Abstract
Based on available evidence showing the antitumoral/antimetastatic activity of the proteolytic fraction (P1G10) from Vasconcellea cundinamarcensis, we analyze possible mechanisms involved in the antimetastasic effect of this fraction and subfractions (CMS1, CMS2) after incubation with B16F10 melanoma cells in vitro and in vivo under sub-apoptotic conditions. The goal was to investigate potential mediators of the antitumoral/antimetastatic effect of P1G10 triggered before the onset of apoptosis. In B16F10 preincubated viable cells, it was observed changes in adhesion to ECM (extracellular matrix), reduced activity of metalloproteases and invasivity, reduction of pAkt and pErk mostly affecting the rate of lung metastasis in mice injected with B16F10 treated cells. In most of these assays the effects depend of the proteolytic activity of the fractions. Unexpectedly, the CMS2-IAA (CMS2 with proteolytically activity inhibited by iodoacetamide), enhanced pErk phosphorylation and increased procaspase-3 levels. The invasivity of B16F10 was impaired following incubation with the proteolytic fraction without affecting cell viability under the conditions analyzed. In conclusion, CMS2 reduces in vitro cell invasion and metastasis in murine melanoma B16F10.
Similar content being viewed by others
Introduction
There is abundant evidence that plant proteolytic enzymes display various biological activities which can be therapeutically useful. The prototype protein of the group is papain, a cysteine proteinase whose 3D structure was identified more than fifty years ago1. In fact, papain is frequently cited for its therapeutic properties in skin lesions, as anti-inflammatory, antitumoral, removal of carious tissue, and digestive aid. Bromelain, another plant cysteine proteinase from A. comosus displays anti-inflammatory effect and antitumoral activity in several in vivo and in vitro models2,3,4.
Our group has characterized the biochemical and pharmacological properties of cysteine proteinases from Vasconcellea cundinamarcensis, ex Carica candamarcensis Hook 1835. V. cundinamarcensis, a member of the Caricaceae family, common to many areas in South America contains a fruit rich in proteolytic enzymes whose activity declines along with fruit maturation. V. cundinamarcencis latex exhibits between 5-8-fold higher activity than C. papaya, thus motivating our interest for these enzymes. The fruit is consumed cooked to quench the effect of residual proteolytic enzymes.
A cysteine proteinase enriched fraction (P1G10) from V. cundinamarcensis, displays numerous activities, such as fibrinogenolytic, fibrinolytic and antithrombotic activities5, tissue repair in cutaneous6,7,8,9, gastric lesions10,11 and anti-inflammatory in TNBS-induced colitis12.
The antitumor activity of P1G10 was first reported in non-metastatic B16F1 melanoma cell line and associated to loss of cell adhesion, apoptosis and reduced tumor angiogenesis13. Chromatographic separation of the two major proteolytic components in P1G10 (CMS1, CMS2) shows that only CMS2 is antimetastatic in murine B16F10 melanoma model14. We describe here the effect of P1G10 and sub-fractions CMS1 and CMS2 inducing melanoma cell detachment and possible mediators involved in the antimetastatic effect.
Materials and methods
Animals
Male C57BL/6J mice (8–10 weeks), obtained from the Centro de Bioterismo (CEBIO/ICB/UFMG), were housed at 22 ± 2°C under a 12/12 h light/dark cycle with free access to food and water. Animals were acclimatized to the laboratory environment for at least 24 h before assays. All animal handling and experimental procedures were conducted with prior approval by the Ethics Committee in Animal Experimentation at the Federal University of Minas Gerais (Process # 229/2013, 18 August 2015) and in accordance with the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020) and ARRIVE guidelines.
Reagents
RPMI-1640 culture medium was supplied by Gibco (Gaithersburg, MA, USA). 0.25% (w/v) trypsin/1 mM EDTA, fetal bovine serum (FBS), penicillin–streptomycin (PS) and phosphate-buffered saline (PBS) were obtained from Invitrogen (Carlsbad, CA, USA). 3-[4,5- Dimethyl-2-thiazolyl]-2,5-diphenyltetrazoliumbromide (MTT), Bradford reagent, bromophenol blue, calcium chloride, CM Sephadex C-50, Comassie blue R-250, dithiothreitol (DTT), dimethyl sulfoxide (DMSO), ethylenediamine tetra acetic acid disodium (EDTA), gelatin, glycerol, iodoacetamide (IAA), L-cysteine, Nα-Benzoyl-DL-arginine 4-nitroanilide hydrochloride (BAPNA), paraformaldehyde (PFA), p-chlorine mercury benzoate (pCMB), propidium iodide (PI), resazurin sodium salt, Sephadex G-10, sodium acetate, sodium citrate, sodium chloride, sulfate dodecyl sodium (SDS), Tris-HCl, Tripan blue and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Luminata™ Forte, Millicoat™ ECM Screening and QCM™ Collagen Cell Invasion Assay kits were purchased from Millipore (Burlington, MA, USA). Primary antibodies against pAkt (Ser473), Akt, pErk 1/2 (Thr 202/Tyr 204), Erk and procaspase-3, and β-actin, as well as the secondary antibodies [anti-rabbit immunoglobulin G (IgG), horseradish peroxidase (HRP)-linked antibodies] were obtained from Cell Signaling (Danvers, MA, USA). 30% acrylamide/Bis solution 19:1 was purchased from Bio-Rad (Hercules, CA, USA). Alexa Fluor 555 Protein Labeling Kit was obtained from Molecular Probes (Eugene, OR, USA). Hoescht 33,342 and Wheat Germ Agglutinin Alexa Fluor™ 488 conjugate were purchased from Thermo Fischer Scientific (Waltham, MA, USA).
Production of P1G10, CMS1 and CMS2 fractions
Twenty grams of dried latex from V. cundinarmarcensis immature fruits were dissolved in 100 mL of 1 M sodium acetate pH 5.0 containing 25 mM L-cysteine, 5 mM DTT and 10 mM EDTA. This mixture was incubated for 30 min with gentle agitation at room temperature, followed by centrifugation in Sorvall, rotor SS-34, for 10 min at 10,000 g. The supernatant was filtered through gaze and the filtrate was chromatographed through a Sephadex G-10 column previously equilibrated with 1 M sodium acetate (pH 5.0)15. The proteolytic fraction peak 1 (P1G10) was pooled and incubated with pCMB to reversible inhibit the proteolytic activity. The unreacted pCMB was removed by dialysis against 100 mM acetate buffer, pH 5.0. The resulting solution was chromatographed onto a CM Sephadex C-50 equilibrated and rinsed after sample application with 0.1 M sodium acetate. The protein was eluted with a linear gradient between 0.1 and 1.2 M sodium acetate, and the protein fractions monitored at 280 nm and the amidase activity with BAPNA substrate. The first and second main pool of protein peaks (CMS1 and CMS2, respectively) were recovered, concentrated by ultrafiltration (10,000 Da pore size) and stored at − 20 °C until use15. The fractions P1G10, CMS1, and CMS2 were subjected to denaturing gel electrophoresis (SDS/PAGE 12%) for qualitative evaluation of the chromatographic processes (Fig. S1). To inhibit proteolytic activity, aliquots of 2 mg/mL of P1G10, CMS1 or CMS2 were adjusted to 5 mM DTT and homogenized at 4 ºC with gently stirring. Then, 2 mM IAA were added to each aliquot and the mixture further incubated for 100 min16. Each sample was then dialyzed against 2,500 mL deionized water at 4 ºC for 48 h (changing the water every 12 h) to eliminate excess of inhibitor. The amidase residual activity of each aliquot was determined as above.
Cell culture
B16F10 cell line, a murine high-metastatic melanoma, was a gift from the Instituto Ludwig de Pesquisa sobre o Câncer (Sao Paulo, Brazil) while CHO (Chinese Hamster Ovary), a normal epithelial cell and BHK 21 (Baby Hamster Kidney), a normal fibroblast, were purchase from ATCC. All cell lines were grown in RPMI medium with 1% penicillin/streptomycin supplemented with 10% heat-inactivated FBS. Cells were cultured at 37ºC in a humidified 5% CO2 atmosphere.
In vivo metastatic murine model
A murine metastatic model was established according to Fidler I.J17. Briefly, animals were subcutaneously injected with B16F10 cells suspension (5 × 104 cells/mouse), in the right ear. After fifteen days, primary tumors were surgically removed and then, animals randomly distributed into seven treatment groups (n = 10 animals/group): control (saline), P1G10, CMS1 or CMS2 fractions (1 or 5 mg/Kg). Treatments started immediately after ear excision and were administered daily by cervical subcutaneous injection. After a 21-day period, mice were euthanized in a CO2 chamber and the number of lung metastatic points scored. The results are expressed as mean ± standard error. Following the normality test, they were analyzed by one-way ANOVA followed by Bonferroni post hoc test (GraphPad Prism v.9.5.0).
Cell viability assay
Cytotoxic effect of P1G10, CMS1 and CMS2 fractions was evaluated by MTT (3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-diphenytetrazoliumromide) assay18. Briefly, B16F10, CHO and BHK-21 cells, at exponentially growing phase, were seeded in 96-well plates (2 × 103 cells/well) and incubated with different concentrations (0.1–1000 µg/mL) of sterile P1G10, CMS1 or CMS2 fractions for 24 h. At the end of this period, MTT 0.5 mg/mL was added onto each well and incubated at 37°C with gentle stirring for 4 h. The cells were then lysed with DMSO and the absorbance determined at 540 nm using an ELISA reader. The half maximal cytotoxic concentration (CC50) was determined after nonlinear regression analysis of experimental data.
Deadhesion assay
B16F10 cells were seeded at a density of 104 cells/100 µL/well in 96 polystyrene plates and maintained in culture conditions. After 24 h, cells were exposed to P1G10, CMS1 or CMS2 fractions (1, 10, 30 or50 µg/mL) or control solution (RPMI 1640/10% FBS) for up to 24 h. Deadhered cells in the supernatant were transferred to a new 96 polystyrene plate and resazurin was added to each well to a final concentration of 10 µg/mL. Cells that remained attached were rinsed twice with PBS and added 100 µL of 10 µg/mL resazurin dissolved in RPMI 1640/10% FBS. After 4 h, the viability of adhered and deadhered cells was determined by the difference in absorbance (Abs570 – Abs600), after blank subtraction.
Adhesion to ECM components and invasion assay
B16F10 cells were seeded at a density of 104 cells/100 µL/well in 96 polystyrene plates and maintained in culture conditions. After 24 h, cells were exposed to 30 µg/mL of P1G10, CMS1, CMS2 fractions or control solution (RPMI 1640/10% FBS) for 24 h. Deadhered cells (in supernatant) were washed twice with PBS 1X, resuspended in RPMI 1640 and used in adhesion and invasion assays. Adhesion to ECM was assessed after seeding viable cells (2 × 104 cells/100 µL), scored by Tripan blue, onto ECM (vitronectin, fibronectin, laminin, collagen I or collagen IV) coated plates and maintained in culture conditions for 1 h. Adhered cells were stained with crystal violet as recommended by the manufacturer. In order to evaluate cell invasion, 2.5 × 105 cells/250 µL were seeded into 8 μm pore inserts coated with collagen placed in wells containing RPMI 1640/10% FBS. After 48 h, invasive cells were stained with crystal violet as recommended by the manufacturer. Stained cells, in each assay, were quantified at 540 nm in a plate reader. The results were expressed as percentage of treated cells relative to untreated control cells (assigned 100% adhesion) after blank subtraction.
Gelatin zymography
Gelatinolytic activity of matrix metalloproteinase 2 (MMP-2, 72 kDa type IV collagenase or gelatinase A) and metalloproteinase 9 (MMP-9, 92 kDa type IV collagenase or gelatinase B) was assessed by 0.1% gelatin zymography, as previously described19. B16F10 cells were treated with intact or IAA inhibited P1G10, CMS1 or CMS2 fractions, at 30 µg/mL for 24 h. Then, supernatants were recovered by centrifugation at 400 x g, 5 min, to remove deadhered cells and 5 µL of this solution incubated with 4.2 µL electrophoresis sample buffer (63 mM Tris, pH 6.8, 2% SDS, 10% glycerol, 0.0025% bromophenol blue) for 10 min at room temperature. Samples were electrophoresed through 10% SDS-PAGE gels containing 0.1% gelatin, at 90 V. Removal of SDS was done by gel washing with 2.5% triton X-100 for 1 h at room temperature followed by incubation overnight in buffer (50 mM Tris–HCl, pH 7.6, containing 10 mM CaCl2 and 0.15 M NaCl). After gel staining with Coomassie blue R-250 and distaining with a methanol-acetic acid mix, gelatinolytic activity is evident as clear bands over a deep blue background. Molecular mass standards (BioRad Laboratories, Hercules, CA) were electrophoresed in parallel with samples to assess protein size. Digital images and densitometric analyses of the gels were obtained with ImageQuant LAS4000 (GE Healthcare Life Sciences, UK), and the MMPs activity was quantified with FIJI software (National Institute of Health, MD, USA).
Flow cytometer analysis
DNA fragmentation as indicator of apoptosis was assessed by cell cycle analysis of total DNA content, as described20, with slight modifications. B16F10 cells were exposed to P1G10, CMS1 or CMS2 fractions (1–75 µg/mL) or RPMI 1640/10% FBS (control) up to 24 h, in culture conditions. Then, detached cells, obtained from the supernatant, were suspended in 0.3 mL hypotonic fluorochrome solution containing 50 µg/mL propidium iodide and 0.1% Triton X-100 in 0.1% sodium citrate. After 2–4 h incubation at 4°C in the dark, nuclear fluorescence (10,000 events) was measured in a FACSCalibur™ flow cytometer (Becton-Dickinson, Mountain View, CA, USA) and analyzed in FlowJo v10 software (FlowJo LCC, OR, USA).
Western blotting
Lysates from detached cells after 24 h exposure to 30 µg/mL P1G10, CMS1 or CMS2 fractions and total cells after similar treatment to the inhibited fractions (P1G10-IAA, CMS1-IAA or CMS2-IAA) were lysed with NP-40 buffer (150 mM sodium chloride, 1% Triton X-100, 50 mM Tris pH 8.0 and 1% protease inhibitor cocktail). Cells exposed to RPMI 1640/10% FBS were used as negative control. Equal amounts of lysate protein (30 µg) were heat-denatured, resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes. The membrane was blocked for 2 h with 5% non-fat milk at room temperature and incubated overnight with primary antibodies (1:1000), including pAkt (Cell Signaling, #4691), Akt (Cell Signaling, #4060), pErk 1/2 (Santa Cruz, sc-16982), Erk (Santa Cruz, sc-292838), procaspase-3 (Cell Signaling, #9662) and β-actin (Cell Signaling, #4967). ColorBurst™ Electrophoresis Marker (Sigma-Aldrich, C1992) was used as molecular size marker (10 µL). The membrane was then incubated with secondary antibodies (anti-rabbit IgG, HRP-linked antibodies; 1:1000) for 2 h at room temperature. Bands were visualized with Luminata™ Forte on ImageQuant LAS 4000. Band densities were analyzed using the FIJI software (National Institute of Health, MD, USA) and were normalized using the density of β-actin. In the results, the blots for each hybridization were removed from the total membranes and framed. Total membranes in different contrasts are available in the supplementary material.
Lung colonization model
A murine model was applied according to21. Briefly, B16F10 cells were exposed to P1G10, CMS1 or CMS2 fractions at 30 µg/mL, or the control with RPMI 1640/10% FBS for 24 h in culture conditions. After that period, cells were harvested with 1x PBS + 0.03% (w/v) EDTA. Cells were rinsed with 1x PBS pH 7.4 and, after centrifugation at 400 x g, 5 min, 100 µL of viable cell suspension (5 × 105 cells/mouse) were intravenously injected via tail vein (10 animals/group). Animals that received cells and no protease treatment represent the control group. After 15 days, mice were euthanized in a CO2 chamber and the number of visible lung metastatic foci quantified. The results are expressed as mean ± standard error. Following the normality test, they were analyzed by one-way ANOVA followed by Bonferroni post hoc test (GraphPad Prism v.9.5.0).
CMS2 fraction Alexa 555-labeling
To investigate CMS2 cellular localization, the fraction was labeled with Alexa Fluor 555 Protein Labeling Kit, according to instructions provided by the manufacturer. In brief, 50 µL of 1 M sodium bicarbonate buffer (pH 8.5) were added to 500 µL of 2 mg/mL CMS2 solution, followed by incubation with the reactive dye for 15 min at room temperature. To remove excess dye, the reaction mixture was passed through a BioGel P-30 fine size resin (Bio-Rad). B16F10 cells, grown in a 4-well glass slide, were treated with CMS2-Alx-555 30 µg/mL at 2, 4–24 h intervals and then, labeled with Hoescht 33,342 (10 µg/mL) and Wheat Germ Agglutinin, Alexa Fluor™ 488 conjugate (5 µg/mL) for 10 min. All confocal images were acquired using an inverted microscope Nikon Eclipse Ti-A1 without modifications.
Results
P1G10 and CMS2 fractions reduce pulmonary metastasis
Antimetastatic activity of P1G10 and derived fractions CMS1 and CMS2 was assessed in murine melanoma B16F10 model. Figure 1a shows that animals treated with P1G10 1 or 5 mg/Kg reduced their pulmonary metastasis by 64% and 80% respectively, but only at 5 mg/Kg attained significance (p < 0.05). Similarly, 5 mg/Kg CMS2 reduced 80% metastasis (p < 0.01) (Fig. 1a and b). On the other hand, animals treated with 1 or 5 mg/Kg of CMS1 showed no significant reduction in pulmonary metastases (Fig. 1a), suggesting that the bulk antimetastatic activity in P1G10 allocates to CMS2.
Antimetastatic effect of P1G10, CMS1 and CMS2 fractions in murine melanoma. B16F10 cells (5 × 104 cells/100 µL) were inoculated subcutaneously in the ear and surgically removed after 15 days. Then, mice (n = 10/group) were daily treated with P1G10, CMS1 or CMS2 fractions (1 or 5 mg/kg) or saline (control – Ctr) for 21 days. (a ) Percentage of pulmonary metastases. (b) Representative images of pulmonary metastases in control group and CMS2 fraction (1 or 5 mg/kg) treated animals. Data present means ± SD; *p < 0.05, **p < 0.01, ANOVA, Bonferroni’s post-hoc test compared to control.
P1G10 and CMS2 fractions display selective cytotoxicity against melanoma B16F10
In order to evaluate the selective cytotoxicity against B16F10 melanoma, cell viability was determined after exposure to P1G10 or its derived fractions, and the effect compared with non-tumorigenic cells (CHO and BHK-21). P1G10 displayed lower CC50 than CMS1 or CMS2 fractions, regardless the cell type; its cytotoxicity was 20-fold stronger than CMS2 (non-significant) and 950-fold superior than CMS1 in melanoma cells. Cytotoxicity of CMS1 did not discriminate between tumor and non-tumor cells (457–470 µg/mL). In turn, CMS2 showed selective toxicity towards melanoma B16F10 cells (CC50 = 10.5 µg/mL) compared to epithelial and fibroblast untransformed cell lines (CC50 > 400 µg/mL) (Table 1).
P1G10, CMS1 and CMS2 fractions impairs cell adhesion
B16F10 melanoma grown on polystyrene plates deadhered when exposed to P1G10 (Fig. 2a), CMS1 (Fig. 2b) or CMS2 (Fig. 2c) fractions (1–50 µg/mL) between 2 and 24 h. Cell detachment was time and concentration dependent; the initial effect seen 2 h after exposure to 10 µg/mL P1G10 (11%, p < 0.05), 30 µg/mL CMS1 (52%, p < 0.05) and 1 µg/mL CMS2 (15%, p < 0.05). At 30 µg/mL, detachment attained 84% for P1G10 (p < 0.05) after 24 h, like CMS1 at 16 h, and was 100% for CMS2 (p < 0.05). Cells in supernatants remain fully viable under each experimental condition and the percentage of dead cells was similar for every condition.
Cell deadhesion after incubation with P1G10, CMS1 and CMS2 fractions. B16F10 cells were treated with P1G10 (a), CMS1 (b) or CMS2 (c) fractions at 1–50 µg/mL for up to 24 h. At the end of each period, the supernatant was transferred to a second plate and incubated with 100 µL/well of 10 µg/mL resazurin both; the plate with supernatant cells and the plate with cells adhered. After 4 h incubation at 37 ºC, the absorbance was measured at 570/600 nm and expressed as percentage of control group. Dead cells were calculated as 100% - (viable cells in the supernatant % + viable adhered % cells). Data represent the mean of three independent triplicate samples ± SD. Two-way ANOVA, Dunett’s post-hoc test compared to respective control group (Ctr) for viable cells in the supernatant (*p < 0.05) and viable adhered cells (#p < 0.05).
Sub-diploid DNA in B16F10 cell supernatants exposed to P1G10, CMS1 or CMS2 fractions (1–75 µg/mL) for up to 24 h (Fig. 3 and Fig. S2) was quantified to assess DNA damage as marker of apoptosis concomitant with loss of adhesion. All fractions enhanced sub-diploid DNA at high concentrations (≥ 50 µg/mL) and extended incubation periods. The efficacy of P1G10 and CMS1 to produce sub-diploid DNA was limited (Fig. 3a and b), < 35%; while sub-diploid DNA attained 65% at 75 µg/mL CMS2 for 24 h, confirming the distinct effect induced by this fraction (Fig. 3c).
Sub-diploid DNA in B16F10 cells exposed to P1G10, CMS1 and CMS2 fractions. B16F10 cells were treated with P1G10 (a), CMS1 (b) or CMS2 (c) at 1–75 µg/mL up to 24 h. At the end of treatment, cells were resuspended in propidium iodide hypotonic solution. After 4 h incubation at 4 °C in darkness, the fluorescence of individual nuclei was measured using a FACS flow cytometer and DNA fragmentation was analyzed by FlowJo Software. Data present means of the three independent triplicate samples ± SD. **p < 0.01; ***p < 0.001; ****p < 0.0001; ANOVA, Bonferroni’s post-hoc test compared to control group (Ctr).
Based on the results showing that P1G10, CMS1 and CMS2 fraction at 30 µg/mL, induce strong deadhesion of B16F10 cells, without increasing the content of sub-diploid DNA, this condition was chosen in further analyses. The relevance of the proteolytic activity for these effects was investigated in the iodoacetamide (IAA) inhibited fractions. P1G10-IAA, CMS1-IAA and CMS2-IAA did not alter B16F10 adhesion when exposed to 30 µg/mL for 24 h, showing the dependence of its proteolytic activity to promote cell detachemnt (Fig. S3).
We then verified the influence of cell detachment on the ability of B16F10 cells to bind ECM. In these assays, detached melanoma cells exposed to 30 µg/mL P1G10, CMS1 and CMS2 fractions were assayed to probe their adherence to ECM components (Fig. 4). B16F10 cells display higher adherence to vitronectin, fibronectin and collagen I than for laminin and collagen IV, as seen in the control group. Preincubation of B16F10 with each of the fractions impaired to varying degrees adhesion to vitronectin, laminin and fibronectin; the largest decline (72%) induced by CMS2. No change in adhesion with collagens type I and IV is observed with any of the fractions.
B16F10 cells adhesion on extracellular matrix (ECM) components after P1G10, CMS1 and CMS2 fractions treatment. Deadhered cells after exposure at 30 µg/mL of P1G10, CMS1 or CMS2 fractions for 24 h were plated on wells coated with ECM components. After 1 h adhered cells were stained with violet crystal and the absorbance measured at 540 nm. (a) Representative images of cell adhesion to ECM, (b) Adhesion percentage at ECM. Data present means of the three independent triplicate samples ± SD; ANOVA, Bonferroni’s post-hoc test compared to control (Ctr) group (*p < 0.05; **p < 0.01; ***p < 0.001), P1G10 (##p < 0.01; ###p < 0.001; ####p < 0.0001) or CMS1 (++p < 0.01; +++p < 0.001; ++++p < 0.0001).
CMS1 and CMS2 fractions reduced melanoma invasion capacity
In order to evaluate the effect of P1G10 and its derived fractions (CMS1 and CMS2) on B16F10 invasion ability, a well-established in vitro assay referred as collagen matrix invasion assay22 was assessed. We found that 30 µg/mL P1G10 did not impair invasion of B16F10 cells, but CMS1 and CMS2 fractions reduced it by 33% (p < 0.01) and 55% (p < 0.01) respectively (Fig. 5).
B16F10 cells invasion on collagen-based membrane after P1G10, CMS1 and CMS2 fractions treatment. Deadhered cells after exposure at 30 µg/mL of P1G10, CMS1 or CMS2 fractions for 24 h were challenge to cross an 8 μm pore collagen-based membrane. After 48 h, cells on the opposite side of membrane were stained with violet crystal and the absorbance measured at 560 nm. (a) Representative images of cell invasion; (b) Percentage of cell invasion; Data present means of the three independent triplicate samples ± SD; **p < 0.01; ANOVA, Bonferroni’s post-hoc test compared to control group (Ctr).
In B16F10 cells treated with P1G10, CMS1 or CMS2 fractions at 30 µg/mL for 24 h, secretion of MMP2 and MMP9 was assessed by zymography. The gelatinolytic activity of MMP2 was reduced between 54 and 63% (p < 0.05) (Fig. 6a and c), and for MMP9 70 to 78% (p < 0.0001) (Fig. 6b and c). Meanwhile, the proteolytically inhibited fractions, by IAA, did not significantly change the activity of MMP2 and MMP9 in B16F10 cells (Fig. 6).
Gelatinolytic activity of secreted matrix metalloproteinase (MMP) 2 (a and c) and 9 (b and c) by B16F10 cells exposed to P1G10, CMS1 and CMS2 fractions. B16F10 cells were treated with actives or iodoacetamide inhibited P1G10, CMS1 or CMS2 fractions at 30 µg/mL for 24 h. Then, supernatants of culture were used to assess MMP2 and MMP9 gelatinolytic activities. Data present means of the three independent triplicate samples ± SD. *p < 0.05; ****p < 0.0001; ANOVA, Bonferroni’s post-hoc test compared to control group (Ctr).
Survival and apoptosis related proteins are modulated by P1G10, CMS1 and CMS2 fractions
The possible effect by P1G10, CMS1 or CMS2 fractions and the corresponding IAA-inhibited fractions on mediators of apoptosis and mitosis were analyzed in supernatants of treated B16F10 cell lysates. Compared to the untreated control, P1G10, CMS1 and CMS2 fractions reduced pAkt phosphorylation by 53% (p < 0.001), 61% (p < 0.0001) and 65% (p < 0.0001), respectively (Fig. 7a), while the inhibited fractions had no effect on pAkt, like the control group.
Erk phosphorylation was reduced between 78 and 85% (p < 0.05) following treatment with each of the three fractions (Fig. 7b). The reduction of pErk induced by the proteolytic fractions is consistent with pAkt changes and should arrest cell proliferation. Instead, in B16F10 cells treated with P1G10 or CMS1 inhibited with IAA, Erk phosphorylation was similar to untreated control, confirming the relevance of the proteolytic activity on Erk changes. Remarkably, inhibited CMS2 fraction with IAA, increased pErk 2.2-fold relative to the control (p < 0.001), and 10-fold relative to uninhibited CMS2 (p < 0.0001). While procaspase-3 in B16F10 cells was not influenced by P1G10 or CMS1 within 24 h, its level declined 61%, (p < 0.01) in presence of CMS2 fraction, stressing the pro-apoptotic effect induced by this fraction. Moreover, in B16F10 cells exposed to CMS2-IAA, procaspase-3 increased 1.7-fold compared to the control (p < 0.01) and 4.6-fold relative to the active CMS2 (p < 0.0001).
Effects of P1G10, CMS1 and CMS2 fractions in survival and apoptotic related proteins. Deadhered cells after exposure at 30 µg/mL of P1G10, CMS1 or CMS2 fractions for 24 h had Akt (a), Erk (b) and pro-Caspase 3 (c) levels determined by western blot. Blots were reprobed with β-actin to confirm equal loading. The immunoblot signals (d, representative blot) were cropped from different blots (see Supplementary information) and were quantified by densitometry and mean data from independent experiments were normalized to the results. The blots for each hybridization were removed from the total membranes and framed. Total membranes in different contrasts are available in the supplementary material Data present means of the three independent triplicate samples ± SD. ANOVA, Bonferroni’s post-hoc test compared to control, Ctr (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.00001) or between groups (#p < 0.05; ###p < 0.001; ####p < 0.0001).
P1G10, CMS1 and CMS2 fractions reduces lung colonization by B16F10 cells
We analyzed the capacity of viable B16F10 detached cells to form lung colonies after exposure to 30 µg/mL of P1G10, CMS1 or CMS2 fractions, by injection into the tail vein of C57Bl6 animals and scoring colonies 15 days later (Fig. 8a). A strong reduction in number of lung colonies was seen; 73% (p < 0.001) for P1G10, 92% (p < 0.0001) for CMS1 and 98% (p < 0.0001) in CMS2 treated cells, relative to the control (Fig. 8b).
Lung colonies in mice after B16F10 cells exposure to P1G10, CMS1 or CMS2 fractions. Detached and viable B16F10 cells (5 × 105 cells/100 µL) were injected by tail vein in C56Bl6 (n = 8/group) after 24 h exposure to P1G10, CMS1 or CMS2 fractions at 30 µg/mL. At 15th day, animals were sacrificed and lung colonies were visually quantified. (a) Representative images of lung colonies (b) Pulmonary metastasis percentage. Data present means ± SEM. ***p < 0.001; ****p < 0.0001; ANOVA, Bonferroni’s post-hoc test compared to control.
CMS2 fraction displays membrane and intracellular localization in B16F10 cells
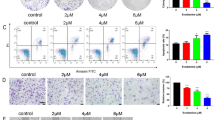
The loss of adhesion and cell rounding observed when B16F10 cells are exposed to CMS2 fraction suggests that proteases may target the cell membrane. We thereby analyzed its potential distribution after labeling with Alexa Fluor™ 555 (CMS2-Alx-555). B16F10 cells exposed to 30 µg/mL CMS2-Alx-555 show membrane and intracellular localization at each interval (2 h, 4 h and 24 h) and changes in cellular morphology featuring progressive increase in cell rounding (Fig. 9a). Indeed, a significant increase in CMS2-Alx-555 positive cells as well as in fluorescence intensity was observed after 4 h of exposure (Fig. 9b).
CMS2 sub-fraction cellular localization in B16F10 cells. Melanoma cells were exposed CMS2 labeled with Alexa Fluor™ 555 (CMS2-Alx-555) at 30 µg/mL, Hoescht 33,342 and Wheat Germ Agglutinin Alexa Fluor™ 488. (a) Images were acquired by confocal microscopy (60x magnitude) after 2 h, 4 h and 24 h exposure. (b) CMS2-Alx-555 positive cells and relative florescent intensity was analyzed by ImageJ software. Data present means ± SEM. ANOVA, Bonferroni’s post-hoc test compared to 0 h (***p < 0.001) or 2 h (####p < 0.0001).
Discussion
The antitumor activity of P1G10 (subcutaneous, s. c.) in B16F1 murine melanoma model has been demonstrated13 along with the antimetastatic activity of CMS2 administration, in an intravenous (i.v.) B16F10 melanoma murine model14, like here. Using now CMS2 treated B16F10 viable cells in the i.v. model, lung metastasis becomes near to 50% reduced compared to the i.v. melanoma model in which CMS2 was given subcutaneously14. The extra reduction suggests that CMS2 treated B16F10 cells, loss additional metastatic efficacy. The antimetastatic activity of CMS2, confirmed here, implies that CMS2 conveys the antitumoral/antimestatic determinants initially found in P1G10. Meanwhile, the lack of significant anti-metastatic effect in CMS1, also confirms previous results14.
Regarding the cytotoxicity of non-purified plant extracts, according to U.S. National Cancer Institute (NCI) and Geran’s protocol, we used to consider as highly cytotoxic activity when CC50 ≤ 20 µg/mL, moderately cytotoxic for CC50 ranged between 21 and 200 µg/mL, weakly cytotoxic for CC50 ranged between 201 and 500 µg/mL and not cytotoxic for CC50 > 501 µg/mL23. According to this categorization, P1G10 and CMS2 have highly cytotoxic activity against B16F10 cells. In view of this sorting, CMS2 is not cytotoxic for normal cell lines, as its CC50 is > 400 µg/mL, implying a selective action towards B16F10. Cytotoxicity of CMS1 and CMS2 fractions analyzed by Lemos et al.14 in melanocytic cells (B16F10 and Melan-a), confirm that CMS2 displays higher cytotoxicity than CMS1. The 20-fold stronger toxicity of P1G10 versus CMS2 fraction, although non-significant might be attributed to a cytotoxic component in P1G10, inactivated during the chromatographic step to produce CMS2.
The overall tendency shows that CMS2 has increased ability to detach B16F10 cells. Bromelain, the cysteine proteinase from A. comosus, reversible inhibits adhesion in glioma cells and leukocite without affecting cell viability, similar to here2,24. It appears that the efficacy of V. cundinamarcensis latex fractions is superior to bromelain, as maximal deadhesion observed with bromelain was around 50% while CMS2 attained 100% detachment under similar conditions. A similar loss in adhesion to ECM components was observed when the metastatic inhibitor galactin-9 was preincubated with B16F10 or Colon-26 cells before ECM binding25 arguing for the relevance of these interactions in metastasis. Cell adhesion to ECM components is primarily done by integrins; this interaction occurs not only in melanoma as in other events during tumor progression; i.e., angiogenesis, invasion, survival and apoptosis26,27. Apoptosis caused by inappropriate binding between integrin and ECM is known as anoikis28 and many non-epithelial cancers, such as breast cancer26 and melanoma29, are resilient to anoikis. Inhibition of apoptosis is critical during tumor development and contributes to the neoplastic phenotype of tumor. Deletion of tumor suppressor genes PTEN and p53 and overexpression of oncogenes ras, raf, rac and src provide the cell situation for the anoikis phenotype30,31. Interestingly, CMS2 fraction appears as more efficacious at inducing sub-diploid DNA (Fig. 3c), albeit CMS1 has 4-fold higher proteolytic activity than CMS2 arguing for a specific cleavage driven by CMS2 fraction affecting the increase in sub-diploid DNA.
Invasion is a crucial property attributed to metastatic cells, and plays a key role during tumor cell migration32. It encompasses detachment from the primary tumor site, passage through the extracellular matrix, intravasation and extravasation from the vascular system33. The role played by metalloproteases (MMPs) during migration through ECM has been stressed, as they are referred “markers of aggressiveness” in melanoma34. Bromelain also inhibits invasion and migration of glioma and breast cancer cells24 and in a cholangiocarcinoma cell line model, bromelain and papain decreased invasion and migration of malignant cells, as well35. In similar assays with B16F10 cells in vitro and in vivo, a reduction of MMP2 and MMP9 activities by natural and synthetic compounds, was associated to a decrease of tumor invasion and metastasis36,37,38. MMP9 is related to enhanced migration and invasion of murine melanoma cell line B16F10 via interaction with cell surface proteins including α5β1 and α4β1 integrin39,40. It is possible that membrane integrin levels, proteolytically modified by CMS2 fraction prevents an increase in MMP9 activity and these assays are in the agenda.
Inhibition of MMP-9 activity by the proteolytic fractions is not restricted to B16F10 cells, as we have shown that in burn skin model and TNBS-induced colitis, P1G10 inhibits the activity of MMPs9,12. The outcome of MMPs inhibition affects spreading of B16F10 cells in the tumor model while in the wounded dermal skin model it accelerates healing. It is unclear if plant proteolytic fractions directly or indirectly inhibit MMPs activity. In experiments with B16F10 cells and its invasive variant B16BL6 cells, it was demonstrated that increased cell adhesion to matrix components induces secretion of MMP9 and is related with the expression of β1,6 branching enzyme N-acetylglucosaminyltransferase-V (GnT-V)41. Increased branching of cell surface integrins and cadherins inhibits cell adhesion and increases migration, thus facilitating metastasis42. Experiments to analyze if the proteolytic fractions interfere with β1,6 branching of membrane glycoproteins could answer this question.
In adhered cells, the interaction between integrins and ECM leads to activation of cytoplasmic kinases; one of them, focal adhesion kinase (FAK) plays a role in integrin-mediated signaling. Following activation, it binds to src kinases triggering activation of the mitogenic pathway via PI3K, Akt, Erk and Mapk43. On the other hand, if integrin-ECM interactions are lost, structural change featuring cell rounding favors activation of Fas receptors resulting in apoptosis44. Inhibition of pAkt favors the apoptotic pathway and inhibit cell proliferation according to the proposed role for Akt45. Ablation of this effect by the inhibited fractions links the proteolytic activity in fractions to Akt phosphorylation. Evaluating in these cells the status of PIP3K, PDK1 and PTEN affecting Akt phosphorylation is required to confirm this hypothesis.
In proliferation assays in a non-tumorigenic cell line of murine fibroblast L92946, it was demonstrated that the proliferative effect of isoforms, CMS2MS2 and CMS2MS3, both components of CMS2 was independent of the proteolytic activity and encompassed Erk phosphorylation. A comparison between both experiments reveals that in B16F10 assays, the concentration of CMS2 is 30 µg/mL, while the concentration of isoforms depicting proliferative effect in L929 fibroblasts was near to 1000-fold lower. Considering that CMS2MS2 and CMS2MS3 account for 20–30% of isoforms in CMS2, each one represents here, 5–10 µg/mL of the CMS2 fraction. We propose that at 30 ug/mL CMS2 fraction, it predominates the proteolytic effect, while at ng/mL CMS2MS3 prevails the proliferative effect.
The enhanced apoptosis by CMS2 fraction was expected (Fig. 2) since it induced a stronger cell detachment than the other fractions. The decline in procaspase-3 following incubation with CMS2 fraction is interpreted as an activation of apoptosis, and its reversal in B16F10 cells incubated with CMS2 inhibited by IAA supports the role of the proteolytic activity on apoptosis. Procaspase-3 also has a non-apoptotic function related to fibronectin secretion and morphology, adhesion and migration control regulating cellular apoptotic threshold47; the raise in procaspase-3 by CMS2-IAA and Erk phosphorylation seems to contribute to a “survival phenotype”. In sum these findings show that P1G10, CMS1 and CMS2 fractions affect the steadiness between proliferative and apoptotic pathways in treated B16F10 cells, as they inhibit cell proliferation by dephosphorylating Erk and Akt and promote apoptosis evidenced by a decline in procaspase-3. The contrasting effects of CMS2 suggest that this fraction or its isoforms modulate the proliferative/antiproliferative response.
The in vitro treatment of B16F10 cells with P1G10 and its derived fractions had reduced lung colonization capacity when then was intravenously injected (Fig. 8). This effect was stronger for CMS2 as well was its antimetastatic activity shown here (Fig. 1). Preliminary results suggest that CMS2MS3 isoform, isolated from CMS2, reduces the levels of integrins in B16F10 cells and decrease its adhesion to ECM components, which might explain that the B10F16 cells, although viable, did not reverse the “induced damage” and kept lower metastatic capacity. It also corroborates earlier data showing that mice given B16F10 cells i.v. and subcutaneously treated with 2.5 or 5 mg/Kg CMS2 fraction for 15 days, had reduced lung colonization while CMS1 had no significant effect14.
The hypothesis of a direct action of CMS2 on cell surface adhesion molecules is corroborated by localization of CMS2-Alx-555 on the cell membrane. Indeed, a rapid increase in intracellular and mitochondrial Ca+ 2 has been observed in B16F10 cells after approximately 15s exposure to CMS2MS3, an isoform from CMS2 (preliminary results) suggesting internalization of the molecule. Thus, the ability of CMS2 or its sub-products to cross the cell membrane does not preclude its action on intracellular targets involved in cell adhesion, proliferation and cell survival.
In conclusion, our study demonstrated that proteases contained in the CMS2 fraction are responsible for the antimetastatic activity shown by the P1G10 fraction of the V. cundinamarcensis latex. In addition to selective cytotoxicity for the tumor cell line, the loss of adhesion promoted by CMS2 fraction downregulates proliferative pathway and MMPs secretion, dependent on proteolytic activity and related to its intracellular or membrane localization. Together, these activities contribute to reduction of cell invasion, in vitro, and metastasis in murine melanoma B16F10 model.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Drenth, J. et al. Structure of papain. Nature218, 929–932. https://doi.org/10.1038/218929a0 (1968).
Tysnes, B. B. et al. Bromelain reversibly inhibits invasive properties of glioma cells. Neoplasia3, 469–479. https://doi.org/10.1038/sj.neo.7900196 (2001).
Báez, R., Lopes, M. T. P., Salas, C. E. & Hernández, M. In vivo antitumoral activity of stem pineapple (Ananas comosus) bromelain. Planta Med. 73, 1377–1383. https://doi.org/10.1055/s-2007-990221 (2007).
Debnath, R. et al. Bromelain plus peroxidase reduces non-hodgkin lymphoma progression in invivo via up-regulation of antioxidant enzymes and modulating apoptotic protein expression. Nutr. Cancer72, 1200–1210. https://doi.org/10.1080/01635581.2019.1670217 (2020).
Bilheiro, R. P. et al. The thrombolytic action of a proteolytic fraction (P1G10) from Carica candamarcensis. Thromb. Res. 131, e175–e182. https://doi.org/10.1016/j.thromres.2013.01.028 (2013).
Gomes, F. S. L. L. et al. Wound-healing activity of a proteolytic fraction from Carica candamarcensis on experimentally induced burn. Burns 36, 277–283. https://doi.org/10.1016/j.burns.2009.04.007 (2010).
Lemos, F. O. et al. Skin-healing activity and toxicological evaluation of a proteinase fraction from Carica candamarcensis. Eur. J. Dermatol. 21, 722–730. https://doi.org/10.1684/ejd.2011.1466 (2011).
Freitas, K. M. et al. Healing activity of proteolytic fraction (P1G10) from Vasconcellea cundinamarcensis in a cutaneous wound excision model. Biomed. Pharmacother. 96, 269–278. https://doi.org/10.1016/j.biopha.2017.09.109 (2017).
Freitas, K. M. et al. P1G10, the proteolytic fraction from Vasconcellea cundinamarcensis, stimulates tissue repair after acute exposure to ultraviolet B radiation. Int. J. Mol. Sci. 20, 4373. https://doi.org/10.3390/ijms20184373 (2019).
Mello, V. J. et al. The gastric ulcer protective and healing role of cysteine proteinases from Carica candamarcensis. Phytomedicine 15, 237–244. https://doi.org/10.1016/j.phymed.2007.06.004 (2008).
Araujo e Silva, A. C. et al. Role of gastric acid inhibition, prostaglandins and endogenous-free thiol groups on the gastroprotective effect of a proteolytic fraction from Vasconcellea cundinamarcensis latex. J. Pharm. Pharmacol. 67, 133–141. https://doi.org/10.1111/jphp.12318 (2015).
Albuquerque, R. M. et al. The proteolytic fraction from Vasconcellea cundinamarcensis latex displays anti-inflammatory effect in a mouse model of acute TNBS-Induced Colitis. Sci. Rep. 10, 3074. https://doi.org/10.1038/s41598-020-59895-3 (2020).
Dittz, D. et al. Antiangiogenesis, loss of cell adhesion and apoptosis are involved in the antitumoral activity of proteases from V. cundinamarcensis (C. candamarcensis) in murine melanoma B16F1. Int. J. Mol. Sci. 16, 7027–7044. https://doi.org/10.3390/ijms16047027 (2015).
Lemos, F. et al. Cysteine proteases from V. cundinamarcensis (C. candamarcensis) inhibit melanoma metastasis and modulate expression of proteins related to proliferation, migration and differentiation. Int. J. Mol. Sci. 19, 2846. https://doi.org/10.3390/ijms19102846 (2018).
Teixeira, R. D. et al. The proteolytic activities in latex from Carica candamarcensis. Plant Physiol. Biochem. 46, 956–961. https://doi.org/10.1016/j.plaphy.2008.06.010 (2008).
Rawlings, N. D. & Salvesen, G. Handbook of Proteolytic Enzymes1, (Academic, 2012).
Fidler, I. J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 38, 2651LP–2660 (1978).
Denizot, F. & Lang, R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 89, 271–277. https://doi.org/10.1016/0022-1759(86)90368-6 (1986).
Kupai, K. et al. Matrix metalloproteinase activity assays: importance of zymography. J. Pharmacol. Toxicol. Methods61, 205–209. https://doi.org/10.1016/j.vascn.2010.02.011 (2010).
Riccardi, C. & Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc.1, 1458 (2006).
Vantyghem, S. A., Postenka, C. O. & Chambers, A. F. Estrous cycle influences organ-specific metastasis of B16F10 melanoma cells. Cancer Res.63, 4763–4765 (2003).
Hulkower, K. I. & Herber, R. L. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics3, 107–124. https://doi.org/10.3390/pharmaceutics3010107 (2011).
Niksic, H. et al. Cytotoxicity screening of thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci. Rep.11https://doi.org/10.1038/s41598-021-92679-x (2021).
Hikisz, P. & Bernasinska-Slomczewska, J. Beneficial properties of bromelain. Nutrients 13 (2021).
Hirashima, M. et al. Galectin-9 suppresses tumor metastasis by blocking adhesion to endothelium and extracellular matrices. Glycobiology18, 735–744. https://doi.org/10.1093/glycob/cwn062 (2008).
Pang, X. et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal. Transduct. Target. Ther.8, 1. https://doi.org/10.1038/s41392-022-01259-6 (2023).
Huang, R. & Rofstad, E. K. Integrins as therapeutic targets in the organ-specific metastasis of human malignant melanoma. J. Exp. Clin. Cancer Res.37, 92. https://doi.org/10.1186/s13046-018-0763-x (2018).
Khan, S. U., Fatima, K. & Malik, F. Understanding the cell survival mechanism of anoikis-resistant cancer cells during different steps of metastasis. Clin. Exp. Metastasis39, 715–726. https://doi.org/10.1007/s10585-022-10172-9 (2022).
Neuendorf, H. M., Simmons, J. L. & Boyle, G. M. Therapeutic targeting of anoikis resistance in cutaneous melanoma metastasis. Front. Cell. Dev. Biol.11 (2023).
Adeshakin, F. O. et al. Mechanisms for modulating anoikis resistance in cancer and the relevance of metabolic reprogramming. Front. Oncol.11 (2021).
de Sousa Mesquita, A. P. et al. Acquisition of anoikis resistance promotes alterations in the Ras/ERK and PI3K/Akt signaling pathways and matrix remodeling in endothelial cells. Apoptosis22, 1116–1137. https://doi.org/10.1007/s10495-017-1392-0 (2017).
Sulekha Suresh, D. & Guruvayoorappan, C. Molecular principles of tissue invasion and metastasis. Am. J. Physiol. Cell Physiol.324, C971–C991. https://doi.org/10.1152/ajpcell.00348.2022 (2023).
Entenberg, D., Oktay, M. H. & Condeelis, J. S. Intravital imaging to study cancer progression and metastasis. Nat. Rev. Cancer23, 25–42. https://doi.org/10.1038/s41568-022-00527-5 (2023).
Kwon, M. J. Matrix metalloproteinases as therapeutic targets in breast cancer. Front. Oncol.12 (2023).
Müller, A. et al. Comparative study of antitumor effects of bromelain and papain in human cholangiocarcinoma cell lines. Int. J. Oncol.48, 2025–2034. https://doi.org/10.3892/ijo.2016.3411 (2016).
Guo, J. et al. Cysteine protease inhibitor S promotes lymph node metastasis of esophageal cancer cells via VEGF-MAPK/ERK-MMP9/2 pathway. Naunyn Schmiedebergs Arch Pharmacol. https://doi.org/10.1007/s00210-024-03014-w (2024).
Hosseini, F. et al. Inhibition of melanoma cell migration and invasion by natural coumarin auraptene through regulating EMT markers and reducing MMP-2 and MMP-9 activity. Eur. J. Pharmacol.971, 176517. https://doi.org/10.1016/j.ejphar.2024.176517 (2024).
Li, Z. et al. The role of MMP-9 and MMP-9 inhibition in different types of thyroid carcinoma. Molecules 28. (2023).
Alford, V. M. et al. Targeting the hemopexin-like domain of latent matrix metalloproteinase-9 (proMMP-9) with a small molecule inhibitor prevents the formation of focal adhesion junctions. ACS Chem. Biol.12, 2788–2803. https://doi.org/10.1021/acschembio.7b00758 (2017).
Sil, H., Sen, T. & Chatterjee, A. Fibronectin-integrin (α5β1) modulates migration and invasion of murine melanoma cell line B16F10 by involving MMP-9. Oncol. Res.19, 335–348. https://doi.org/10.3727/096504011X13079697132925 (2011).
Ranjan, A., Bane, S. M. & Kalraiya, R. D. Glycosylation of the laminin receptor (α3β1) regulates its association with tetraspanin CD151: impact on cell spreading, motility, degradation and invasion of basement membrane by tumor cells. Exp. Cell. Res.322, 249–264. https://doi.org/10.1016/j.yexcr.2014.02.004 (2014).
Nagae, M. et al. Structure and mechanism of cancer-associated N-acetylglucosaminyltransferase-V. Nat. Commun.9, 3380. https://doi.org/10.1038/s41467-018-05931-w (2018).
Kamranvar, S. A., Rani, B. & Johansson, S. Cell cycle regulation by integrin-mediated adhesion. Cells 11 (2022).
Voss, A. K. & Strasser, A. The essentials of developmental apoptosis. F1000Res 9. (2020).
Shariati, M. & Meric-Bernstam, F. Targeting AKT for cancer therapy. Expert Opin. Investig. Drugs28, 977–988. https://doi.org/10.1080/13543784.2019.1676726 (2019).
Gomes, M. T. R. et al. Isolation of two plant proteinases in latex from Carica candamarcensis acting as mitogens for mammalian cells. Planta Med.71, 244–248. https://doi.org/10.1055/s-2005-837824 (2005).
Brentnall, M. et al. Procaspase-3 regulates fibronectin secretion and influences adhesion, migration and survival independently of catalytic function. J Cell Sci 127:2217 LP – 2226. https://doi.org/10.1242/jcs.135137(2014).
Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Contributions
DD contributed to the study design, literature search, experimental and manuscript writing; MTPL and CESB participated in the study design, the writing of the manuscript and provided financial support; GBM, HMCO and IPN contributed to data acquisition and analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval was obtained from the Ethics Committee in Animal Experimentation at the Federal University of Minas Gerais (Process # 229/2013, 18 August 2015).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dittz, D., Nunes, I.P., de Castro Oliveira, H.M. et al. Loss of adhesion impairs invasiveness and cell survival, contributing to the antimetastatic effect of cysteine proteases from Vasconcella cundinamarcensis in melanoma. Sci Rep 15, 20845 (2025). https://doi.org/10.1038/s41598-024-73489-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73489-3