40 patients (80%) in the non-echogenic needle group (p = 0.22). There were no statistically significant differences for the secondary outcomes (Table 2).
Carry over effect
There was a tendency that access time was reduced during the course of the trial. However, this was not statistically significant (Fig. 2).
The figure illustrates a possible carryover effect for anesthesiologists (AN) 1, 2, 3, and 4. The venous access time data from both study groups were pooled and analyzed for each anesthesiologist. The green dots represent procedures with echogenic needles, and the red dots the procedures with non-echogenic needles. Regression analyses were not significant.
Adverse events
One patient (non-echogenic needle group) experienced pneumothorax during the procedure. The patient had to be treated with a thoracic drainage during the first two postoperative days. No clinical or radiological sign of pneumothorax was observed in any of the other patients. In three patients in each group (6%), arterial puncture occurred without further clinical implications. No other adverse events were observed.
Discussion
In this prospective, randomized, controlled trial we compared echogenic with non-echogenic needles for ultrasound-guided infraclavicular axillary vein cannulation using an in-plane needle approach. A 19% reduction of the venous access time (primary outcome) was not statistically significant. There were no statistically significant differences for the secondary outcomes.
Compared to the landmark guided techniques for subclavian venous access, the puncture of the vein in most long-axis ultrasound guided procedures is more lateral. In a position lateral to the outer border of the first rib the vessel is correctly referred as axillary vein8. In our manuscript, we therefore use the term infraclavicular axillary vein cannulation.
One patient in the non-echogenic needle group suffered pneumothorax during axillary vein cannulation and was treated with a chest tube. The arterial puncture frequency was equally distributed in both study groups (three per group). It can be argued that the use of echogenic needles could prevent pleural puncture. However, the number of 50 patients per study group and the occurrence of a single complication do not allow a valid conclusion to be drawn. Hence, it remains unclear whether or to what degree echogenic needles can improve safety.
Nichols et al. investigated the effect of different needle angles on needle visibility using an in-plane needle approach9. The authors found that both echogenic and non-echogenic needles were easily visualized at 90° angles of insonation (with a needle insertion perpendicular to the ultrasound beam). For a steep needle approach with insonation, angles between 35° and 15° echogenic needles were superior. Hence, the angle of needle insertion relative to the ultrasound beam affects the general visibility of the needle and thereby the possible benefit that can be obtained by echogenic coating. Using an in-plane approach with a linear transducer for infraclavicular axillary vascular access, the needle insertion point is rather lateral, resulting in higher insonation angles (usually above 35°). This might explain why echogenic needles have not proven favorable in our study.
In obese patients, lower insonation angles and a generally more difficult sonoanatomy is found10. While the average weight of our patients was 77 kg in both groups, a study in a more obese population might potentially show superior results for the echogenic needle.
So far, only a few studies have investigated the effect of echogenic needles for vascular access6,7,11. Crum and colleagues compared echo-enhanced needles with standard needles in vascular access phantoms11. The authors found a decreased incidence of posterior wall punctures for both in-plane and out-of-plane procedures when echo-enhanced needles were used. However, they found no significant differences between the echo-enhanced needles and standard needles according to first-pass success, visibility of the needle tip at the time of puncture, total number of attempts, or number of redirections. The visibility of echogenic needles has been investigated in two studies by Stefanidis et al.6,7. While an in-plane approach was used for subclavian vein catheterization, an out-of-plane approach was used for internal jugular vein catheterization. They reported that the use of echogenic needles significantly improved cannula visibility and decreased access time. These results are in contrast to the findings in our present study.
The above-mentioned studies have been conducted in more than a decade ago. Since then, ultrasound technology has continuously improved12,13. Modern ultrasound units with an improved image quality facilitate the visualization and needle identification in the 2D ultrasound image. This could explain a more pronounced effect of echogenic needles in older studies.
Echogenic needles are commonly used for ultrasound-guided peripheral nerve blocks13. The use of echogenic needles reduced procedure time and patient discomfort compared with a nerve stimulating catheter system14. In a systematic review, Hovgesen et al., demonstrated that echogenic needles improved needle visualization by ultrasound images compared with standard needles15. However, neither time consumption, success rate, or other parameters indicating needle control were evaluated.
The negative results of our study refer to 18 G needles that are commonly used for central venous access. Echogenic coating could make a difference in smaller needles, e.g. 21 G needles used for peripheral nerve blocks, which are more difficult to visualize by ultrasound. Further, our results refer to ultrasound-guided infraclavicular axillary CVC placement using an in-plane approach in a peri-operative situation and should not be transferred to other contexts such as placement of CVC with an out-of-plane approach or in emergency situations.
The results from the four consultant anesthesiologists performing venous cannulation in our study showed a considerable spread while the differences in venous access time almost reached the expected difference of 30% used for the sample size calculation. Venous access time ranged from 6 to 440 s and 6 to 925 s for the echogenic needle and the control group respectively. Hence, a size might sample size of 100 patients might have been too small to provide a firm conclusion.
Besides the use of echogenic needles, several needle-tracking technologies have been introduced to enhance the visualization of injection needles4. These technologies are based on magnetic field generators, camera technology, or piezoelectric sensors registration16,17,18,19. Needle-tracking technologies have shown a greater benefit for out-of-plane procedures compared with in-plane approaches4,19. It is possible that echogenic needle coating is more beneficial for out-of-plane procedures. Of note, studies on ultrasound guided short-axis out-of-plane subclavian vein cannulation has shown higher success rates and less complications compared with a long-axis in-plane procedure in a previous study in cardiac surgical patients20.
The single center study design and the limited sample size are the most important limitations of our study. Complication rates associated with ultrasound-guided vascular access procedures are relatively low. It would therefore require a large number of patients to discover significant differences in safety parameters. Further, all operators in our study had some experience in ultrasound-guided procedures. Yet, echogenic needles might be more beneficial among less experienced colleagues. Many needle tracking technologies are apparently more beneficial for novices21,22. Some heterogeneity of the ultrasound skills among the operators in our study might have masked the impact of echogenic needles.
For practical and logistical reasons, only four anesthesiologists performed ultrasound-guided procedures. Including more operators in our study may have increased generalizability. However, the results may have been even more heterogeneous (with higher standard deviations), requiring a much larger sample size to demonstrate significant differences between the study groups.
Venous access time, defined as the time from insertion of the needle (skin puncture) until aspiration of venous blood in the syringe, was the main outcome in this study. Other outcomes like the number of attempts, success in first attempt, number of skin punctures, or numbers of adverse events might be clinically more relevant. However, procedural time has previously been considered an appropriate proxy marker for needle control in many studies23,24,25. The outcome has been used as a surrogate measure for the degree of skill and ease in manual performance. A short procedural time combined might indicate better needle control and increased safety19,26. It can also be assumed that increased number off attempts or skin punctures are having an immediate effect on the procedure time.
It can be questioned whether subjective experiences (quality of needle visualization, ease of needle placement) are relevant outcomes in an interventional study. Subjective outcomes might have a greater risk of bias and do not necessarily reflect the real effect of an intervention. Of note, no significant differences were found for the quality of needle visualization and ease of needle placement between the two groups in the present study.
In this study, echogenic needles did not significantly improve needle control or safety when used for infraclavicular axillary vein cannulation with an in-plane needle approach. The results indicate that standard needles are appropriate for ultrasound guided infraclavicular axillary vascular access in a perioperative situation. Studies with larger sample sizes should be performed to confirm these findings.
Methods
This randomized controlled trial was conducted between 12/01/ 2022, and 23/07/2022, at Oslo University Hospital. Ethical approval was provided by the Committee for Medical Research Ethics, Region South-East, Oslo, Norway, on 04/02/2020 (Ethical Committee Number 85090). The study was registered at ClinicalTrials.gov (NCT05045352) first posted 16/09/2021 and complied with the declaration of Helsinki. Good clinical practice was followed throughout the study. All patients gave written informed consent.
Participants
One hundred patients aged 18 years or more undergoing a surgical procedure with the requirement of a central venous catheter, were included in the study. Participants had to speak and understand Norwegian language to be included in the study. Written informed consent was obtained from all the participants. Skin disease or infection affecting the whole-body surface or the area of examination, untreated coagulopathy, and known vascular abnormality were exclusion criteria.
Procedure
All patients received general anesthesia with endotracheal intubation. Standard monitoring included a pulse oximeter, non-invasive or invasive blood pressure monitor, and electrocardiography. Trendelenburg position was applied before central venous catheterization. Four consultant anesthesiologists performed the venous access in the study. All the anesthesiologists had solid experience in ultrasound guided procedures and regularly performed central venous access. Before participating in the study, they had to perform at least 20 ultrasound guided subclavian catheterizations with an in-plane needle approach in a standardized way as defined in the study protocol.
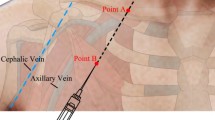
A Venue Go™ ultrasound system (GE HealthCare, Mexico) with a linear array L4-12T transducer was used for ultrasound guided needle placement. The transducer was placed above the pectoral muscles, caudally and close to the clavicle. During a pre-scan the axillary vein, axillary artery and the surrounding anatomy were visualized. The depth from the skin to the superficial border of the axillary vein was then registered. Aseptic preparation included a skin scrub with chlorhexidine 0.5%, a sterile transducer cover, and sterile ultrasound gel (Mikrotek Medical Inc, St Paul, US). Vascular access was performed with an in-plane needle approach and a long axis visualization of the axillary vein (Fig. 3 and supplemental video 1). After venous puncture and blood aspiration a guidewire was advanced into the infraclavicular axillary vein. Using Seldinger technique, a three-lumen central venous catheter (Arrow Multi-Lumen Central Venous Catheterization Set, Teleflex, US) was inserted.
Vascular access of the infraclavicular axillary vein was performed with an in-plane needle approach and a long axis visualization of the axillary vein. Echogenic and non-echogenic and standard needles were used according to randomized allocation. The figure shows a infraclavicular axillary cannulation with an echogenic needle (VascularSono 18 G × 70 mm; Pajunk GmbH, Germany).
Needles
Both echogenic needles (VascularSono 18 G × 70 mm) and standard needles (Vascular cannula 18 G × 70 mm) were manufactured by Pajunk® (Pajunk GmbH, Germany). The echogenic needles had a cornerstone geometry embossed into the needle surface over a length of 20 mm at the distal part of the needle. Standard and echogenic needles had the same appearance and could not be distinguished without close and thorough examination.
Randomization and blinding
The study had a prospective, randomized controlled study design. Patients were allocated in two groups of equal size by using a list of random numbers, according to the Moses–Oakford algorithm27. Block randomization was performed with block sizes of ten to ensure equal numbers of echogenic and non-echogenic procedures for each anesthesiologist. The block size was not known to the investigators. Sealed opaque envelopes with sequential numbering were prepared by a staff member not involved in the further conduct of the study, containing either a standard or an echogenic needle according to randomized allocation. The envelopes were opened by an assistant before the vascular access procedure. Hence, the anesthesiologist performing the procedures was initially blinded for randomization until eventually recognizing cornerstone geometry during ultrasound visualization of the needle. Observers measuring outcome variables were blinded for randomized allocation and could not see the ultrasound image during the procedure.
Outcome and assessments
The primary outcome was access time defined as the time from insertion of the needle (skin puncture) until aspiration of venous blood. Secondary outcomes were total procedure time defined as the time from insertion of the needle until the central venous catheter had been advanced over the guidewire. Other secondary outcomes were success in first needle attempt, number of needle attempts, number of skin punctures, change of site for vascular access, successful catheter placement evaluated by x-ray, adverse events, and the subjective experience of the anesthesiologist of the needle visualization, as well as the performance of the needle procedure. The consecutive measurements of the access time were analyzed to evaluate a possible learning curve and carryover effect for each anesthesiologist.
Measurement of access time was done with a stopwatch by an observer unaware of the needle type used for the procedure. The time used for the pre-scan was not included. The number of needle attempts was counted by the observer. Each time the needle was withdrawn at least 0.5 cm and consecutively reinserted a needle attempt was registered. The number of skin punctures were also recorded. We defined success in first attempt as a single skin puncture and successful venous access.
Immediately after performing the central venous access procedure the anesthesiologists were asked to evaluate the quality of the visualization of the needle. A Numeric Rating Scale (NRS) from 0 to 10 was used for graduation, where 10 signified very good needle visualization, and 0 indicated that the needle was not possible to visualize. They were also asked to grade the ease of the needle placement using a NRS (10 = easy needle placement, 0 = not possible to place the needle).
All adverse events, including intra-arterial puncture, hematoma or pneumothorax were recorded. Chest x-rays were performed after the surgical procedure to verify the catheter placement and exclude pneumothorax.
Statistical analysis
Statistical analysis was done using Stata/SE V.15.1 (StataCorp LLC, College Station, TX, US). The predefined main outcome variable was venous access time. Based on a previous study on ultrasound guided subclavian central venous catheter a standard deviation of 50% could be expected6. We considered a reduction of 30% in access time as clinically significant. A sample size of 90 patients would be sufficient to detect a difference in means of 6 s, assuming average time consumptions of 20 s (mean1) and 14 s (mean2) and a common standard deviation of 10 s using a one-sample t-test with alpha 0.05 and beta 0.8. To allow for missing data or dropouts, 100 patients were included.
The data was tested for normal distribution using Q–Q plots and histograms. Non-normal distributed data (access time, total procedure time, number of skin punctures, number of redirections, experience of needle visualization, and ease of needle placement) were presented with median, interquartile range (IQR), and range. Wilcoxon rank sum test was used to analyze these data. Continuous data (baseline characteristics) were presented with means and standard deviation and ANOVA was used for data analysis. Binary outcomes (success in the first attempt, change of site for vascular access, correct catheter placement evaluated by x-ray) were presented as counts, ratios, and percentages while Chi square tests were used for analysis. Linear regression analysis was used to assess each anesthesiologist learning curves and carryover effect. Group allocation blinding was broken after the completion of the statistical analysis.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Rezayat, T. et al. Ultrasound-guided cannulation: Time to bring subclavian central lines back. West. J. Emerg. Med.17(2), 216–221. https://doi.org/10.5811/westjem.2016.1.29462 (2016).
Brass, P., Hellmich, M., Kolodziej, L., Schick, G. & Smith, A. F. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst. Rev.1(1), 006962. https://doi.org/10.1002/14651858.CD006962.pub2 (2015).
Brusasco, C. et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes. Surg.19(10), 1365–1370. https://doi.org/10.1007/s11695-009-9902-y (2009).
Scholten, H. J., Pourtaherian, A., Mihajlovic, N., Korsten, H. H. M. & Bouwman, R. A. Improving needle tip identification during ultrasound-guided procedures in anaesthetic practice. Anaesthesia72(7), 889–904. https://doi.org/10.1111/anae.13921 (2017).
Wiesmann, T. et al. Compound imaging technology and echogenic needle design: Effects on needle visibility and tissue imaging. Reg. Anesth. Pain Med.38(5), 452–455. https://doi.org/10.1097/AAP.0b013e31829730d5 (2013).
Stefanidis, K. et al. Optimization of cannula visibility during ultrasound-guided subclavian vein catheterization, via a longitudinal approach, by implementing echogenic technology. Crit. Care Res. Pract.2012, 617149. https://doi.org/10.1155/2012/617149 (2012).
Stefanidis, K. et al. Echogenic technology improves cannula visibility during ultrasound-guided internal jugular vein catheterization via a transverse approach. Crit. Care Res. Pract.2012, 306182. https://doi.org/10.1155/2012/306182 (2012).
Johnson, D. Pelvic girdle, shoulder region and axilla. In Gray’s Anatomy: The Anatomical Basics of Clinical Practice 40th edn (ed. Standring, S.) 818 (Churchill Livingstone, Edinburgh, 2008).
Nichols, K., Wright, L. B., Spencer, T. & Culp, W. C. Changes in ultrasonographic echogenicity and visibility of needles with changes in angles of insonation. J. Vasc. Interv. Radiol.14(12), 1553–1557. https://doi.org/10.1097/01.rvi.0000099527.29957.a6 (2003).
Pépin, J. L. et al. Prevention and care of respiratory failure in obese patients. Lancet Respir. Med.4(5), 407–418. https://doi.org/10.1016/s2213-2600(16)00054-0 (2016).
Crum, T., Adhikari, S., Lander, L. & Blaivas, M. Do echo-enhanced needles make a difference in sonographically guided vascular access?. J. Ultrasound Med.33(4), 623–628. https://doi.org/10.7863/ultra.33.4.623 (2014).
Ortiz, S. H. C., Chiu, T. & Fox, M. D. Ultrasound image enhancement: A review. Biomed. Signal Process. Control7(5), 419–428 (2012).
Sen, S. et al. Recent technological advancements in regional anesthesia. Best Pract. Res. Clin. Anaesthesiol.33(4), 499–505. https://doi.org/10.1016/j.bpa.2019.07.002 (2019).
Brookes, J. et al. Comparative evaluation of the visibility and block characteristics of a stimulating needle and catheter vs an echogenic needle and catheter for sciatic nerve block with a low-frequency ultrasound probe. Br. J. Anaesth.115(6), 912–919. https://doi.org/10.1093/bja/aev351 (2015).
Hovgesen, C. H., Wilhjelm, J. E., Vilmann, P. & Kalaitzakis, E. Echogenic surface enhancements for improving needle visualization in ultrasound: A PRISMA systematic review. J. Ultrasound Med.41(2), 311–325. https://doi.org/10.1002/jum.15713 (2022).
Umbarje, K., Tang, R., Randhawa, R., Sawka, A. & Vaghadia, H. Out-of-plane brachial plexus block with a novel SonixGPS(TM) needle tracking system. Anaesthesia68(4), 433–434. https://doi.org/10.1111/anae.12213 (2013).
Gadsden, J., Latmore, M. & Levine, D. M. Evaluation of the eZono 4000 with eZGuide for ultrasound-guided procedures. Expert Rev. Med. Devices12(3), 251–261. https://doi.org/10.1586/17434440.2015.995095 (2015).
Najafi, M., Abolmaesumi, P. & Rohling, R. Single-camera closed-form real-time needle tracking for ultrasound-guided needle insertion. Ultrasound Med. Biol.41(10), 2663–2676. https://doi.org/10.1016/j.ultrasmedbio.2015.05.016 (2015).
Kasine, T. et al. Needle tip tracking for ultrasound-guided peripheral nerve block procedures-an observer blinded, randomised, controlled, crossover study on a phantom model. Acta Anaesthesiol. Scand.63, 1055–1062. https://doi.org/10.1111/aas.13379 (2019).
Vezzani, A. et al. A randomized clinical trial of ultrasound-guided infra-clavicular cannulation of the subclavian vein in cardiac surgical patients: short-axis versus long-axis approach. Intensive Care Med.43(11), 1594–1601. https://doi.org/10.1007/s00134-017-4756-6 (2017).
McLeod, G. A. et al. An initial evaluation of the effect of a novel regional block needle with tip-tracking technology on the novice performance of cadaveric ultrasound-guided sciatic nerve block. Anaesthesiahttps://doi.org/10.1111/anae.14851 (2019).
McVicar, J., Niazi, A. U., Murgatroyd, H., Chin, K. J. & Chan, V. W. Novice performance of ultrasound-guided needling skills: Effect of a needle guidance system. Reg. Anesth. Pain Med.40(2), 150–153. https://doi.org/10.1097/AAP.0000000000000209 (2015).
Johnston, D. F. & Stafford, M. Dominant hand operating probe vs needle: A comparison study of ultrasound-guided needle placement in phantom models. Anaesthesia70(8), 969–974. https://doi.org/10.1111/anae.13070 (2015).
Marhofer, P. & Fritsch, G. Safe performance of peripheral regional anaesthesia: The significance of ultrasound guidance. Anaesthesia72(4), 431–434. https://doi.org/10.1111/anae.13831 (2017).
Koscielniak-Nielsen, Z. J., Stens-Pedersen, H. L. & Lippert, F. K. Readiness for surgery after axillary block: Single or multiple injection techniques. Eur. J. Anaesthesiol.14(2), 164–171 (1997).
Kåsine, T. et al. Ultrasonographic needle tip tracking for in-plane infraclavicular brachialis plexus blocks: A randomized controlled volunteer study. Reg. Anesth. Pain Med.45(8), 634–639. https://doi.org/10.1136/rapm-2020-101349 (2020).
Meinert, C. L. Clinical Trials: Design (Oxford University Press, 1986).
Acknowledgements
We want to thank Kristin Lühr Villa and Anders Steigan Aasheim at The Department of Critical Care and Emergency medicine, Oslo University Hospital, Oslo; Norway for their support with observations, logistics and practical arrangement for our study.
Author information
Authors and Affiliations
Contributions
T.K., A.R.S. and L.A.R. planned and designed the study. T.K. and A.R.S. collected and screened the data. H.T.L., L.G., M.M. and R.S. included patients and perfomed the procedure. T.K. and A.R.S. performed statistical analysis and calculations. T.K., L.A.R., M.M., H.T.L., L.G., R.S. and A.R.S. wrote and edited the manuscript. A.R.S. and T.K. designed the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kåsine, T., Rosseland, L.A., Myhre, M. et al. Echogenic needles versus non-echogenic needles for in-plane ultrasound-guided infraclavicular axillary vein cannulation, a randomized controlled trial. Sci Rep 14, 22258 (2024). https://doi.org/10.1038/s41598-024-72620-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72620-8