Abstract
Basalt-hosted monocentric agates from Bou Hamza (Morocco) were examined to unravel a discrete relationship between moganite content, water content, and the abundance of various types of chalcedony/quartz microtextures, so that the agate-forming process is refined. Herein, moganite content is noticeably low in length-slow chalcedony (quartzine, up to 18 wt.%), as compared with the co-occurring length-fast (LF) chalcedony (up to 31 wt.%). Such a type of disparity reflects a stabilization of the crystallization front during the formation of chalcedonic bands. Recrystallization-related quartz types (i.e. feathery and mosaic/jigsaw puzzle) show low and high moganite content (0 and up to 34 wt.%, respectively) since they have likely originated from different silica precursors (i.e. opaline and chalcedonic, respectively). Meanwhile, the initial formation of mosaic quartz can be exclusively witnessed in the so-called zebraic variety of length-fast chalcedony (i.e. with twisted fibres) that should be hence regarded as a ,,metastable” variety of silica though. The range of total water content remains quite stable across chalcedonic bands (0.5–0.6 wt%) and reaches the highest values (0.7 wt%) in feathery quartz. However, there is rather a general negative relationship between the content of molecular water and silanol-group water. This can stem from the recycling of water species following the condensation reaction of silanols within the agate geodes. The agates originated from Fe-Ti-bearing solutions that co-precipitated goethite, hematite, and rutile, but also contain minor low-grade carbonaceous material.
Similar content being viewed by others
Introduction
Agate represents a multi-coloured, gem-quality, and banded variety of chalcedony, i.e. optically-fibrous and microcrystalline (i.e. consisting of ca. 50–100 nm size crystallites aligned in fibres) variety of α-quartz intermixed in variable proportions with another but metastable silica polymorph, i.e. moganite1,2,3,4. Overall, this type of deposits is abundant in both acidic (e.g. porphyries) and basic (e.g. basalts) volcanic rocks and their tuffs where it occurs as nodules, veins, or irregular bodies associated with other low-temperature secondary products, such as calcite, iron oxides, clay group species (celadonite, kaolinite, illite-smectite, dickite), pectolite, fluorite, etc5,6,7,8. However, minor occurrences of banded chalcedony can still involve host sedimentary rocks, e.g. shales and siltstones9. Overall, two possibilities arise in the discussion on the origin agates: either they crystallize via the influx of discrete, silica supersaturated and partially polymerized solutions that may trigger spiral growth of chalcedony10,11 or emerge from viscous precursor (i.e. lumps of antecedent gel) infilling the cavity prior to the development of multi-coloured chalcedonic bands12,13. Meanwhile, the formation of these deposits is a complex and multi-step process that embraces recrystallization of metastable silica phases such as opal and moganite14, fluid boiling and mixing7,15, and/or self-purification and self-organization mechanisms12,13,16, followed by variations in temperature, pH, and Si concentration in the mother fluid/gel. The temperature of agate formation, though not precisely determined, is rather low and roughly falls below 120 ºC, as constrained by e.g. O isotopic studies17,18 and/or the presence of low-grade carbonaceous material7. There is also some data indicating higher temperature values (above 150 ºC) as constrained using e.g. fluid inclusion data5. These high-temperature values can, however, correspond to the burial-induced re-equilibration mechanism19. In turn, the origin of colouration within chalcedonic bands can be ascribed to the presence of solid inclusions20, trace element impurities21, and/or the Mie scattering effect related to nano-textural features of chalcedony22. Textural maturation of the agates in geological time is a well-known phenomenon that increases crystallite size and silica crystallinity index, as well as enhances the removal of water and subsequent moganite/opal→quartz transformation14, followed by changes in cathodoluminescence properties of chalcedony23. Furthermore, according to Kinnunen and Lindqvist24, agates can even serve as indicators for impact structures (or young volcanic craters) in shield areas. Geochemical data, particularly the presence of positive Eu anomaly and shape of REE patterns, supports the hypothesis that agate-forming processes are linked to the syn- or post-volcanic alterations (e.g. feldspar alterations) of host volcanic rocks7,25, with or without the influence of meteoric waters. Extraordinary concentrations of U have also been reported in some agate deposits due to the contemporaneous accumulation of Si and U (note the latter occurs as uranyl ions) – Götze et al.26.
In this study, we present an integrated microscopic and spectroscopic, Raman (RS), and Fourier-transformed infrared (FTIR), study of the agates from Bou Hamza (Morocco) hosted by Triassic basaltic rocks. Particularly, we aim to elucidate whether there is a relationship between moganite content and the abundance of distinctive chalcedony types (i.e. length-fast/normal and length-slow/quartzine), as well as recrystallization-related quartz micro-textures (i.e. mosaic/jigsaw puzzle and feathery, both typical of epithermal vein systems, but also found in agate deposits). Moreover, water contents including molecular water (H2Omol) and silanol-group water (H2OSiOH) were calculated in a semi-quantitative way for these chalcedony types and silica micro-textures. Consequently, we hypothesise about the possible stages of agate-forming processes and their textural maturation, as well as the possible environment that favours the precipitation of a given type of chalcedony within agate nodules.
Remarks on silica nomenclature
Silica polymorphs found in the agates include different types of opal (A, C, and/or CT), moganite, and low-temperature α-quartz, though fibrous silica (e.g. chalcedony) can be treated as either mixture of α-quartz and moganite or simply water-rich microcrystalline α-quartz marked by a relatively low refractive index and density27. Based on microscopic properties, chalcedony can be further subdivided into length-fast (LF), where the c axis is situated perpendicular to the fibres, and length-slow (LS) with the c axis parallel to the fibres. Length-fast (LF) chalcedony can be additionally subdivided into ,,normal” non-twisted and twisted. The latter is also referred as ,,zebraic” or ,,wrinkle-banded” as it shows a mottled extinction pattern under a polarizing microscope (using crossed polars). This pattern is caused by the twisting of the c axis around the fibres and can originate from e.g. screw dislocations and ionic substitutions11,28,29,30. Prismatic to isometric megaquartz (> ca. 20 μm), as well as microquartz (< ca. 20 μm) are other common agate constituents. The term pseudochalcedony/microflamboyant quartz is sometimes used to describe chalcedonic silica that has lost its microscopically-visible fibrosity28 at the expanse of granularity. In the following text, we use the terms of recrystallization-related quartz microtextures typical of epithermal vein systems proposed by Dong et al.31, such as mosaic/jigsaw (marked by sinusoidal/interpenetrating crystal boundaries) and feathery/flamboyant (recognized by undulose/patchy extinction and/or the presence of impurities distributed along the external margin of the crystals). Especially, such a type of micro-textures has been recently reported in vein agates7, though they seem to be also common in other agate types32.
Water in chalcedony
Chalcedonic silica can contain up to ca. 2.0-2.5 wt.% of water, found in two forms, i.e. free molecular water (H2Omol) and chemically-bound water (so-called silanol-group water; H2OSiOH)33,34. The former occurs as inclusions and can be adsorbed in pores and interstices, whereas the presence of silanols is intimately related to the defects and crystal boundaries. Minor structural (charge-compensating) silanols, connected with the presence of elemental substitutions/defects in quartz, can also be distinguished, though their role in chalcedonic silica is rather insignificant35. Following FTIR observations, fundamental vibrations for H2Omol and H2OSiOH are observed in mid infrared range. Herein, molecular water shows a broad band centred around ca. 3400 cm-1, followed by a sharp shoulder (e.g. ca 3585 cm-1) peculiar to silanol-group water36,37. These water species also show individual and well-resolved bands in the near-infrared range, i.e. at. ca. 5200 cm-1 and 4500 cm1, respectively. These bands are separated from each other and thus, unlike the region 3750 − 3000 cm-1, can be used for direct measurements of water distribution. Additionally, the position of these bands may shift towards lower wavelengths since silanols can form hydrogen bonds with each other and/or molecular water. This type of hydrogen-bonded silanols is also referred to ,,type B” following the nomenclature of Flörke et al.38. Noteworthy, isolated (non-involved in hydrogen bonding) silanole shows an additional band at around 3745 cm-139. Finally, the co-existence of quartz particles, water species, and defects can be somehow visualized as agglomerated ,,aqua-complexes”40.
Geological background
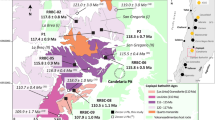
The geology of Marocco, a country in the northwestern corner of Africa, was ultimately established during the Mesozoic-Cenozoic Wilsonian cycle of plate tectonics, which caused the Pangea break-up, the opening of the Central Atlantic and Alpine Tethys Oceans, and completed with the Tethys closure and Alpine belt formation41. The climax of the rifting was marked by a short-lived, but voluminous basaltic magmatism, defined as the Central Atlantic Magmatic Province (CAMP). The magmatic activity was mainly developed on the African side of the rift where it was recorded by the presence of numerous dikes, sills, and lava flows, mostly dated at 200 ± 1Ma41. The deposits of agate mineralization are found within the Upper Triassic doleritic basalts of the High Atlas (Tizi-n-Tichka-Agouim-Al Hama, Asni, Sidi Rahal) or in valleys between the Middle Atlas and the High Atlas (Kerrouchen, Bou Hamza). The Bou Hamza agate deposit is located several kilometres to the east of Zaida town and to the north of Mibladen town, both situated in the Midelt province, Drâa-Tafilalet region in Morocco (Fig. 1). In general, the study area comprises Paleozoic basement with metasediments, metavolcanics, and amphibolites42. These rocks were intruded by granitic rocks (e.g. gabbro-diorites and granodiorites) during the Variscan Orogeny (spanning the period from ca. 360 to 280 Ma) and subsequently overprinted by thermal metamorphism. Palaeozoic rocks are unconformably overlain by Mesozoic and Cenozoic deposits including Upper Triassic doleritic basalts (containing agate mineralization described in the following text) and siliciclastic deposits, followed by Jurassic-Cretaceous carbonate series (e.g. limestones, dolostone, and marls). Miocene-Quaternary alluvial, carbonate, and siliciclastic deposits represent the youngest deposits in the study area.
(a) The position of the study area in North Africa; (b) Main lithological units exposed in the vicinity of Bou Hamza agate deposit (after42, modified).
Results
Field description
The majority of agates from Bou Hamza represent nodular onion-like type (amygdale-filling), though some of them occupy fissures/cracks and thus resemble vein agate species. The agates chiefly appear as monocentric bodies with more or less visible quartz-rich centres. The size of these individual bodies does not exceed several centimetres in diameter. The outermost regions of the agates start with a thin, bluish, and translucent chalcedonic layer, followed by alternating (opaque to transparent) orange-to-yellow chalcedonic bands (Fig. 2a-b). Additionally, orange to yellow areas sometimes contain infiltration channels/escape tubes (i.e. osculum). Locally, white-coloured regions, either in the form of bands or randomly distributed oval-shaped speckles, have been observed (Fig. 2c). Moss-type samples, i.e. remarkably rich in inclusions (celadonite, Fe- and/or Mn-oxides), occur to the lesser extent (Fig. 2d). The agates are hosted by aphyric, fine-crystalline, massive, and dark-coloured volcanic rocks (Fig. 2a-c).
(a-d) Examples of agate bodies from Bou Hamza. (a, b) Red to orange monocentric agates; Note the presence of infiltration channel (osculum) and bluish translucent chalcedonic layer in the outermost region of agate bodies; (c) Vein agate with alternating orange and yellow bands followed by drusy quartz in the centre; (d) Moss agate with celadonite and iron oxide impurities, followed by wall-lining chalcedony and drusy quartz in the centre.
Petrography and cathodoluminescence
Host basaltic rocks
The agate bodies are hosted by either typical basaltic rocks (Fig. 3a) or strongly silicified host rock matrix (skarn-like) containing anhedral quartz, oval-shaped iron oxides, and calcite (Fig. 3b). The basaltic rocks exhibit aphyric (i.e. devoid of phenocrysts), fine-crystalline to aphanitic, massive, and non-foliated microtexural fabrics. Primary minerals have been strongly affected by ubiquitously secondary alterations. The corresponding secondary products that fill interstices within volcanic glass and/or appear as pseudomorphs include celadonite (green under PPL), possible smectite-group species (yellowish under PPL), and quartz. T-shaped dendritic crystals of ilmenite and some relicts of mafic minerals (possibly pyroxene) are still visible in thin sections (Fig. 3a).
(a) Photomicrograph of the contact between chalcedonic silica (Cha) found in agate nodule and host basaltic rocks composed of relict pyroxene (Px), celadonite (Cel), and dendritic ilmenite (Ilm). Note the latter is indicative of rapid-cooling crystallization conditions; (b) Strongly silicified agate host rock with quartz, iron oxides, and calcite adjacent to some of the agate bodies. Note PX and PPL symbols refer to crossed polars and plane-polarized light, respectively.
Agates
The relationship between agate colouration and their microscopic features is linked in Figs. 4 and 5, showing the macroscopic appearance of the samples and their corresponding textural characteristcs, respectively. The agates are predominately composed of two types of chalcedonic silica, i.e. ,,normal” (non-twisted) LF and ,,zebraic” (twisted) LF chalcedony. The former type occurs in the outermost regions of agate bodies (translucent, bluish to colourless in hand specimens - Fig. 4), where it appears as wall-lining (paraboloidal/bundle-like), and/or minor spherulitic aggregates (Fig. 5a). This chalcedony type can be also observed in the inner regions of agate bodies - herein, it is either colourless or red to orange due to the presence of disseminated inclusions of Fe compounds. ,,Normal” LF chalcedony is also marked by the presence of infiltration channels and locally exhibits truncations of particular bands, as well as surficial bleaching (i.e. whitening) effects – Fig. 5b and c, respectively. LF ,,zebraic” chalcedony (chiefly white to colourless, but also slightly brownish in the macroscale - Fig. 4) occupies inner parts of agate bodies and mostly lacks iron oxide impurities. It is also interbedded with scarce amounts of LS chalcedony (quartzine) forming relatively thin bands (up to ca. 0.2 mm) – Fig. 5d. Megaquartz appears as prismatic or isometric crystals up to ca. 0.2 mm in length. The inner regions of agate nodules are occasionally filled with bladed or isometric calcite that reveals the signs of replacement by Si-rich fluids as shown in Fig. 5e. Two types of quartz microtextures were recognized during petrographic observations, i.e. feathery and mosaic (jigsaw-puzzle) – see Fig. 5f and, 5 g, respectively. The former is white and nearly opaque (similar to milky quartz) in the macro-scale (Fig. 4) and shows random distribution within agate bodies. Otherwise, mosaic quartz is translucent, greyish, bluish, and/or colourless in hand specimens (Fig. 4). It chiefly develops at the expense of LF ,,zebraic” chalcedony where applicable, resulting in the progressive vanishing of fibrous fabrics typical of chalcedony and subsequent introduction of granular fabrics typical of water-poor silica (i.e. quartz) (Fig. 5h).
Polished agate plates used for microscopic and spectroscopic measurements. Note the relationship between agate colouration and the presence of particular chalcedony/quartz types. For microscopic images of particular chalcedony/quartz types, the reader is further referred to the next figure (i.e. Figure 5). Abbreviations: nCha - ,,normal” non-twisted length-fast chalcedony with bundle-like or spherulitic microscopic fabrics; tCha - twisted (,,zebraic”) length-fast chalcedony; Qzn - length-slow chalcedony (quartzine); mQz - mosaic (jigsaw puzzle) quartz (i.e. with interpenetrating grain boundaries); fQz - feathery quartz (with a peculiar undulose/wavy extinction); Cal - calcite; Qz - drusy quartz (megaquartz).
Photomicrographs of agate samples. (a, b) Wall-lining LF ,,normal” chalcedony (nCha) impured with iron oxides along the banding. Note the bands are interrupted (black dotted line) and or show the effects of surficial bleaching (red arrow), as well as the formation of bundle-like chalcedony due to the infiltration of newly-formed Si-rich fluids along the pre-existing chalcedonic bands (yellow arrow); (c) Paraboloidal (bundle-like) and spherulitic ,,normal” (non-twisted) LF chalcedony (nCha) reflecting an initial growth of crystals at the cavity walls3; note the presence of oval-shaped inclusions comprising iron oxides (red arrow); (d) Alternating chalcedonic bands composed of LS chalcedony (quartzine; Qzn) and LF twisted ,,zebraic” chalcedony (tCha), followed by prismatic megaquartz (Qz); note the inset photomicrograph (lower left) with a gypsum plate inserted to the microscope; (e) Corrosion of calcite (Cal) via the influx of siliceous fluids; (f) Feathery quartz (fQz) embedded within silica matrix consisting of mosaic quartz (mQz) and remnants of chalcedony (possibly LF); (g) ,,Normal” LF chalcedony surrounded by mosaic quartz (mQz); (h) Triple-point junction between polygonal spherules made of LF ,,zebraic” chalcedony (lower right). Note the partial vanishing of fibrosity (upper left), as well as the pervasive conversion of fibrosity into granularity (upper right) that results in the appearance of mosaic/jigsaw puzzle microtexture.
Regarding cathodoluminescence features of the samples, megaquartz exhibits reddish to weak blue cathodoluminescence (Fig. 6a). Chalcedonic silica displays similar weak reddish emission, whilst mosaic quartz is dull-blue luminescent, with traces of agate-related banding (not visible under polarizing microscope) - Fig. 6b-c. Feathery quartz shows a whitish to pinkish CL signal and spherulitic outlines underlined by changes in CL signal (Fig. 6c). Calcite unravels orange-red patchy-zoned CL colours (possibly Mn2+-activated) - Fig. 6d. These colours are quenched in some places due to the possible Fe impurity.
Cathodoluminescence images of agate samples. (a) Reddish luminescent twisted LF chalcedony (tCha), followed by blue to red luminescent megaquartz (Qz); (b) Feathery quartz (fQz; white to pinkish CL) associated with dark-blue luminescent mosaic quartz (mQz); (c) Feathery quartz impregnated with orange-yellow luminescent calcite; Note the possible original outline of chalcedonic (or opaline) bands was preserved (yellow arrow). (d) Two generations of calcite (Cal), i.e. orange-yellow (patchy-zoned) and non-luminescent Fe-rich found in association with megaquartz (Qz); Note the orange-yellow CL of calcite suggest an oxygen-poor environment and the corresponding presence of Mn at the divalent stage that acts as a luminescence activator in carbonate minerals.
Raman spectroscopy
Moganite distribution
Both moganite and α-quartz (low-temperature) were identified in agate nodules owing to the presence of marker bands at 501 cm-1 and 464 cm-1, respectively (Fig. 7). According to43, these bands can be assigned to the symmetric stretching–bending vibrations (A1 mode).
Examples of Raman spectra deconvolution in the spectral range 600 –300 cm-1, along with calculated moganite content, collected from megaquartz, various types of chalcedonic silica, and quartz microtextures.
Overall, the calculated moganite content varies between 0.0 and 31.8 wt% depending on the presence of specific chalcedony type and/or quartz microtexture (Table 1). Particularly, the spectra obtained from prismatic megaquartz from the agate cores, as well as feathery quartz revealed the presence of an almost pure α-quartz with only a little moganite admixture. A fairly low but detectable moganite content (av. 9.3 wt%; up to ca. 17.5 wt%) was observed in the bands made of LS chalcedony (quartzine). In contrast, the co-occurring zebraic chalcedony contains up to 30.9 wt% (av. 23.7 wt%) of moganite, though its concentrations vary within the individual chalcedonic bands (Fig. 8). Not only constant, but also quite high moganite concentrations were reported for normal non-twisted LF chalcedony (~ 20.8 wt%). Finally, mosaic quartz with remnants of unrecrystallized chalcedony is also noticeably rich in moganite (i.e. 28.6–34 wt%).
(a) Photomicrograph of alternating LF ,,zebraic” chalcedony and LS chalcedony (quartzine), coupled with (b) the intensity of moganite-related Raman band at ca. 501 cm-1 The range of the calculated moganite content (wt%) for particular chalcedonic bands (labelled 1–10) was included at the bottom. Note two spectra with the highest and the lowest intensity of the moganite-related band (501 cm-1) were selected in each of these bands, and further used during the deconvolution procedure. For the comparison of moganite content in chalcedonic silica and quartz microtextures, the reader is also referred to Table 1.
Solid inclusions
Fe-bearing compounds embrace hematite and goethite, responsible for the red and yellow colouration of chalcedonic bands, respectively (Fig. 9). Other minor impurities are rutile (centre part of agate body, in association with calcite) and poorly-organised carbonaceous material (CM) found in alternating LF ,,zebraic”–quartzine chalcedonic bands.
Raman spectra for solid inclusions found in agate nodules involving (a) hematite, (b) goethite, (c) rutile, and (d) poorly organised carbonaceous material that yields a deposition temperature of around 74 °C, as calculated based on FWHM of the disorder-related D2 band of amorphous carbon at ca. 1600 cm-1. Note that the spectrum was decomposed without graphite-related G-band as proposed by Kouketsu et al.50 for poorly-organised CM.
Hematite forms characteristic red-coloured irregularly shaped aggregates. The spectrum of hematite is marked by numerous, diagnostic bands at 223, 242, 289, 408, 497, 609, and 655 cm−1 (Fig. 9a). The bands at 242, 289, 408, and 609 cm−1 were assigned to Fe-O symmetric bending vibrations (Eo mode), while the weaker lines at 223, 497, and 655 cm−1 come from Fe-O symmetric stretching vibrations (A1g mode)44. The maximum of the most intensive and broad band occurs at 1322 cm−1 and is attributed to a second-order 2LO mode with 2Eu symmetry due to defects in the hematite lattice45. The presence of goethite is proved by marker band at 387 cm−1 due to Fe-O-Fe/-OH symmetric stretching vibrations44 and minor lines at 246, 301, 485, 551, 686, and 1007 cm−1 (Fig. 9b)46. Rutile manifests its occurrence by the characteristic stretching bands at 142 (B1g), 447 (Eg), and 611 (A1g) cm−1 (Fig. 9c)47. The broad band with a maximum of 232 cm−1 is due to the multiple phonon scattering processes48.
The CM depicts two first-order broad bands at ca. 1350 and 1600 cm−1 (Fig. 9d). These bands are commonly referred to as D and G bands, and can be ascribed to the presence of disordered carbon and/or graphite, respectively49. Following the deconvolution procedure proposed by Kouketsu et al.50, the calculated ,,metamorphic” temperature of CM (i.e. 74 ºC) is typical for amorphous carbon.
Fourier transformed infra-red spectroscopy – water content
Water is ubiquitously presented in chalcedonic silica and quartz microtextures found in the agate nodules. FTIR spectra in the range of 5500 –4000 cm-1 show the presence of marker bands for both molecular water H2Omol and silanol groups H2OSiOH, found at ca. 5200 and 4500 cm-1, respectively (Table 2; Fig. 10). They can be assigned to bending-stretching modes33,34,51,52. Additionally, the silanols are likely involved in hydrogen bonding as the marker band for these species is quite broad and splits into two more or less visible sub-bands (i.e. ca. 4500 and 4350 cm-1).
Examples of near-infrared (NIR) spectra for particular types of chalcedonic silica and quartz microtextures in the range of 5500 –4000 cm-1, where marker bands for molecular water and silanol groups (SiOH) are visible. Note the broadening of the silanol-related band and its shift towards lower wavelengths, as induced by hydrogen bonding.
In general, the observed total water content (cH2OTOT; note c prefix refers to content in the entire text) covers a narrow range of 0.38–0.67 wt.% as compared with relatively scattered moganite distribution. The content of molecular water (cH2Omol) in LF ,,zebraic” chalcedony falls in the range of 0.35–0.51 wt%, while the content of silanol-group water (cH2OSiOH) is variable and ranges between 0.06 and 0.17 wt% (Table 3). Otherwise, ,,normal” LF chalcedony bears relatively low and constant cH2Omol (up to 0.24 wt%), accompanied by roughly high and constant cH2OSiOH (up to 0.24 wt%) and corresponding high cH2OSiOH/cH2Omol ratio up to 1.09. Regarding LS chalcedony, cH2Omol is fairly oscillating (0.32–0.49 wt%), though cH2OSiOH displays even larger distributions (i.e. 0.00-0.19 wt%). Feathery quartz contains the highest observed cH2Omol (up to 0.56 wt%) and quite variable cH2OSiOH (0.07–0.14 wt%). Mosaic quartz (with chalcedony remnants) contains between 0.24 and 0.37 wt% of H2Omol, but its cH2OSiOH (0.14–0.24 wt%) is higher relative to LS chalcedony, LF ,,zebraic” chalcedony, and feathery quartz, but lower or similar in comparison with LF ,,normal” chalcedony.
According to Fig. 11a, the correlation between cH2Omol and cH2OSiOH is rather negative for chalcedonic silica including LF and LS chalcedony species. Particularly, this negative relationship is also well-developed (i.e. R-squared = 0.91) once projection points for LS chalcedony (quartzine) only are considered. The cH2Omol and cH2OSiOH in mosaic quartz (+ remnants of chalcedony) and feathery quartz follow rather positive and negative relationships (Fig. 11b), respectively. Figure 11c shows that cH2OTOT is tightly clustered in LS chalcedony and LF ,,normal” chalcedony since it falls in the range of 0.46–0.51 and 0.45–0.48 wt%, respectively. Whereas LF ,,zebraic” chalcedony, as well as recrystallization-related quartz microtextures, i.e. feathery and mosaic, reveals a relatively wide range of cH2OTOT, i.e. 0.46–0.62, 0.55–0.67, and 0.42–0.61 wt% (Fig. 11c), respectively. These variations are still lowers as compared with scattered moganite distribution.
Relationship between contents of molecular water and silanol-group water in particular types of (a) chalcedonic silica and (b) quartz microtextures; Note a well-developed negative correlation for projection points of LS chalcedony (quartzine); (c) Range of total water content (i.e. molecular water + silanol groups) in chalcedonic silica and quartz microtextures.
Discussion
Moganite and water participation in chalcedonic silica
The abundance of different types of chalcedonic silica, i.e. length-fast (LF) and length-slow (LS), has been reported in agates and other siliceous sediments such as cherts53 worldwide, though the latter chalcedony type appears to be generally less common in nature. Nevertheless, to the best of our knowledge, their formation conditions and possible linkage with specific crystallization conditions (e.g. pH, temperature, the presence of specific elements in parental fluids, etc.) are not entirely understood. It was believed that the emergence of LS chalcedony could be attributed to alkaline/sulphate-rich crystallization conditions as provided by e.g. pre-existing (now silicified) evaporitic regimes, whilst LF chalcedony would crystallize under opposite (i.e. relatively more acidic/non-sulphate) geological environments54,55. These empirical-based suggestions were later disputed by several authors, who found LS chalcedony in various (including non-evaporate) geological settings56. Meanwhile, both chalcedony types contain not only α-quartz, but also moganite admixture57. Given the fact that moganite is a metastable phase, it can be intimately transformed into quartz along with geological time and hence emerges in relatively young deposits58,59. Meanwhile, Heaney60 further proposed that moganite crystallization can be enhanced by Fe-rich and/or alkaline solutions, whilst Gislason et al.61 concluded that arid (water-poor) environment inhibits age-related moganite →quartz transformation and can account for high contents of the former polymorph in some deposits.
The agates from Bou Hamza are chiefly made of chalcedonic silica including LF ,,normal” (non-twisted), as well as LF ,,zebraic” (twisted) chalcedony intermixed with minor LS chalcedony (quartzine), followed by prismatic megaquartz. Herein, moganite and total water (molecular H2O + silanol-group water) contents, measured in situ using combined Raman (RS) and Fourier-transformed infra-red (FTIR) spectroscopy, range between 0.00 and 30.9 wt% and 0.38–0.62 wt%, respectively. Overall, the measured moganite content is quite typical as compared with the other agate deposits of variable ages14, even though its variations are quite large within the investigated chalcedony types. Conversely, total water contents are relatively low as they can reach up to 2.5 wt% in moganite-rich chalcedonic silica from agate deposits worldwide29,33,38.
It should be underlined that the study on the agates from Bou Hamza revealed a hitherto unrecognized spatial relationship between the presence of particular chalcedony types and moganite concentration. Particularly, LS chalcedony (quartzine) is remarkably poor in moganite (0.0-17.5 wt.%) relative to not only adjacent LF ,,zebraic” (twisted) chalcedony (up to 30.9 wt%), but also ,,normal” (non-twisted) LF chalcedony (up to 21 wt%). Moganite was, in turn, nearly absent in prismatic megaquartz. Our data thus contradicts the quite recent study of Bustillo57 who noted that there is no correlation between moganite content and the presence of particular chalcedony types (LF and LS). The observed differences in the participation of moganite among various chalcedonic silica can be explained in four ways. (1) Firstly, once its formation is believed to be chemically controlled11, rapid fluctuations of physicochemical conditions of agate-forming fluids (pH, polymerization degree, etc.) were possible. This further infers the formation of moganite-poor LS chalcedony reflects strikingly different conditions (such as Fe-poor, acidic) as compared to those reported for moganite-rich LF chalcedony (i.e. Fe-rich, alkaline). (2) Secondly, the formation of LS chalcedony can correspond to the temporal stabilization of the crystallization front as predicted by the model of Merino et al.13, followed by a self-purification process that would result in the lowering of trace element contents (Fe, but also Al and Na that can participate in the twisted fibres typical of ,,zebraic” LF chalcedony) within the mother fluid/gel. (3) Thirdly, an ongoing moganite→quartz transition through geological time could follow a different rate of recrystallization. ,,Free” molecular water plays a vital role in such transformation2,14,61,62, and can be synthesized via the condensation of neighbourhood silanol groups following the reaction of:
(1) Si–OH + HO–Si→Si–O–Si + H2O34,63,64.
Meanwhile, the presence of silanols (SiOH) and/or the formation of strained Si-O-Si bonds (see reaction 2) has also likely contributed to the prevalent reddish to pinkish cathodoluminescence colours of chalcedony23,65 - see e.g. Figure 6a. (4) Finally, crystallographic orientation and fibre twisting of chalcedony can account for the measured intensity of moganite-related Raman band66. Especially, this mechanism can at least partially explain a relatively broad range of moganite content in LF ,,zebraic” chalcedony.
LF ,,zebraic” chalcedony and quartzine contain variable contents of molecular water and silanol-group water, whereas ,,normal” (non-twisted) LF chalcedony shows quite high content of silanol-group water and low content of molecular water (Fig. 11a). This can at least partially stem from the variable addition of moganite that exhibits higher ratios of silanol-group water to molecular water as compared with α-quartz according to e.g. Miehe and Graetsch67. Another explanation is that relatively fast nucleation and/or low crystallinity of chalcedonic species can result in the accumulation of high amounts of water species, especially silanols35,38. Therefore, the prismatic megaquartz, which likely crystallized from diluted Si-rich solutions, shows the lowest observed total water contents and lacks silanol-group species. Partial recrystallization of LF ,,zebraic” chalcedony could also contribute to variations of cH2OSiOH and cH2Omol. Interestingly, chalcedonic silica (especially quartzine, but also LF ,,zebraic” chalcedony) is marked by relatively variable cH2OSiOH/cH2Omol ratios (Fig. 11a), but its total water content (cH2OTOT) is maintained at a roughly similar level - see Fig. 12. This observation does not remain consistent with the reviewed literature data. According to Flörke et al.38, Miehe and Graetsch67, French et al.10, and Conte et al.36, there is a positive correlation between silanol-group water and molecular water (not visible in Fig. 11a), suggesting a co-evolution of these silica species within chalcedonic bands, instead. The preservation of total water content, followed by decreasing silanol-group water has been, however, so far observed in hydrothermally-treated chalcedony34. Our data invokes that molecular water can be eventually produced from the condensation of silanols (i.e. Si(OH)4→SiO2 + 2H2O, see also reaction 1) in a closed system and does not necessarily leave the agate63 once the reaction is completed. Variable condensation ratios within single chalcedonic types could be eventually affected by pressure variations in geological time, following erosion, uplift, and other tectono-magmatic events, as suggested by Harris15.
According to Conte et al.36, moganite content is negatively correlated with the abundance of water in chalcedonic bands found in agates. Our study neither confirms nor denies such a type of relationship. Total water content is, indeed, slightly higher in moganite-poor LS chalcedony as compared with moganite-rich LF ,,normal” chalcedony (up to 0.51 and 0.48 wt% of H2OTOT, respectively; see Fig. 11c). However, H2OTOT in moganite-rich LF ,,zebraic” chalcedony still covers a relatively wide range of 0.46–0.62 wt%. These micro-range heterogeneities regarding water (but also moganite) contents in the latter chalcedony type can stem from a vigorous crystallization environment and rapid changes during the formation of agate bands.
Moganite and water distribution in quartz microtextures (mosaic and feathery)
The ubiquitous presence of recrystallization-related quartz microtextures (feathery and mosaic) reflects not only the age-related textural maturity of the investigated agates through geological time, but also the presence of metastable silica phases (moganite, opal). It has been debated whether feathery texture can be explained by epitaxial growth31 or recrystallization of pre-existing (moganite-rich chalcedonic and/or amorphous/opaline? ) precursor at elevated temperatures, i.e. exceeding 180°C68,69,70. According to e.g. Li et al.71, opaline silica can contain high (up to ca. 10 wt.%) of water that occurs mostly as molecular water. Thus, feathery quartz containing relatively high contents of molecular water (0.41–0.56 wt.%) and low contents of silanol-group water (0.07–0.14 wt.%), coupled with low moganite admixture, has likely been derived from pristine amorphous (e.g. opaline) rather than chalcedonic silica. Otherwise, mosaic quartz is rich in moganite (up to 34 wt.%) and contains slightly lower contents of molecular H2O and higher contents of silanol-group water (0.24–0.37 and 0.14–0.24 wt.%, respectively). Thus, the origin of this microtexture is likely connected with the transformation of the former chalcedonic precursor, followed by the presence of boiling-related conditions69. Such a type of scenario is further aided by microtextural observations (see e.g. Figure 5h) that revealed the progressive transformation of LF ,,zebraic” chalcedony into mosaic quartz and corresponding loss of petrographic fibrosity at the expense of granularity. Finally, the observed blue CL emission of mosaic quartz is likely related to the healing and creation of lattice defects that may be introduced after fibrosity→granularity transformation23.
The presence of moganite itself is not a sine qua non factor that triggers chalcedony recrystallization and subsequent development of granular silica (i.e. mosaic quartz microtexture). This conclusion stems from the presence of notable moganite admixture (up to 21.1 wt.%) in ,,normal” (non-twisted) LF chalcedony, which is devoid of any signs of recrystallization (i.e. fibrosity →granularity conversion). A key question is what other factors determine LF chalcedony transformation into mosaic quartz within single agate bodies though? These can simply embrace crystallographic-related features of LF ,,zebraic” chalcedony (ionic substitutions, screw dislocation, that provoke fibre twisting)12,35 and/or its crystallization under non-equilibrium conditions. The presence of a Fe-rich environment, as evidenced by relatively common hematite/goethite inclusions in ,,normal” LF chalcedony, could also play some role in the ,,stabilization” of chalcedonic silica.
Nature of agate-forming medium and colouration mechanisms
Solid inclusions recognized in agates from Bou Hamza (i.e. hematite, goethite, rutile) suggest high activities of ferrous iron and titanium during agates formation. These elements could be both mobilized during post-volcanic alterations and leached from magmatic phases such as pyroxene or ilmenite (see Fig. 3a). Carbonaceous material (CM) could be mechanically transported by fluids (i.e. remobilized) due to the prior interaction of agate-forming fluids (post-magmatic fluids and/or meteoric waters) with soil/country rocks. According to Dumańska et al.6 who investigated organic-rich (bituminous) agates from Nowy Kościół (Poland), such a type of organic material can be of algal or algal-humic origin. Hydrothermal origin of CM involving chemical reactions between hydrocarbons and carbon dioxide cannot be, however, entirely excluded72. The formation of agate bodies was accompanied by the crystallization of the former calcite, which was partially replaced by chalcedonic silica. The coexistence of hematite and goethite implies temperature changes (note the stability field of these phases covers similar pH values) during the influx of Si-bearing medium and oxidizing environment during the formation of chalcedonic bands. The presence of goethite may suggest temperatures below ca. 80 °C, whereas hematite is stable above this value according to Yue et al.74.
The colouration of chalcedonic bands found in agates from Bou Hamza reflects the presence of solid inclusions, i.e. hematite- and goethite-rich areas are coloured red and orange, respectively. However, colouration mechanisms seem to be also linked to the presence of quartz microtextures and the corresponding variations in moganite contents and cH2OSiOH/cH2Omol ratios. Especially, feathery quartz depicts milky white colouration and opaque characteristics due to the possible presence of fluid inclusions (and corresponding high measured content of molecular water). Mosaic quartz, in turn, appears in shades of grey to blue and can be rather described as translucent. Zebraic chalcedony is frequently white to brownish, whereas pure inclusion-free LF ,,normal” chalcedony depicts bluish colouration. These changes can be linked to variations of silanol-group and molecular water, as well as recrystallization of silica, though further studies involving a detailed analysis of e.g. silica crystallinity index and crystallite size in particular domains are required. It seems that the presence of solid inclusions is crucial, but not the only factor that affects agate colouration.
The transition from chalcedony (or quartzine) to prismatic megaquartz towards the centre of agate bodies from Bou Hamza has been reported elsewhere in the literature and reflects the lowering of Si content and polymerization degree, along with the repetitive pulses of silica-bearing medium (possibly hydrothermal fluid)10. Regarding ,,normal” LF chalcedony, the involvement of sediment-related fabrics (e.g. deformation/truncation of the bands, inflow tubes) and bleaching effects (see Fig. 5b-c), may suggest the presence of the former viscous precursor and/or later hydrothermal overprint by reducing (e.g. hydrocarbon-rich) fluids, respectively5,75.
Conclusions
-
1.
Recrystallization-related quartz microtextures, such as feathery and mosaic (jigsaw-puzzle), were found in the agates from Bou Hamza. The former type has likely been derived from the transformation of moganite-poor and water-rich (possibly opaline) precursor, while mosaic quartz has rather originated from pristine moganite-rich chalcedonic silica.
-
2.
Length-fast ,,zebraic” chalcedony (i.e. with fibres twisted around the c axis), showing scattered content of moganite, is more prone to loss of its optically-visible fibrosity relative to other chalcedony types, i.e. length-slow chalcedony (quartzine) and ,,normal” non-twisted length-fast chalcedony. Thus, it may serve as a potential marker for the textural maturation of siliceous deposits.
-
3.
Length-slow chalcedony (quartzine) is remarkably poor in moganite as compared with length-fast chalcedony (both twisted ,,zebraic” and non-twisted ,,normal” species). Hence, its presumed crystallization conditions (e.g. alkaline and/or evaporite-related) should be revised.
-
4.
Chalcedonic silica contains variable amounts of water including molecular water (H2O) and hydrogen-bonded silanol-group water (SiOH). The latter is especially concentrated in ,,normal” non-twisted length-fast chalcedony due to the possible rapid nucleation within agate bodies. Meanwhile, the relationship between the content of molecular and silanol-group water in chalcedonic silica shows rather a negative trend that does not concur with the existing literature, but was observed in hydrothermally-treated chalcedony. The direct conversion of silanols into molecular water through geological time, and the corresponding stable level of total water is thus possible within the single agate geodes.
-
5.
Banded structure of the agates, marked by alternating length-fast ,,zebraic” and length-slow chalcedony (quartzine) types, can originate from the influx of hydrothermal fluids, followed by self-organization and/or changes in the crystallization regime. The presence of antecedent viscous (gel-like) precursor and pristine opaline globs could enhance the formation ,,normal” length-fast chalcedony and feathery quartz, respectively. The occurrence of carbonaceous material within agate geodes may eventually support some influence of meteoric waters that interacted with soil and/or country rocks prior to the development of silica mineralization.
-
6.
The colouration of agate bodies is affected not only by the presence of solid inclusions (e.g. orange goethite, red hematite and green celadonite), but also reflects the abundance of certain types of chalcedonic silica/quartz. Bluish colouration is linked to the presence of ,,normal” LF chalcedony with high content of silanol-group water, whereas white colouration is coupled with the presence of feathery quartz, as well as partially recrystallized LF ,,zebraic” chalcedony.
Materials and methods
Optical microscopy and cathodoluminescence (OM-CL)
Polished thin sections were investigated using Olympus BX51 polarizing microscope with a magnification range from 40 to 400 times, supported by optical cathodoluminescence (cold cathode). The latter was aided by CITL Mk5 cold-cathode CL device, which operated at a maximum of 30 kV and 1 mA of beam voltage. CL images were recorded using Canon Digital Camera.
Raman microspectroscopy (RS)
Raman spectra for particular types of chalcedonic silica and quartz microtextures were obtained via Thermo Scientific DXR Raman microscope that operated in a confocal mode and backscatter geometry. The samples were excited by a semiconductor laser with an excitation wavelength of 532 nm. The analytical conditions were as follows: 10 mW power, 25 μm pinhole, 1 min exposure time, and an estimated resolution of 3 cm-1. Moganite content was determined via spectral deconvolution of quartz and moganite-related bands at 464 and 501 cm-1, respectively16. Firstly, the bands were resolved using the Lorentzian function and constant background in the range of 600 –300 cm-1. Subsequently, band integrals I(501) and I(464) were calculated as the full-width half measured (FWHM) multiplied by the band height. FWHM was also additionally corrected for the apparatus function after76. Finally, I(501)/I(464) ratios were determined and plotted on the calibration curve proposed by Schmidt et al.64 – Fig. 13. The spectra with hematite were excluded from further calculations due to the possible overlap of moganite- and hematite-related bands. One should bear in mind that the presence of surface silanols can result in the introduction of a 503 cm-1 band that may still partially overlap the moganite-related band at 501 cm-1 as shown by Schmidt et al.77. Thus, the possible correlations between silanol-group water and moganite content were taken into account during the interpretation of the data.
Calibration curve showing the relationship between moganite content and integral ratio [I(501)/I(464)] of quartz- and moganite-related bands64.
The spectra of solid inclusion were obtained under similar analytical conditions except for carbonaceous material (CM) that required low laser power (around 1 mW) to avoid heating effects78. The temperature of formation was obtained via the spectral deconvolution procedure suggested by50, using the full-width half measured of (FWHM) of the D2 band (around 1600 cm-1).
Fourier-transform infrared spectroscopy (FTIR)
Two double-polished plates (910 and 960 μm thickness) of agate samples were used to obtain transmission FTIR spectra for particular types of chalcedonic silica and quartz microtextures. The spectra were recorded in the range of 6000 –1000 cm-1, using unpolarized radiation provided by Nicolet FT-IR 8700 Spectrometer coupled to a Nicolet Continuum infrared microscope. The spectral resolution was set to 8 cm-1, and 300 scans were co-added for spectra acquisition from variable but representative areas (minimum 30 × 30 μm) maintained by the width of quartz and chalcedonic bands and size of the areas occupied by various quartz microtextures. Water content was calculated semi-quantitatively, following the Lambert-Beer law that explains the relationship between FTIR absorbance intensity and substance concentration. The content of molecular water (cH2Omol) and silanol-group water (cH2OSiOH) were calculated independently based on the absorption bands at ca. 5200 cm-1 and 4500 cm-137, respectively, and according to the equation proposed by Lambert-Beer:
(2) Wt.% (H2O) = mH2O·Abs/(ρ·ε·t)·100%, where:
mH2O - the mass of H2O (i.e. 18 g/mol);
Abs – height of the absorption bands at 5200 cm-1 (H2Omol) and 4500 cm-1 (H2OSiOH); note that baselines for absorbance measurements of the two combination bands were determined as straight lines anchored between the two lowest points on each side of the bands39 ;
ρ - density of the sample, i.e. around 2650 g/L typical for α-quartz;
ε – molar absorption coefficients as provided by Langer and Flörke79, i.e. 1.14 L/mol·cm for H2Omol related band at 5200 cm-1 and 0.76 L/mol·cm for H2OSiOH related band at 4500 cm1;
t – sample thickness (cm).
This procedure was used during the studies of chalcedony, quartz, and opaline silica39,71,80,81,82. One should bear in mind that hydrogen bonding may result in the shift of silanol-related bands toward lower wavenumbers. Thus, the obtained values may be slightly underestimated, though they still reflect variations among various silica types.
Data availability
All data generated or analysed during this study is included in this published article.
References
Ríos, S., Salje, E. K. H. & Redfern, S. A. T. Nanoquartz vs. macroquartz: a study of the α ↔ β phase transition. Eur. Phys. J. B. 20, 75–83 (2001).
Heaney, P. J. & Post, J. E. The widespread distribution of a novel silica polymorph in microcrystalline quartz varieties. Sci. (80-). 255, 441–443 (1992).
Graetsch, H., Flörke, O. W. & Miehe, G. Structural defects in microcrystalline silica. Phys. Chem. Min.14, 249–257 (1987).
Heaney, P. J., Veblen, D. R. & Post, J. E. Structural disparities between chalcedony and macrocrystalline quartz. Am. Mineral.79, 452–460 (1994).
Götze, J., Möckel, R., Pan, Y. & Müller, A. Geochemistry and formation of agate-bearing lithophysae in Lower Permian volcanics of the NW-Saxonian Basin (Germany). Mineral. Petrol.https://doi.org/10.1007/s00710-023-00841-2 (2023).
Dumańska-Słowik, M. et al. Mineralogical and geochemical characterization of the ‘bituminous’ agates from Nowy Kościół (Lower Silesia, Poland). Neues Jahrb fur Mineral. Abhandlungen. 184, 255–268 (2008).
Powolny, T., Dumańska-Słowik, M., Sikorska-Jaworowska, M. & Wójcik-Bania, M. Agate mineralization in spilitized Permian volcanics from Borówno quarry (Lower Silesia, Poland) – microtextural, mineralogical, and geochemical constraints. Ore Geol. Rev.114, 103130 (2019).
Dumańska-Słowik, M. et al. Characteristics and origin of agates from Płóczki Górne (Lower Silesia, Poland): a combined microscopic, micro-raman, and cathodoluminescence study. Spectrochim Acta - Part. Mol. Biomol. Spectrosc.192, 6–15 (2018).
Götze, J., Möckel, R., Kempe, U., Kapitonov, I. & Vennemann, T. Characteristics and origin of agates in sedimentary rocks from the Dryhead area, Montana, USA. Mineral. Mag. 73, 673–690 (2009).
French, M. W., Worden, R. H. & Lee, D. R. Electron backscatter diffraction investigation of length-fast chalcedony in agate: implications for agate genesis and growth mechanisms. Geofluids. 13, 32–44 (2013).
Heaney, P. J. A proposed mechanism for the growth of chalcedony. Contrib. Mineral. Petrol.115, 66–74 (1993).
Wang, Y. & Merino, E. Self-organizational origin of agates: banding, fiber twisting, composition, and dynamic crystallization model. Geochim. Cosmochim. Acta. 54, 1627–1638 (1990).
Merino, E., Yifeng, W. & Deloule, E. Genesis of agates in flood basalts: twisting of chalcedony fibers and trace-element geochemistry. Am. J. Sci.295, 1156–1176 (1995).
Moxon, T. & Ríos, S. Moganite and water content as a function of age in agate: an XRD and thermogravimetric study. Eur. J. Mineral.16, 269–278 (2004).
Harris, C. Oxygen-isotope zonation of agates from Karoo volcanics of the Skeleton Coast, Namibia. Am. Mineral.74, 476–481 (1989).
Götze, J., Nasdala, L., Kleeberg, R. & Wenzel, M. Occurrence and distribution of ‘moganite’ in agate/chalcedony: a combined micro-raman, Rietveld, and cathodoluminescence study. Contrib. Mineral. Petrol.133, 96–105 (1998).
Yan, Q. & Shi, G. Low-Crystallinity Index Chalcedony from Longhua, China: Characteristics and Formation. Lithosphere 2061776 (2023).
Fallick, A. E., Jocelyn, J., Donnelly, T., Guy, M. & Behan, C. Origin of agates in volcanic rocks from Scotland. Nature. 313, 672–674 (1985).
Lueth, V. & Goodell, P. Fluid Inclusion Analysis and Manganese-Iron Oxide Mineralogy of Quartz-Chalcedony Geodes from the Parana Basalts, Rio Grande do Sul, Brazil. in Symposium on Agate and Cryptocrystalline Quartz 53–59 (2005).
Mrozik, M., Götze, J., Pan, Y., Möckel, R. & Mineralogy Geochemistry, and Genesis of agates from Chihuahua, Northern Mexico. Minerals. 13, 687 (2023).
Park, C. S., Shin, H. S., Yi, K., Cho, H. & Kim, Y. Quantitative mapping of trace elements in agate using LA-ICP-MS. J. Anal. Sci. Technol.6, 1–8 (2015).
Lorenzi, R. et al. Atomic and microstructural origin of banded colours in purple-blue variety of agate from Yozgat Province, Turkey. Phys. Chem. Min.49, 1–8 (2022).
Moxon, T. & Reed, S. J. B. Agate and chalcedony from igneous and sedimentary hosts aged from 13 to 3480 Ma: a cathodoluminescence study. Mineral. Mag. 70, 485–498 (2006).
Kinnunen, K. A. & Lindqvist, K. Agate as an indicator of impact structures: an example from Sääksjärvi, Finland. Meteorit Planet. Sci.33, 7–12 (1998).
Götze, J., Tichomirowa, M., Fuchs, H., Pilot, J. & Sharp, Z. D. Geochemistry of agates: a trace element and stable isotope study. Chem. Geol.175, 523–541 (2001).
Götze, J., Gaft, M. & Möckel, R. Uranium and uranyl luminescence in agate/chalcedony. Mineral. Mag. 79, 985–995 (2015).
Gliozzo, E. Variations on the silica theme: classification and provenance from pliny to current supplies. Eur. Mineral. Union Notes Mineral.20, 13–85 (2019).
Cady, S. L., Wenk, H. R. & Sintubin, M. Microfibrous quartz varieties: characterization by quantitative X-ray texture analysis and transmission electron microscopy. Contrib. Mineral. Petrol.130, 320–335 (1998).
Miehe, G., Graetsch, H. & Flörke, O. W. Crystal structure and growth fabric of length-fast chalcedony. Phys. Chem. Min.10, 197–199 (1984).
Comer, J. & Ortoleva, P. Coexistence of twisted and untwisted crystals: an impurity/structural order model with implications for agate patterns. Am. Mineral.92, 1952–1957 (2007).
Dong, G., Morrison, G. & Jaireth, S. Quartz textures in epithermal veins, Queensland - classification, origin, and implication. Econ. Geol.90, 1841–1856 (1995).
Natkaniec-Nowak, L. et al. Fire agate from the deer Creek deposit (Arizona, USA) – new insights into structure and mineralogy. Mineral. Mag. 84, 343–354 (2020).
Flörke, O. W., Köhler-Herbertz, B., Langer, K. & Tönges, I. Water in microcrystalline quartz of volcanic origin: agates. Contrib. Mineral. Petrol.80, 324–333 (1982).
Graetsch, H., Flörke, O. W. & Miehe, G. The nature of water in chalcedony and opal-C from Brazilian agate geodes. Phys. Chem. Min.12, 300–306 (1985).
Frondel, C. Structural hydroxyl in chalcedony (type B quartz). Am. Mineral.67, 1248–1257 (1982).
Conte, A. et al. Hydrothermal genesis and growth of the banded agates from the Allumiere-Tolfa volcanic district (Latium, Italy). Phys. Chem. Min.49, 1–21 (2022).
Fukuda, J. Water in Rocks and Minerals - Species, Distributions, and Temperature Dependences. in Infrared Spectroscopy - Materials Science, Engineering and Technology (ed. Theophanides, T.) 77–96 doi: (InTech, 2012). https://doi.org/10.5772/35668.
Flörke, O. W., Graetsch, H., Martin, B., Röller, K. & Wirth, R. Nomenclature of micro- and non-crystalline silica minerals, based on structure and microstructure. Neues Jahrb fur Mineral. Abhandlungen. 161, 19–42 (1991).
Schmidt, P., Badou, A. & Fröhlich, F. Detailed FT near-infrared study of the behaviour of water and hydroxyl in sedimentary length-fast chalcedony, SiO 2, upon heat treatment. Spectrochim Acta - Part. Mol. Biomol. Spectrosc.81, 552–559 (2011).
Stenina, N. G. Water-related defects in quartz. Bull. Geosci.79, 251–268 (2004).
Michard, A., Saddiqi, O., Chalouan, A. & Frizon de Lamotte, D. Continental Evolution: The Geology of Morocco: Structure, Stratigraphy, and Tectonics of the Africa-Atlantic-Mediterranean Triple Junction (Springer-Verlag, 2008).
Yaagoub, D., Hinaje, S., El Fartati, M. & Gharmane, Y. Analysis of tectonic fracturing in the Mibladen ore deposit (Upper Moulouya, Morocco) and its impact on the Pb–Ba mineralization emplacement. Rend. Lincei Sci. Fis. e Nat.32, 325–342 (2021).
Kingma, K. J. & Hemley, R. J. Raman spectroscopic study of microcrystalline silica. Am. Mineral.79, 269–273 (1994).
Legodi, M. A. & de Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dye Pigment. 74, 161–168 (2007).
Marshall, C. P. Olcott Marshall, A. Raman hyperspectral imaging of microfossils: potential pitfalls. Astrobiology. 13, 920–931 (2013).
Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int.177, 941–948 (2009).
Franck, O. et al. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys.14, 14567–14572 (2012).
Challagulla, S., Tarafder, K., Ganesan, R. & Roy, S. Structure sensitive photocatalytic reduction of nitroarenes over TiO2. Sci. Rep.7, 1–11 (2017).
Beyssac, O. et al. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim Acta - Part. Mol. Biomol. Spectrosc.59, 2267–2276 (2003).
Kouketsu, Y. et al. A new approach to develop the Raman carbonaceous material geothermometer for low-grade metamorphism using peak width. Isl Arc. 23, 33–50 (2014).
Gallas, J. P. et al. Quantification of water and silanol species on various silicas by coupling IR spectroscopy and in-situ thermogravimetry. Langmuir. 25, 5825–5834 (2009).
Chauviré, B., Rondeau, B. & Mangold, N. Near infrared signature of opal and chalcedony as a proxy for their structure and formation conditions. Eur. J. Mineral.29, 409–421 (2017).
El-Hawat, A. S., El-Ghali, M. A. K., McLaren, S. J. & Kemp, S. J. Early silicification of the Cyrenaican chert, Libya: the importance of moganite as a transitional silicon dioxide phase. Sedimentology. 68, 855–880 (2021).
Folk, R. L. & Pittman, J. S. Length-slow chalcedony: a New Testament for Vanished evaporites. J. Sediment. Petrol.41, 1045–1058 (1971).
Milliken, K. The Silicified Evaporite syndrome–two aspects of Silicification History of former Evaporite nodules from Southern Kentucky and Northern Tennessee. J. Sediment. Petrol.49, 245–256 (1979).
Keene, J. B. Chalcedonic quartz and occurrence of quartzine (length-slow chalcedony) in pelagic sediments. Sedimentology. 30, 449–454 (1983).
Bustillo, M. Á., Pérez-Jiménez, J. L., Alonso-Zarza, A. M. B. & Furio, M. Moganite in the chalcedony varieties of continental cherts (Miocene, Madrid Basin, Spain). Spectrosc. Lett.45, 109–113 (2012).
Götze, J., Stanek, K., Orozco, G., Liesegang, M. & Mohr-Westheide, T. Occurrence and distribution of moganite and opal-ct in agates from paleocene/eocene tuffs, El Picado (Cuba). Minerals. 11, 1–13 (2021).
Çalik, A. & Arzoǧullari, U. Occurrence of dendritic agate from Dereyalak village (Eskişehir) - NW of Turkey and its relationship to sepiolite nodules in the region. J. Afr. Earth Sci.97, 99–108 (2014).
Heaney, P. J. Moganite as an indicator for vanished evaporites: a testament reborn? J. Sediment. Res. Sediment. Petrol. Process. 633–638. https://doi.org/10.1306/d4268180-2b26-11d7-8648000102c1865d (1995).
Gislason, S. R. G., Heaney, P. J., Oelkers, E. H. & Schott, J. Kinetic and thermodynamic properties of moganite, a novel silica polymorph sure lator enterin solution fluid exit and sampling heater and insulation d pump H. P. L. C. titanium reactor. Geochim. Cosmochim. Acta61, 1193–1204 (1997).
Moxon, T. A re-examination of water in agate and its bearing on the agate genesis enigma. Mineral. Mag. 81, 1223–1244 (2017).
Constantina, C. & Moxon, T. Agates from Gurasada, southern Apuseni Mountains, Romania: an XRD and thermogravimetric study. Carpathian J. Earth Environ. Sci.5, 89–99 (2010).
Schmidt, P., Bellot-Gurlet, L., Leá, V. & Sciau, P. Moganite detection in silica rocks using Raman and infrared spectroscopy. Eur. J. Mineral.25, 797–805 (2013).
Möckel, R. et al. Trace-element analysis by laser ablation inductively coupled plasma Mass Spectrometry (LA-ICP-MS): a case study for agates from Nowy Kościoł, Poland. J. Sib Fed. Univ. Eng. Technol.2, 123–138 (2012).
Parthasarathy, G., Kunwar, A. C. & Srinivasan, R. Occurrence of moganite-rich chalcedony in Deccan flood basalts, Killari, Maharashtra, India. Eur. J. Mineral.13, 127–134 (2001).
Miehe, G. & Graetsch, H. Crystal structure of moganite: a new structure type for silica. Eur. J. Mineral.4, 693–706 (1992).
Sander, M. V. & Black, J. E. Crystallization and recrystallization of growth-zoned vein quartz crystals from epithermal systems - implications for fluid inclusion studies. Econ. Geol.83, 1052–1060 (1988).
Yilmaz, T. I., Duschl, F. & Di Genova, D. Feathery and network-like filamentous textures as indicators for the re-crystallization of quartz from a metastable silica precursor at the Rusey Fault Zone, Cornwall, UK. Solid Earth. 7, 1509–1519 (2016).
Moncada, D. et al. Mineral textures and fluid inclusion petrography of the epithermal Ag-Au deposits at Guanajuato, Mexico: application to exploration. J. Geochemical Explor.114, 20–35 (2012).
Li, N., Guo, Q., Wang, Q. & Liao, L. Water characterization and structural attribution of different colored opals. RSC Adv.12, 30416–30425 (2022).
Kříbek, B. et al. The origin and hydrothermal mobilization of carbonaceous matter associated with paleoproterozoic orogenic-type gold deposits of West Africa. Precambrian Res.270, 300–317 (2015).
Yue, T. et al. Low-pH mediated goethite precipitation and nickel loss in nickel hydrometallurgy. Hydrometallurgy. 165, 238–243 (2016).
Parry, W. T., Chan, M. A. & Beitler, B. Chemical bleaching indicates episodes of fluid flow in deformation bands in sandstone. Am. Assoc. Pet. Geol. Bull.88, 175–191 (2004).
Irmer, G. On the influence of the apparatus function on the determination of scattering cross sections and lifetime from optical phonon spectra. Exp. Tech. Der Phys.33, 501–506 (1985). (in German).
Schmidt, P., Bellot-Gurlet, L., Slodczyk, A. & Fröhlich, F. A hitherto unrecognised band in the Raman spectra of silica rocks: influence of hydroxylated Si-O bonds (silanole) on the Raman moganite band in chalcedony and flint (SiO 2). Phys. Chem. Min.39, 455–464 (2012).
Henry, D. G., Jarvis, I., Gillmore, G. & Stephenson, M. Raman spectroscopy as a tool to determine the thermal maturity of organic matter: application to sedimentary, metamorphic and structural geology. Earth Sci. Rev.198, 102936 (2019).
Langer, K. & Flörke, O. W. Near infrared absorption spectra (4000–9000 cm – 1) of opals and the role of “water” in these SiO2 -nH2O minerals. Fortschritte der Miner. 1974 52, 17–51 (1974).
Schmidt, P., Lauer, C., Buck, G., Miller, C. E. & Nickel, K. G. Detailed near-infrared study of the ‘water’-related transformations in silcrete upon heat treatment. Phys. Chem. Min.44, 21–31 (2016).
Schmidt, P., Nash, D. J., Coulson, S., Göden, M. B. & Awcock, G. J. Heat treatment as a universal technical solution for silcrete use? A comparison between silcrete from the Western Cape (South Africa) and the Kalahari (Botswana). PLoS One. 12, 1–26 (2017).
Fukuda, J., Peach, C. J., Spiers, C. J. & Nakashima, S. Electrical impedance measurement of hydrous microcrystalline quartz. J. Mineral. Petrol. Sci.104, 176–181 (2009).
Acknowledgements
This work was supported by AGH University of Krakow, research grant no 16.16.140.315. We thank Jacek Szczerba for providing agate samples and Piotr Strzelecki for his assistance during CL observations. We do appreciate all of the comments and suggestions which were provided to the first version of the text by two anonymous Reviewers.
Author information
Authors and Affiliations
Contributions
T.P. and M.D. were responsible for the collection of the agate samples, data acquisition, conceptualization and preparation of the manuscript, M.S. assisted in FTiR measurements and further data interpretation, M.W. was partially responsible for basic petrography.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Powolny, T., Dumańska-Słowik, M., Szczerbowska-Boruchowska, M. et al. Water and moganite participation in agates from Bou Hamza (Morocco). Sci Rep 14, 22473 (2024). https://doi.org/10.1038/s41598-024-73136-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73136-x
Keywords
This article is cited by
-
Resolving colouration mechanisms in gem-quality chalcedony: mineralogical and spectroscopic constraints
Mineralogy and Petrology (2025)