Abstract
The association between vitamin D concentrations and the occurrence of diabetic foot ulcers (DFUs) remains a topic of ongoing debate. In order to provide a comprehensive and updated review, we conducted this meta‐analysis to further investigate the relationship between vitamin D concentrations and DFUs occurrence. The following databases, including Cochrane Library, EMBASE, Web of Science, PubMed, CBM, CNKI, WANFANG DATA and VIP Database, were systematically searched for studies published up to Dec. 20th, 2023. The combined estimation was calculated using both fixed-effects and random‐effects models. The overall effect size was reported as a weighted mean difference (WMD) with a corresponding 95% confidence interval (95%CI). Data analysis was performed utilizing Review Manager 5.4 and Stata 14. The Protocol has been registered in PROSPERO CRD42024503468. This updated meta-analysis, incorporating thirty-six studies encompassing 11,298 individuals with or without DFUs, demonstrated a significant association between vitamin D deficiency/insufficiency and an elevated risk of DFUs occurrence (< 25 nmol/L, OR 3.28, P < 0.00001; < 50 nmol/L, OR 2.25, P < 0.00001; < 75 nmol/L, OR 1.67, P = 0.0003). Vitamin D concentrations were significantly lower in individuals with DFUs compared to those without DFUs (P < 0.00001). Subgroup analyses consistently demonstrated this trend among the older population (> 50 years, P < 0.00001), individuals with long duration of diabetes (> 10 years, P < 0.00001), and those with poor glycemic control (mean HbA1c 8%-9% and > 9%, P < 0.00001).
Similar content being viewed by others
Introduction
Current research indicates that the occurrence of diabetic foot ulcers (DFUs) isnot only associated with traditional factors, such as diabetes‐related peripheral neuropathy (DPN), peripheral arterial disease (PAD), and/or foot deformity1,2, but also with the circulating levels of micronutrients3. In recent years, there has been growing attention towards the role of vitamin D insufficiency and deficiency in DFUs4. The reasonable assumption that vitamin D status may influence the risk of DFUs occurrence is supported by emerging evidence suggesting potential involvement of vitamin D in DPN5,6 and PAD7,8, which are significant contributors to the development of DFUs1,2. Furthermore, pre-clinical studies also provide supporting evidence for a potential role of vitamin D in wound healing9,10.
However, the findings from observational studies have yielded inconsistent results. Recently, two meta-analyses11,12 have reported an inverse association between vitamin D status and DFUs. Nevertheless, these analyses may be subject to potential limitations and were underpowered. Firstly, the incorporation of Chinese-language studies has been constrained, resulting in a limited number of included studies and sample size. Secondly, considering the variability in disease states and objectives, such as osteoporosis, rickets and osteomalacia, vitamin D deficiency is defined as less than 25 nmol/L, 30 nmol/L or 50 nmol/L13. Given the inherent ambiguity surrounding the precise definition of vitamin D deficiency, it is imperative to conduct additional comprehensive investigations to elucidate the potential association between vitamin D deficiency/insufficiency and DFUs. Moreover, despite the presence of a high degree of heterogeneity among studies, sensitivity analysis and subgroup analysis were not conducted, potentially leading to the oversight of significant confounding factors. Additionally, neither of these two meta-analyses provided any information regarding the detection method for vitamin D.
Therefore, it is imperative to recommence this analysis with more rigorous and detailed protocol. Moreover, we assert that our research is more comprehensive and representative owing to the utilization of larger and updated data.
Methods
Protocol and registration
This study presents a systematic review incorporating a meta-analysis conducted in accordance with the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines14 and registered in PROSPERO (CRD42024503468). The reporting adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines15.
Data sources and searches
A comprehensive search was conducted by an experienced researcher (XR) across multiple databases, including Cochrane Library (Cochrane), EMBASE, Web of Science (WOS), PubMed, Chinese BioMedical Literature Database (CBM), CNKI, WANFANG DATA and VIP Database from inception until December 20th, 2023. The search was not limited by language restrictions. Additionally, the WHO International Clinical Trials Registry Platform was consulted to identify ongoing or unpublished potentially eligible trials that met the potential eligibility criteria. In order to ensure a comprehensive search for relevant articles, we manually examined the reference lists of identified trials and systematic reviews. The detailed search strategy can be found in Supplementary Table 1.
Study selection
The eligible studies were independently screened by two authors (WT and DC). In the event of any discrepancies, a consensus was achieved through thoroughly discussion. If an agreement could not be reached, a third experienced researcher (XR) was consulted. In accordance with the PECO strategy, we applied the following inclusion criteria: (1) Participants: adults (age ≥ 18 years) diagnosed with diabetes, irrespective of the presence or absence of DFUs; (2) Exposure: serum vitamin D concentrations (nmol/L or ng/ml) and categorization into deficient, insufficient and normal levels among study participants; (3) Comparison: the prevalence rate for vitamin D deficiency/insufficiency or variations in its levels by considering the presence or absence of DFUs; (4) Outcome: the prevalence of vitamin D deficiency/insufficiency or difference in levels of vitamin D; (5) Cross‐sectional, cohort, or case‐control studies without language or publication status restrictions. Case reports, case series, review articles, qualitative studies, and animal studies were excluded. Additionally, studies with incomplete data, incompatible formats, or non-convertible data were also excluded. The study eligibility assessment was conducted by two authors (WT and DC), with conflicts resolved through discussion with a third author (XR).
Data extraction and quality assessment
We extracted the data using predefined tables. The following information was recorded for each study: first author, year of publication, country where the study was conducted, study design, source of vitamin D in the samples, method used to determine vitamin D levels, sample size, gender distribution, vitamin D levels, age, body mass index (BMI), and HbA1c in both case and control groups. Continuous variables are depicted as mean and standard deviation (SD), categorical variables are depicted as number and percentages. For continuous variables expressed as median and interquartile range (IQR), we converted the data according to the preset method16,17,18,19, and employed estimated mean and standard deviation (SD) for the final quantitative synthesis. Study quality was assessed using the Newcastle–Ottawa quality assessment scale (NOS)20.
Data synthesis and statistical analysis
Continuous variables were pooled using the inverse variance random-effects model and were presented as mean differences with 95% CIs. Dichotomous variables were pooled using the Mantel–Haenszel method and presented as odds ratios (ORs) with 95% CI. The heterogeneity among the included studies was assessed using I2 statistics. According to the results of heterogeneity analysis, appropriate pooled methods were selected: a random‐effects model was employed if I2 > 50%, while a fixed‐effects model was adopted if I2 ≤ 50%. We evaluated the presence of publication bias through visual inspection of funnel plots and both Egger’s and Begg’s linear regression test when more than ten studies were available. To examine potential sources of heterogeneity, several a priori determined subgroup analyses were performed according to age, duration of diabetes and Hemoglobin A1c (HbA1c). Additionally, we used meta-regressions to explore potential confounders that may affect vitamin D concentrations. All data analysis was performed using Review Manager 5.4 and Stata 14, with a significance level set at 0.05.
Results
Literature search results
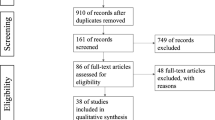
According to the search strategy, a total of 950 citations were initially identified from the eight databases and other sources. After removing duplicate studies (n = 302), we screened 648 potentially eligible records. Subsequently, based on title and abstract screening, 592 studies were excluded. Finally, 36 eligible studies were included in the meta-analysis. The selection process is visually depicted in Fig. 1 using a PRISMA flow diagram.
Study characteristics and quality assessment
The summarized characteristics of the included trials are presented in Table 1. All studies conducted between 2009 and 2023 were of an observational nature. A total of 11,298 participants were enrolled, with 3,450 cases in the DFU group and 7,848 cases in the non-DFU group. Sample sizes ranged from 26 to 4,284 with two studies4,21 surpassing a sample size of over 1,000 participants. Out of the 36 studies included in this meta‐analysis, 31 were conducted in Asia (20 in China, 7 in India, 2 in Iran, 1 each in Indonesia and Jordan), while 4 were carried out across Europe (located respectively in Belarus, Bulgaria, Germany and Greece), and one study was conducted in North America (in the USA). Serum concentrations of 25-Hydroxyitamin D were measured across all thirty-six studies, and reported as either nmol/L or ng/ml (1 ng/mL = 2.5 nmol/L). In eighteen studies, vitamin D status of patients was categorized and prevalence rates were reported for each category. Seven different detection methods for the determination of vitamin D were recorded. The quality assessment of the included studies is presented in Supplementary Table 2.
Variations in vitamin D concentrations and their impact on DFUs
Given the lack of consensus regarding the definition of vitamin D deficiency or insufficiency, we classified vitamin D concentrations into three distinct thresholds based on available data from the included studies, namely vitamin D < 25 nmol/L as Grade A, vitamin D < 50 nmol/L as Grade B and vitamin D < 75 nmol/L as Grade C.
Seven studies yielded data on vitamin D concentrations below 25 nmol/L, with 253 (45.59%) individuals with DFUs and 122 (20.37%) individuals with both diabetes and non-DFUs having concentrations of vitamin D below this threshold. The findings demonstrate a significant association between vitamin D concentrations below 25 nmol/L and the risk of developing DFUs (OR 3.28, 95% CI [2.52, 4.27], P < 0.00001; I2 = 0%, P-heterogeneity = 0.43) (Fig. 2A). As depicted in Fig. 2B, a total of 1545 (77.10%) patients with DFUs and 4317 (65.56%) individuals without DFUs exhibited vitamin D concentrations below 50 nmol/L across thirteen studies, which was also associated with an elevated risk of DFUs (OR 2.25, 95% CI [1.80, 2.80], P < 0.00001; I2 = 55%, P-heterogeneity = 0.009) (Fig. 2B). The absence of publication bias was indicated by the funnel plot as well as the Egger and Begg tests (Egger P = 0.252, Begg P = 0.246) (Supplementary Fig. 2B). In relation to vitamin D concentrations below 75 nmol/L, a total of ten studies involving 253 (45.59%) patients in the DFUs group and 122 (20.37%) patients in the non-DFUs control group were identified. Notably, a statistically significant disparity persisted in the incidence of DFUs between these two groups (OR 1.67, 95% CI [1.26, 2.20], P = 0.0003; I2 = 21%, P-heterogeneity = 0.25) (Fig. 2C). The analysis of bias tests using funnel plots, Egger and Begg tests did not reveal any significant publication bias (Egger P = 0.085, Begg P = 0.283) (Supplementary Fig. 2C).
Vitamin D concentrations and DFUs
As depicted in Fig. 3A, the meta-analysis employed a random-effects model to analyze all 36 studies. The findings demonstrated a significant reduction in serum vitamin D levels among patients with DFUs compared to individuals without DFUs (WMD = − 10.80, 95% CI [− 11.89, − 9.71], P < 0.00001). The heterogeneity tests indicated substantial heterogeneity among the included studies (I2 = 88%, P-heterogeneity < 0.00001). The absence of asymmetry observed in the funnel plot (Fig. 3B) analysis suggests no evidence of publication bias. Furthermore, both the Egger test (P = 0.44) and Begg test (P = 0.205) did not identify any significant small study effects.
The heterogeneity observed in this study was not attributed to any individual study, and a total of eight studies21,24,25,31,35,39,40,55 with a high risk of bias were identified. However, excluding these eight studies that contributed the most to between-study heterogeneity did not significantly alter the results (WMD = − 10.93, 95% CI [− 12.20, − 9.65], P < 0.00001). Furthermore, this exclusion led to a substantial reduction in heterogeneity (I2 = 52%, P-heterogeneity = 0.0007) (Supplementary Fig. 3).
Subgroup analysis
Subgroup analysis was conducted to evaluate the potential interaction between vitamin D and other factors.
Initially, variations in the association between vitamin D and DFUs were observed across different age groups. Among studies with a mean age of 50 years or younger, there was no statistically significant difference in vitamin D concentrations between patients with DFUs and individuals without DFUs (WMD = − 18.77, 95% CI [− 38.77, 1.23], P = 0.07; I2 = 99%, P-heterogeneity < 0.00001). However, in individuals aged over 50 years, the DFUs group exhibited significantly lower vitamin D concentrations compared to the non-DFUs group. Notably, heterogeneity decreased across studies without reaching statistical significance for interaction (WMD = − 10.95, 95% CI [− 11.77, − 10.13], P < 0.00001; I2 = 70%, P-heterogeneity < 0.00001; P-interaction = 0.44) (Supplementary Fig. 4).
The results demonstrated robustness and consistency across various subgroups based on the duration of diabetes, thereby highlighting their generalizability. Furthermore, a notable reduction in heterogeneity was observed specifically within the subgroup characterized by an average diabetes duration exceeding 10 years (WMD = − 10.48, 95% CI [− 13.31, − 7.64], P < 0.00001; I2 = 60%, P-heterogeneity = 0.008) (Supplementary Fig. 5).
Considering the potential interaction between vitamin D concentrations and glycemic control, we further stratified mean HbA1c values into three subgroups (≤ 8%, 8–9% or > 9%). Upon comparing these three subgroups, no significant difference in vitamin D concentrations was observed in patients with or without DFUs when mean HbA1c was less than or equal to 8% (mean HbA1c ≤ 8%, WMD = − 2.06, 95% CI [− 4.18, 0.06], P = 0.06; I2 = 0%, P-heterogeneity = 0.84). However, the vitamin D concentrations were significantly lower in the DFUs group compared to the non-DFUs group, when mean HbA1c values were ranging from 8 to 9% (mean HbA1c 8%-9%, WMD = − 13.63, 95% CI [− 16.51, − 10.76], P < 0.00001; I2 = 89%, P-heterogeneity < 0.00001). In subgroup analysis involving individuals with mean HbA1c values greater than 9%, those with DFUs exhibited significantly lower vitamin D concentrations compared to those without DFUs, and heterogeneity between studies was reduced (mean HbA1c > 9%, WMD = − 9.38, 95% CI [− 10.37, − 8.39], P < 0.00001; I2 = 0%, P-heterogeneity = 0.51). Furthermore, a significant interaction was observed among the three subgroups based on mean HbA1c values (P-interaction < 0.00001) (Supplementary Fig. 6).
Additionally, the meta-regression analysis investigating the influence of geographical location, gender, BMI and research quality on the risk of DFUs did not yield statistically significant results.
Discussion
This meta-analysis of thirty-six observational studies, encompassing patients with or without DFUs, revealed a significant correlation between vitamin D insufficiency and deficiency, and an elevated risk of developing DFUs. Furthermore, lower serum concentrations of vitamin D were found to be associated with an increased susceptibility to DFUs. Additionally, our meta-analysis found a significant association between low serum vitamin D levels and the presence of DFUs. This association was particularly pronounced in older patients with longer diabetes duration and poorer glycemic control.
Although several single-center randomized controlled trials (RCTs)56,57,58,59,60 with a limited sample size have been conducted, the current evidence on this topic primarily relies on observational studies. Therefore, it is important to emphasize that our findings do not necessarily establish a direct causal relationship between vitamin D and DFUs. It is worth noting that there are two evident confounding factors that imped causal inference. Firstly, it is well-established that vitamin D synthesis primarily occurs in the skin following exposure to ultraviolet B radiation from sunlight61. Therefore, engaging in appropriate outdoor activities becomes crucial for maintaining normal serum vitamin D levels. The decline in outdoor activities among patients with DFUs and those at high risk cannot be overlooked, as it may contribute to the observed low serum vitamin D levels. Secondly, a significant inverse association has been reported between serum vitamin D and obesity62, which is particularly relevant considering the majority of type 2 diabetic patients are overweight or obese. However, numerous significant potential mechanisms revealed by preclinical studies may hypothetically elucidate the augmented risk of DFUs in individuals with both diabetes and low vitamin D concentrations. Firstly, vitamin D plays a pivotal role in regulating the proliferation, migration, and differentiation of keratinocytes. In addition to serving as the primary source of vitamin D, the keratinocytes in the epidermis and hair follicle express all the necessary enzymes for vitamin D metabolism63. Conditional VDR knockout mice exhibit impaired self-renewal, activation, and migration of epidermal stem cells due to inhibition of β-catenin signaling, resulting in delayed re-epithelialization and wound closure64. Furthermore, topical application of vitamin D has been demonstrated to enhance wound healing65. Additionally, vitamin D plays a pivotal role in modulating immune and inflammatory responses. Supplementation with vitamin D can attenuate the expression of proinflammatory cytokines such as IL-6, IL-1β, MCP-1 and TNFα by inhibiting AMPK activation and nuclear NF-κB phosphorylation66. On the contrary, the normal inflammatory response is facilitated through interaction with the TGF-β signaling pathway and enhancement of VDR-Smad3 complex formation67. Besides, vitamin D exerts its influence on the innate immune system by impacting antigen-presenting cells (APC) and inducing expression of antimicrobial peptides (AMPs)68, while also regulating adaptive immunity by modulating the activities of T-helper cells, T-regulatory cells and B cells69. Thirdly, it can enhance angiogenesis by upregulating the expression of pro-angiogenic factors such as vascular endothelial growth factor A, hypoxia-inducible factor-1α and angiogenin70. Moreover, vitamin D deficiency may contribute to clinical diabetic small fibre neuropathy through its impact on nerve growth factor (NGF), which governs phenotype and sensitivity of nociceptor fibres71, while chronic administrations of vitamin D has been shown to alleviate behavioral scores associated with neuropathic pain in rats72. These mechanisms substantiate the ongoing interest among clinical researchers in exploring the relationship between vitamin D and DFUs, thereby highlighting the increasing attention garnered by the clinical significance of vitamin D supplementation in DFUs. Therefore, despite the potential influence of various factors such as outdoor activities and obesity on serum vitamin D levels in patients with DFUs, pertinent data from preclinical investigations still substantiate the plausible causal roles of vitamin D in the pathophysiology of DFUs.
However, the current available observational studies on vitamin D and DFUs have yielded inconsistent findings. While the majority of studies indicated lower vitamin D concentrations in patients with DFUs compared to those without DFUs, a minority of others conducted in India27, Greece47 and China31,73 reported no statistically significant difference. Interestingly, a cross-sectional study conducted in Iran22 even yielded conflicting findings. Notably, in 2019, Jiezhi Dai et al.74 performed the inaugural meta-analysis on this subject matter, encompassing seven studies involving 1,115 patients. Their analysis revealed a significant decrease in vitamin D levels among patients with DFUs (MD − 13.47 nmol/L, 95% CI [− 16.84, − 10.10], P < 0.00001; P-heterogeneity = 0.34, I2 = 12%) and established an association between severe vitamin D deficiency and an elevated risk of DFUs (OR 3.22, 95% CI [2.42 − 4.28], P < 0.00001; P-heterogeneity = 0.64, I2 = 0%). However, the limited number of included studies and inadequate sample size of researched objects constrain the strength of their evidence. In recent years, two similar meta-analyses have been reported11,12. However, regrettably, neither of them encompassed studies published in Chinese. Moreover, studies yielding non-significant results27,31,73 were also excluded, potentially introducing bias. Additionally, the lack of standardization in the units used for vitamin D measurements in these two reviews may potentially impact the reliability of results. For instance, Xin Li et al.’s11 meta-analysis published in 2023 reported vitamin D concentrations as ng/ml in six out of fifteen included studies26,39,41,44,45,47, while others utilized nmol/L. Furthermore, in another meta-analysis conducted by Juan Lin et al.12 during the same year, there were inaccuracies in evaluating sample sizes for two of twelve studies included55,75. Lastly, the high heterogeneity observed across studies highlighted by both reviews was not addressed through subgroup or meta-regression analysis. This oversight may have overlooked significant potential confounding factors.
There are two significant distinctions between previous reports and our study. Firstly, we conducted a comprehensive evaluation of all relevant studies published in Chinese, which were included in the current analysis. Additionally, we incorporated three recently published studies from Oct 2023 with an aggregate sample size exceeding 800 patients that were previously unavailable for inclusion. These additions facilitated the acquisition of more extensive data, thereby enhancing statistical power. Secondly, to investigate potential sources of heterogeneity, we performed sensitivity, subgroup analyses, and meta‐regression analyses to augment the credibility and reliability of our findings. Furthermore, by implementing more detailed grading system for vitamin D concentrations, we aimed to explore any possible linear relationship between vitamin D levels and the risk of DFUs.
In addition to conventional comparison, given the substantial heterogeneity (I2 = 88%) observed in the overall forest plot and the multitude of influencing factors associated with vitamin D, we further conducted subgroup analyses based on age, duration of diabetes, and glycemic control.
An intriguing finding from the subgroup analysis based on age is that a significant disparity in vitamin D levels was observed only among individuals aged 50 years or older (P < 0.00001), whereas no such difference was found in patients younger than 50 years (P = 0.07). Furthermore, the heterogeneity (I2 = 70%) substantially decreased within the subgroup of older patients (> 50 years), although no statistically significant differences were observed between subgroups (P > 0.05). The decline in vitamin D concentrations with advancing age can be attributed to various factors, including variations in dietary intake, metabolic dysfunctions, and diminished vitamin D receptors76,77. Consequently, there is a significant degree of interindividual variation in vitamin D concentrations, which tends to increase with advancing age. Notably, perimenopausal and postmenopausal women exhibit a higher prevalence of lower serum levels of vitamin D78, potentially contributing to inconsistent findings observed across different age subgroups.
In the subgroup analysis of varying durations of diabetes, patients with DFUs exhibited comparatively lower concentrations of vitamin D compared to those without DFUs in the subgroup with a disease duration exceeding 10 years, resulting in a significant reduction in heterogeneity among studies. These findings suggest that the duration of diabetes may contribute to the observed heterogeneity.
In the subgroup analysis of different glycemic control statuses, a statistically significant difference in vitamin D levels was only observed in the population with poor blood sugar control (mean HbA1c 8%-9% and > 9%, P < 0.00001), but not in those with acceptable glycemic control (mean HbA1c ≤ 8%, P = 0.06), accompanying with a significant differences between the subgroups (P-interaction < 0.00001). Actually, the impact of vitamin D on glycemic control continues to garner attention, as evidenced by a recent RCT demonstrating its preservation of vitamin D on pancreatic β-cell function79. Overall, Vitamin D supplementation has been shown to have a beneficial effect in reducing insulin resistance80 and improving glycemic control81. This finding partially elucidates why significant disparities in vitamin D levels were observed solely among individuals with suboptimal glycemic control.
Furthermore, we conducted a comprehensive meta-regression analysis accounting for potential confounding factors such as geographical location, gender, BMI and research quality that could potentially impact the outcomes of vitamin D studies. However, despite our diligent efforts, we did not find any significant associations. Unfortunately, owing to the unavailability of data, we were unable to account for several potentially significant confounding factors, including blood draw season, diabetes medication usage, physical activity levels and duration of outdoors exposure during summer. Additionally, it is worth noting that vitamin D receptor polymorphism also exerts a crucial influence on both vitamin D status and vitamin D metabolism82,83.
We conducted comprehensive subgroup and sensitivity analyses to thoroughly investigate the sources of heterogeneity, and our results remained robust even after adjusting for confounding factors or conducting additional sensitivity analyses. Based on these findings, it is imperative to consider implementing routine screening for vitamin D concentrations in individuals with diabetes, particularly those with DFUs. Furthermore, it is advisable to administer vitamin D supplementations to individuals exhibiting inadequate or deficient levels of this nutrient, particularly among elderly patients with long-standing diabetes and suboptimal glycemic control. Based on animal models, the latest research findings84,85 support the involvement of vitamin D in wound healing, while there remains a debate regarding routine vitamin D supplementation in patients with DFUs. However, careful consideration must be given while interpreting findings derived from subgroup analysis and meta-regression utilizing average indicators due to the absence of individual-level data. For instance, it should be noted that studies involving participants with a mean age of 50 years or younger may also include individuals aged over 50 years in both DFUs and non-DFUs groups. Conversely, a similar situation could arise in subgroups with a mean age exceeding 50 years. Therefore, caution should be exercised when interpreting the findings derived from subgroup analysis and meta-regression.
However, it is crucial to meticulously consider several limitations of the present study. Firstly, the analysis was solely conducted on trial-level data, without access to individual-level data. This limitation also applies to the subgroup analysis. For example, our subgroup analysis relied on mean HbA1c values in order to ascertain whether the disparity in vitamin D concentrations is confined to subjects with poor glycemic control. However, the inclusion of individual-level data would enhance its reliability and strengthen the findings. Secondly, due to limited available data, a comprehensive investigation into sources of heterogeneity was not possible. Consequently, potential confounding factors such as ulcers severity, dietary habits, and other variables could not be adequately accounted for. It is important to note that these included studies encompassed both outpatients and inpatients, while those with inpatients may introduce bias towards more severe ulcers. Thirdly, there was a lack of consistency in the methodology employed for assessing serum vitamin D concentrations across the studies included, as several studies omitted reporting this crucial information. Additionally, there was a substantial variation in absolute values of vitamin D concentrations among the included studies. Fourthly, the utilization of case–control and cross‐sectional designs instead of RCTs hindered the establishment of causal relationship. Moreover, the imprecise temporal sequence between the occurrence of DFUs and assessment of vitamin D status also limited the ability to establish causality. Therefore, it is crucial to interpret these findings with caution.
Conclusions
The findings of this meta-analysis suggest that patients with DFUs exhibit lower serum vitamin D concentrations compared to those without DFUs, particularly among the elderly population with long-standing diabetes and poor glycemic control. Vitamin D deficiency and insufficiency may potentially contribute to the increased susceptibility to developing DFUs. However, the causal relationship between vitamin D status and DFUs remains unclear. Given the underlying supporting mechanisms, this clinical issue holds significant practical implications. Therefore, if maintaining adequate vitamin D concentrations proves beneficial in preventing DFUs, vitamin D supplementation could serve as a safe, cost-effective, and widely accessible potential therapeutic approach for individuals with DFUs. Nevertheless, further investigation is warranted to establish a definitive causal association between vitamin D status and the development of DFUs.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Bus, S. A. et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes Metab. Res. Rev.2023, e3651 (2023).
Schaper, N. C. et al. Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab. Res. Rev.2023, e3657 (2023).
Kurian, S. J. et al. The association between micronutrient levels and diabetic foot ulcer: A systematic review with meta-analysis. Front. Endocrinol. (Lausanne)14, 1152854 (2023).
Tang, W. et al. Association between vitamin D status and diabetic foot in patients with type 2 diabetes mellitus. J. Diabetes Invest.https://doi.org/10.1111/jdi.13776 (2022).
Li, K. et al. How vitamins act as novel agents for ameliorating diabetic peripheral neuropathy: A comprehensive overview. Ageing Res. Rev.91, 102064 (2023).
Pang, C. et al. Vitamin D and diabetic peripheral neuropathy: A multi-centre nerve conduction study among Chinese patients with type 2 diabetes. Diabetes Metab. Res. Rev.39(7), e3679 (2023).
Yuan, J. et al. Vitamin D deficiency is associated with risk of developing peripheral arterial disease in type 2 diabetic patients. BMC Cardiovasc. Disord.19(1), 145 (2019).
Hammad, R. et al. Non-classical monocytes frequency and serum vitamin D(3) levels are linked to diabetic foot ulcer associated with peripheral artery disease. J. Diabetes Invest.https://doi.org/10.1111/jdi.14048 (2023).
Bikle, D. & Christakos, S. New aspects of vitamin D metabolism and action - addressing the skin as source and target. Nat. Rev. Endocrinol.16(4), 234–252 (2020).
Bikle, D. D. Role of vitamin D and calcium signaling in epidermal wound healing. J. Endocrinol. Invest.46(2), 205–212 (2023).
Li, X. et al. The relationship between vitamin D deficiency and diabetic foot ulcer: A meta-analysis. Int. Wound J.https://doi.org/10.1111/iwj.14177 (2023).
Lin, J. et al. Association between vitamin D deficiency and diabetic foot ulcer wound in diabetic subjects: A meta-analysis. Int. Wound J.20(1), 55–62 (2023).
Bertoldo, F. et al. Definition, assessment, and management of Vitamin D inadequacy: suggestions, recommendations, and warnings from the italian society for osteoporosis, mineral metabolism and bone diseases (SIOMMMS). Nutrients14(19), 4148 (2022).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA283(15), 2008–2012 (2000).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med.151(4), W65-94 (2009).
Shi, J. et al. Detecting the skewness of data from the five-number summary and its application in meta-analysis. Stat. Methods Med. Res.32(7), 1338–1360 (2023).
Wan, X. et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol.14, 135 (2014).
Luo, D. et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res.27(6), 1785–1805 (2018).
Shi, J. et al. Optimally estimating the sample standard deviation from the five-number summary. Res. Synth. Methods11(5), 641–654 (2020).
Wells G.A., et al., The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute 2011. p1–12.
Xiao, Y. et al. Association between vitamin D status and diabetic complications in patients with type 2 diabetes mellitus: A cross-sectional study in Hunan China. Front. Endocrinol. (Lausanne)11, 564738 (2020).
Afarideh, M. et al. Raised serum 25-hydroxyvitamin D levels in patients with active diabetic foot ulcers. Br. J. Nutr.115(11), 1938–1946 (2016).
Atoum, M. F. et al. Relationship of serum vitamin D levels with diabetic foot in patients with type 2 diabetes mellitus: A cross-sectional study. Int. J. Low Extrem. Woundshttps://doi.org/10.1177/15347346231205641 (2023).
Bao, C. Clinical analysis of 25-hydroxyvitamin D, D-dimer, glycosylated hemoglobin and type 2 diabetic foot. Feet Health Care.28(03), 49–50 (2019).
Chen, X. et al. Analysis of the association between serum levels of 25(OH)D, retinol binding protein, and Cyclooxygenase-2 and the disease severity in patients with diabetic foot ulcers. Int. Wound J.https://doi.org/10.1111/iwj.14502 (2023).
Dai, J. et al. Association between serum 25-OH-vitamin D and diabetic foot ulcer in patients with type 2 diabetes. Front. Nutr.7, 109 (2020).
Danny Darlington, C. J. et al. Evaluation of serum vitamin D levels in diabetic foot infections: A cross-sectional study in a tertiary care center in South India. Iran J. Med. Sci.44(6), 474–482 (2019).
Deng, H. et al. The effects of vitamin D deficiency on patients with diabetic foot infection. Chin. J. Prev. Control Chronic Dis.23(10), 743–745 (2015).
Feldkamp, J. et al. Severe vitamin D3 deficiency in the majority of patients with diabetic foot ulcers. Horm. Metab. Res.50(8), 615–619 (2018).
Gupta B, S.K.S., In vitro study of role of vitamin D on macrophages dysfunction in patients with diabetic foot infection. Int. J. Adv. Res. (2016).
Hong, G. et al. Clinical research of 25-hydroxyvitamin D, D-dimer, glycosylated hemoglobin and diabetic foot. China Practical Med.12(02), 4–6 (2017).
Ignatovich, I.N., Kondratenko, G.G. & Dobrovol’skaia Iu, V. [Low level of 25-OH-vitamin D as a marker of critical ischemia in case of diabetic foot syndrome]. Khirurgiia (Mosk)2014(3), 11–14.
Kota, Inflammatory markers in diabetic foot and impact of vitamin D deficiency. 2013.
Li, Q. et al. Analysis of serum hepcidin levels and related in patients with diabetic lower extremity vascular disease and foot ulcer. J. Chin. Phys.23(5), 674–678 (2021).
Li, R. et al. Correlation analysis of serum 25(OH)D, RBP, COX-2 levels and disease severity in elderly diabetic foot patients. Int. J. Lab. Med.44(07), 884–886 (2023).
Lu, A., et al. The relationship between neurotic foot ulcers and serum vitamin D in type 2 diabetes mellitus. J. Inner Mongolia Med. Univ.41(04), 399–401+404 (2019).
Luo, L. et al. Relationship between serum TLR9 and 25-hydroxyvitamin D in patients with type 2 diabetic foot ulcer. Chin. J. Diabetes.27(06), 413–416 (2019).
Najafipour, F. et al. Role of serum vitamin D level in progression of diabetic foot ulcer. J. Clin. Diagn. Res.https://doi.org/10.7860/JCDR/2019/39974.12689 (2019).
Priyanto, M. H. et al. Comparison of vitamin D levels in diabetes mellitus patients with and without diabetic foot ulcers: An analytical observational study in Jakarta, Indonesia. Int. Wound J.20(6), 2028–2036 (2023).
Shen, H. et al. Association between serum vitamin D and diabetic foot risk. J. Gannan Med. Univ.35(02), 261–264 (2015).
Tang, Y. et al. Level of 25-hydroxyvitamin D and vitamin D receptor in diabetic foot ulcer and factor associated with diabetic foot ulcers. Diabetol. Metab. Syndr.15(1), 30 (2023).
Tiwari, S. et al. Prevalence and severity of vitamin D deficiency in patients with diabetic foot infection. Br. J. Nutr.109(1), 99–102 (2013).
Tiwari, S. et al. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br. J. Nutr.112(12), 1938–1943 (2014).
Tiwari, S. et al. Association of vitamin D with macrophage migration inhibitory factor and interleukin-8 in diabetic foot infection. Chron. Diabetes Res. Practicehttps://doi.org/10.4103/cdrp.cdrp_6_21 (2022).
Todorova, A. S. et al. Vitamin D status in a bulgarian population with type 2 diabetes and diabetic foot ulcers. Int. J. Low Extrem. Wounds21(4), 506–512 (2022).
Tong, L. et al. Association of serum TGF-β1, 25(OH) vitamin D and vitamin B12 levels with disease severity and healing rate of patients with diabetic foot ulcer. J. North Sichuan Med. College.38(10), 1358–1361 (2023).
Tsitsou, S. et al. Evaluation of vitamin D levels in patients with diabetic foot ulcers. Int. J. Low Extrem. Wounds22(1), 27–35 (2023).
Wang, F. et al. A retrospective analysis of the relationship between 25-OH-vitamin D and diabetic foot ulcer. Diabetes Metab. Syndr. Obes.15, 1347–1355 (2022).
Wang, F., et al. Correlation between serum 25-OH-vitamin D Level and diabetic foot ulcer in elderly diabetic patients (2022).
Wang, J. et al. Characteristics of bone metabolism indexes in diabetic foot patients and analysis of the effect of surgical treatment. Zhejiang Clin. Med. J.24(9), 1363–1366 (2022).
Wu, L. et al. Analysis of related influencing factors of osteoporosis in diabetic foot patients. Diabetes New World.09, 230 (2015).
Yoho, R. M. et al. A comparison of vitamin D levels in nondiabetic and diabetic patient populations. J. Am. Podiatr. Med. Assoc.99(1), 35–41 (2009).
Zhang, Z. et al. Analysis of related influencing factors of osteoporosis in diabetic foot patients. Shandong Med. J.53(21), 75–77 (2013).
Zhang, D. et al. Correlations between serum TLR-9, 25-(OH) D3 levels and blood glucose indexes and disease severity in patients with diabetic foot infection. J. North Sichuan Med. College.37(03), 320–323 (2022).
Zubair, M. et al. 25-Hydroxyvitamin D [25(OH)D] levels and diabetic foot ulcer: is there any relationship?. Diabetes Metab. Syndr.7(3), 148–153 (2013).
Maggi, S. et al. Vitamin D deficiency, serum leptin and osteoprotegerin levels in older diabetic patients: an input to new research avenues. Acta Diabetol.51(3), 461–469 (2014).
Mozaffari-Khosravi, H., Haratian-Arab, M., MoeinTavakkoli, H. & Nadjarzadeh, A. Comparative effect of two different doses of vitamin D on diabetic foot ulcer and inflammatory indices among the type 2 diabetic patients a randomized clinical trial. Iranian J. Diabetes Obes.8(4), 164–171 (2017).
Razzaghi, R. et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J. Diabetes Complicat.31(4), 766–772 (2017).
Ajoinish, K. et al. To study the effect of oral vitamin D supplements on wound healing in patient with diabetic foot ulcer and its effect on lipid metabolism. Int. J. Res. Pharmac. Sci.11(2), 2701–2706 (2020).
Halschou-Jensen, P. M. et al. Improved healing of diabetic foot ulcers after high-dose vitamin D: A randomized double-blinded clinical trial. Int. J. Low Extrem. Woundshttps://doi.org/10.1177/15347346211020268 (2021).
Janoušek, J. et al. Vitamin D: sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit. Rev. Clin. Lab. Sci.59(8), 517–554 (2022).
Karampela, I. et al. Vitamin D and obesity: current evidence and controversies. Curr. Obes. Rep.10(2), 162–180 (2021).
Bikle, D. D. Vitamin D metabolism and function in the skin. Mol. Cell. Endocrinol.347(1–2), 80–89 (2011).
Oda, Y. et al. Vitamin D receptor is required for proliferation, migration, and differentiation of epidermal stem cells and progeny during cutaneous wound repair. J. Invest. Dermatol.138(11), 2423–2431 (2018).
Lu, X. et al. Effects of topical 1,25 and 24,25 vitamin D on diabetic, vitamin D deficient and vitamin D receptor knockout mouse corneal wound healing. Biomolecules13(7), 1065 (2023).
Chang, E. Effects of vitamin D supplementation on adipose tissue inflammation and NF-κB/ampk activation in obese mice fed a high-fat diet. Int. J. Mol. Sci.23(18), 10915 (2022).
Luderer, H. F. et al. Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-β signaling during the inflammatory response to cutaneous injury. Endocrinology154(1), 16–24 (2013).
Lin, R. Crosstalk between vitamin D metabolism, VDR signalling, and innate immunity. Biomed. Res. Int.2016, 1375858 (2016).
Vanherwegen, A. S., Gysemans, C. & Mathieu, C. Regulation of immune function by vitamin D and its use in diseases of immunity. Endocrinol. Metab. Clin. North Am.46(4), 1061–1094 (2017).
Trujillo, V. et al. Calcitriol promotes proangiogenic molecules in keratinocytes in a diabetic foot ulcer model. J. Steroid Biochem. Mol. Biol.174, 303–311 (2017).
Shillo, P. et al. Reduced vitamin D levels in painful diabetic peripheral neuropathy. Diabet. Med.36(1), 44–51 (2019).
Banafshe, H. R. et al. Vitamin D supplementation attenuates the behavioral scores of neuropathic pain in rats. Nutr. Neurosci.22(10), 700–705 (2019).
Huang, R. et al. Relationship between serum vitamin level and diabetes and diabetic foot. J. Hainan Med. Univ.25(16), 1227–1231 (2019).
Dai, J. et al. Vitamin D and diabetic foot ulcer: a systematic review and meta-analysis. Nutr. Diabetes9(1), 8 (2019).
Çağlar, S. et al. Osteoprotegerin and 25-hydroxy vitamin D levels in patients with diabetic foot. Eklem Hastalik Cerrahisi29(3), 170–175 (2018).
Gallagher, J. C. Vitamin D and aging. Endocrinol. Metab. Clin. North Am.42(2), 319–332 (2013).
Ferri, E. et al. Vitamin D in physiological and pathological aging: Lesson from centenarians. Rev. Endocr. Metab. Disord.20(3), 273–282 (2019).
Kostecka, D. et al. Body Composition analysis in perimenopausal women considering the influence of vitamin D, menstruation, sociodemographic factors, and stimulants used. Int. J. Environ. Res. Public Health19(23), 15831 (2022).
Yan, X. et al. Combination therapy with saxagliptin and vitamin D for the preservation of β-cell function in adult-onset type 1 diabetes: a multi-center, randomized, controlled trial. Signal Transduct. Target Ther.8(1), 158 (2023).
Li, X. et al. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Nutrients10(3), 375 (2018).
Wu, C. et al. Vitamin D supplementation and glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Metabolism73, 67–76 (2017).
Zaki, M. et al. Association of vitamin D receptor gene polymorphism (VDR) with vitamin D deficiency, metabolic and inflammatory markers in Egyptian obese women. Genes Dis.4(3), 176–182 (2017).
Levin, G. P. et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA308(18), 1898–1905 (2012).
Ma, Y. et al. 1,25(OH)(2)D(3) improves diabetic wound healing by modulating inflammation and promoting angiogenesis. J. Steroid Biochem. Mol. Biol.239, 106477 (2024).
Wu, Y. et al. Effects of vitamin D status on cutaneous wound healing through modulation of EMT and ECM. J. Nutr. Biochem.134, 109733 (2024).
Acknowledgements
This study was financially supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University [Grant No. ZYGD18025], the West China Nursing Discipline Development Special Fund Project, Sichuan University [Grant No. HXHL20005], and Health commission of Sichuan province (Grant No. 372 23LCYJ042).
Funding
The 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University, (Grant no: ZYGD18025); the West China Nursing Discipline Development Special Fund Project, Sichuan University, (Grant no: HXHL20005); Health commission of Sichuan province (Grant no: 372 23LCYJ042).
Author information
Authors and Affiliations
Contributions
XR and WT devised the research plan, demonstrating their expertise in study design. WT and DC conducted a comprehensive review of relevant studies, ensuring a thorough examination of existing literature, and meticulously extracted the data with great attention to detail. GL and WT performed rigorous data analysis using advanced statistical methods, enhancing the reliability of the findings. The manuscript was skillfully composed by WT, showcasing her proficiency in scientific writing. LC, SS, CW and YG diligently revised the manuscript to improve its clarity and coherence. All authors have thoroughly reviewed and given their approval to the final version of this scholarly work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, W., Chen, D., Chen, L. et al. The correlation between serum vitamin D status and the occurrence of diabetic foot ulcers: a comprehensive systematic review and meta‐analysis. Sci Rep 14, 21932 (2024). https://doi.org/10.1038/s41598-024-73133-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73133-0