Abstract
Hyperuricemic nephropathy (HN) is renal injury caused by hyperuricemia (HUA). While sleeve gastrectomy (SG) has shown promise in improving renal injury in patients with obesity-related HN, the mechanisms are not fully understood. This study induced an obesity-combined HN model in male ob/ob mice and measured serum uric acid (SUA), creatinine, and other biochemical indicators 6 weeks post-surgery. Renal histological changes were evaluated through staining techniques, and the study also assessed renal adenosine monophosphate-activated protein kinase (AMPK) and nuclear factor erythroid 2-related factor 2 (Nrf2) phosphorylation levels and urate transporter ABCG2 expression. In vitro experiments involved Nrf2 knockdown in AMPK-activated HK-2 cells and ChIP to confirm Nrf2 binding to the ABCG2 promoter. Results showed that SG reduced SUA levels, serum creatinine, and blood urea nitrogen, increased p-AMPK, p-Nrf2 protein, and ABCG2 expression, and alleviated renal fibrosis and inflammation. In vitro, Nrf2 knockdown down-regulated ABCG2 expression, and ChIP confirmed Nrf2’s role in ABCG2 transcription. The study suggests that SG may improve renal injury in HN mice by modulating the AMPK/Nrf2 pathway and upregulating ABCG2 transcription.
Similar content being viewed by others
Introduction
The rising prevalence of obesity, coupled with its association with numerous complications, has made it a critical global health issue1. The association between obesity and hyperuricemic nephropathy (HN) has been demonstrated by many researchers2,3,4. In China, with the changes in people’s lifestyles and diets, the prevalence of hyperuricemia (HUA) has been increasing year by year, and has now become the second largest metabolic disease after diabetes mellitus5. Uric acid (UA) is the end product of purine metabolism in humans, and is predominantly excreted by the kidneys. When UA is overproduced or the kidneys do not have enough function to excrete it, UA accumulates in the body, leading to HUA6. Prolonged UA elevation results in kidney damage, causing HN7,8, which is also a direct contributor to the development and progression of chronic kidney disease (CKD)9. However, monotherapy with uric acid-lowering drugs for comorbid obesity and HUA often proves suboptimal, with presenting certain limitations10,11. Current clinical approaches to HN primarily involve UA-lowering drugs such as allopurinol, febuxostat, and benzbromarone; however, their applicability is limited due to varied side effects, including urate stones, hepatic and renal calculi, and hepatic dysfunction12.
Bariatric surgery stands as an efficacious intervention for severe obesity, offering benefits beyond weight reduction, such as amelioration of associated metabolic disorders13,14. Sleeve gastrectomy (SG), a prevalent bariatric procedure, offers distinct advantages over traditional pharmacotherapy in managing obesity, particularly in alleviating HUA, insulin resistance, and regulating blood glucose and lipid profiles15. As one of bariatric procedures, SG has been shown to significantly reduce serum uric acid (SUA) levels and gout attack frequency in obese patients with HN by reducing body weight16,17 and to improve renal function in the long term postoperatively. As one of the means to lower uric acid in patients with severe obesity, bariatric surgery has progressively gained acceptance within both societal and academic spheres18,19. However, the specific mechanism is still unclear. Therefore, exploring the mechanism by which bariatric surgery reduces SUA and improves HN will help to develop new drugs for the treatment of HN.
Renal urate excretion accounts for approximately two-thirds of total urate excretion, and insufficient excretion is the main cause of HUA20. ATP-binding cassette subfamily G member 2 (ABCG2), a well-recognized HUA locus, plays a crucial role in renal and intestinal urate excretion, As one of the major urate transporters in the kidney, ABCG2 dysfunction is a risk factor for HUA21. In addition to ABCG2, other urate transporter in the kidney such as urate transporter 1 (URAT1) and glucose transporter 9 (GLUT9) also play important roles in impaired urate excretion and the development of HUA22,23,24,25, organic anion transporter 1 (OAT1), organic anion transporter 3 (OAT3) can promote the secretion of UA in the kidney26.
This study aimed to determine whether SG enhances uric acid excretion by upregulating the AMPK/Nrf2 pathway and promoting the expression of uric acid transporters. Additionally, we investigated whether these effects could ameliorate renal injury associated with obesity-combined hyperuricemic nephropathy by improving renal inflammation and histopathological changes.
Materials and methods
Animals
Six-week-old male leptin-deficient obese mice (ob/ob) mice were obtained from Beijing HFK Bioscience Company., Ltd. (Beijing, China). The mice had free access to food and water under a constant temperature of 22 °C and a 12-hour light/dark cycle. After adaptive feeding for 1 week, the ob/ob mice were randomly divided into three groups; one group was fed with standard chow, no other intervention (as a blank control group, n = 12), while the remaining two groups received intraperitoneal injections of potassium oxonate and intragastric hypoxanthine for 14 consecutive days to induce a hyperuricemic nephropathy model, subsequently divided into the SG group (n = 12) and sham surgery (SHAM) group (n = 12). The latter was based on previous studies27,28,29,30.
Surgical procedures
The established HN mouse model was randomly subjected to SG or sham surgery. The mice fasted for 12 h prior to surgery. Following induction and maintenance anesthesia with isoflurane (3% and 2%, respectively), SG and SHAM operations were performed according to standard procedures31. In the SG group, a midline incision was made from the sternum to the middle of the abdomen, the abdominal cavity was opened, and the stomach was gently manipulated with a wet cotton swab. Microscissors were used to detach it from the surrounding connective tissues and separate the greater curvature from the cardia to the pylorus. The majority of the gastric body was resected, leaving about 20% of the original gastric volume intact. After careful inspection, the residual stomach was sutured intermittently with No. 7 − 0 silk suture, and the abdominal incision was closed with No. 4 − 0 silk suture. In the SHAM group, the abdomen was opened to expose the stomach and small intestine, and the small intestine was manipulated with forceps, and then the abdominal incision was closed to match the duration of the SG procedure.
Postoperatively, mice were housed in individual cages and the diet was gradually transitioned from a liquid diet to a chow diet. Vital signs were monitored, and surgery-related complications were closely observed. Food intake and body weight were recorded weekly. On the 41st postoperative day, urine samples were collected through metabolic cages. On the 42nd postoperative day, after blood collection from the retro-orbital venous plexus, the mice were killed by intraperitoneal injection of pentobarbital, and then the kidney tissue was quickly harvested. This tissue was subsequently subjected to both histological and molecular biological analysis (Fig. 1).
All methods were carried out in accordance with relevant guidelines and regulations.
Biochemical analysis for serum and urine
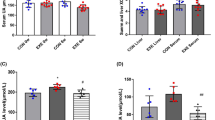
The blood samples were centrifuged (1000 xg, 20 min, 4 °C), and the serum was collected and placed in a centrifuge tube at room temperature. Urine supernatant was also obtained by centrifugation (1000 xg, 20 min, 4 °C). UA and creatinine levels in serum and urine were measured using elisa kits (Finetest, Wuhan, China), respectively, and blood urea nitrogen (BUN) levels were measured only by blood samples, and these results were used to calculate fractional UA excretion (FEUA). FEUA = (urinary uric acid × serum creatinine)/(serum uric acid × urine creatinine) × 100%.
Histological evaluation
Kidney tissues were fixed in 4% paraformaldehyde and then made into paraffin blocks. After deparaffinization and dehydration, hematoxylin-eosin (HE) staining, Masson staining and periodic acid-Shiff (PAS) staining of the kidney tissue were performed to evaluate the standard histology, Collagen distribution, degree of fibrosis, and morphology. The Pannoramic 250 digital slide scanner produced by 3DHISTECH (Hungary) was used to collect images of the slices. For each slice, the gross lesions of all tissues were first observed under a low-magnification microscope, and then the area to be observed was selected to collect images and the specific lesions were observed.
Immunohistochemistry
After dewaxing and hydrating the paraffin sections, immerse them in citrate buffer (PH 6.0) for antigen retrieval. Add fetal calf serum blocking solution and block at room temperature for 20 min. Excess blocking solution was removed, and the primary antibody against ABCG2 was applied to the sections, which were then allowed to sit flat. The sections were placed in a humidified chamber and incubated overnight at 4 °C. Afterward, the sections were incubated with the secondary antibody at 37 °C for 30 min, followed by three washes with phosphate buffer saline (PBS) (5 min each). Positive immunoreactivity was visualized using diaminobenzidine (DAB) at room temperature. Finally, the sections were lightly counterstained with hematoxylin, and images were captured from randomly selected cortical areas using a microscope imaging system.
Quantitative RT-PCR
Total RNA was isolated using the FastPure Cell/Tissue Total RNA Isolation Kit V2 (Vazyme, Nanjing, China) following the manufacturer’s instructions. One microgram of RNA was reverse transcribed using the Revert Aid First Strand cDNA Synthesis Kit (Vazyme, Nanjing, China), following the provided protocol. Reverse Transcription Polymerase Chain Reaction (RT-PCR) assays were performed on a Lightcycler 480 (Roche, Germany). The primer list is shown in Table 1.
Western blot
Protein extracts from HK-2 cells and kidney tissues were denatured in lysis buffer and then loaded onto 10% SDS-PAGE gels for electrophoresis. The resolved proteins were transferred onto a PVDF membrane (Millipore, MA, USA). Following a 2-hour blocking period with 5% skim milk in TBST at room temperature, the membrane was incubated with primary antibodies overnight at 4 °C. The primary antibodies used targeted the following proteins: ABCG2 (1:1000), URAT1 (1:1000), phospho-AMPK (p-AMPK) (1:1000), t-AMPK (1:500), phospho-Nrf2 (p-Nrf2) (1:1000), t-Nrf2 (1:1000), GLUT9 (1:1000), OAT1 (1:2000), OAT3 (1:2000), and β-actin (1:50000). Subsequently, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000) for 2 h at room temperature. Protein bands were visualized using an ECL detection kit (Epizyme, Shanghai, China), and their intensities were quantified with ImageJ software.
Cell culture and transfection
HK-2 cells were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. Lentiviral vectors targeting Nrf2 (siNrf2-1, siNrf2-2, and siNrf2-3) and a negative control (NC) were procured from Shanghai Genechem Co., Ltd. (Shanghai, China). The sequences of the lentiviral shRNA constructs were as follows: siNrf2-1, 5′-GACATACTTTGGAGGCAAGAT-3′; siNrf2-2, 5′-GGCATTTCACTAAACACAAGT-3′; siNrf2-3, 5′-GGTTGAGACTACCATGGTTCC-3′; and the Vector sequence was 5′-TTCTCCGAACGTGTCACGT-3′. 48 h after transfection, the siRNA showing the best interference with gene expression was selected for RT-qPCR and Western blot assays.
Cell counting Kit-8 (CCK-8) assay
The viability of HK-2 cells after treatment with different concentrations of A-769,662 was investigated using the CCK-8 assay. After the drugs acted for 24 h and 48 h respectively, the supernatant was discarded. Dilute CCK-8 reagent 1:10 in serum-free medium, add 110 µL/well of diluted CCK-8 working solution; shake the culture plate gently several times, and continue culturing for 2 h at 37 °C and 5% CO2. The absorbance value of each well was measured using a microplate reader at a wavelength of 450 nm.
Immunofluorescence staining
Cell slides were placed in a staining tray, washed with PBS, and then covered with a membrane-permeabilizing solution and incubated at room temperature for 10 min. Subsequently, fetal bovine serum was added dropwise for blocking non-specific binding at room temperature for 20 min. Primary antibodies were applied dropwise to the slides, which were then placed flat in a humidified chamber and incubated at 4 °C overnight. Then add secondary antibody dropwise, incubate at 37 °C for 30 min, DAPI was then applied dropwise for nuclear staining, followed by a 10-minute incubation at room temperature. The slides were mounted using an anti-fade reagent. Images were captured using a fluorescence scanning and browsing software. First observe all tissues at low magnification in random slices, and then collect microscopic images. Use the Image-J image analysis system to measure the fluorescence intensity (IntDen) and area (Area) of all collected images, and calculate the average fluorescence intensity (Mean gray value, Mean) of each image; use statistical analysis software to perform single analysis on the average. Data are presented as the mean ± standard error of the mean (± SEM) and were analyzed using two-way ANOVA to determine statistical significance.
Chromatin immunoprecipitation analysis and qPCR (ChIP-qPCR) analysis
The Chromatin Immunoprecipitation (ChIP) analysis was performed using the PierceTM Magnetic ChIP Kit (Thermo Fisher Scientific, Waltham, MA, USA). Renal cancer cells (786-O) were cross-linked with 1% formaldehyde for 15 min, followed by quenching with a 10X glycine solution for 5 min at room temperature to terminate the reaction. The lysate is then used to disrupt the nuclear membrane. Chromatin was transferred to a 1.5 ml microcentrifuge tube for immunoprecipitation with specific antibodies. Finally, DNA-protein cross-linking was reversed, and the purified DNA was analyzed by quantitative PCR (qPCR).
Statistical analysis
All data were analyzed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA) and are presented as the mean ± standard error of the mean (± SEM). The Shapiro-Wilk test was used to assess whether the data of each group were normally distributed. Two-way ANOVA was used for groups with normal distribution, and Kruskal-Wallis test was used for groups with non-normal distribution to analyze the differences between groups. For post hoc multiple comparison tests, data from ANOVA were analyzed using Tukey’s HSD test, and data from Kruskal-Wallis test were analyzed using Dunn post-test. p < 0.05 was considered statistically significant.
Results
SG improves the general characteristics and renal function indicators of obese mice with hyperuricemic nephropathy
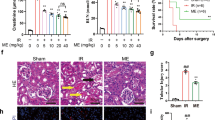
Postoperatively, the body weight of ob/ob mice in both the SG and SHAM groups decreased compared to the CON group. However, while the SHAM group’s weight began to recover after one week, the SG group maintained a more sustained weight reduction (Fig. 2A). The food intake of the SG group and SHAM group began to gradually recover 1 week after surgery (Fig. 2B). Six weeks post-surgery, the SHAM group exhibited increased levels of SUA, serum creatinine, and BUN compared to the CON group (Fig. 2C, E, G), and decreased levels of urinary uric acid, urinary creatinine, and FEUA (Fig. 2D, F, H). Conversely, the SG group displayed reduced levels of SUA, serum creatinine, and BUN compared to the SHAM group, along with increased urinary uric acid, urinary creatinine, and FEUA (Fig. 2C-H).
Effects of sleeve gastrectomy on basic characteristics, serum uric acid levels and renal function markers in ob/ob mice with hyperuricemic nephropathy. (A) body weight, (B) food intake, (C) serum uric acid, (D) urinary uric acid, (E) serum creatinine, (F) urine creatinine, (G) blood urea nitrogen, (H) fractional excretion of uric acid. Data are presented as the mean ± SEM. αP < 0.05, ααP < 0.01 CON vs. SG, *P < 0.05, **P < 0.01 CON vs. SHAM; #P < 0.05, ##P < 0.01 SHAM vs. SG, n = 12 for each group. CON, control group; SHAM, sham group; SG, sleeve gastrectomy group.
Kidney histopathology was improved in sleeve gastrectomy group
HE staining and PAS staining revealed that, compared with the CON group, the SHAM group exhibited pronounced glomerular mesangial proliferation and tubular degeneration along with necrosis, whereas these pathological features were markedly attenuated in the SG group (Fig. 3A). Masson’s trichrome staining demonstrated that renal fibrosis was significantly increased in the SHAM group compared to the CON group, while the SG group showed a reduction in fibrotic changes compared to the SHAM group (Fig. 3A).
Morphological changes in ob/ob mice with hyperuricemic nephropathy following sleeve gastrectomy. (A) Representative images of kidney tissue sections stained with HE, PAS, and Masson (original magnification, ×400; scale bars, 50 μm). (B) Quantitative analysis: the Image-Pro Plus 6.0 image analysis system (Media Cybernetics, Inc., USA) was used to determine the positive expression area (Area) in the collected pictures. (C) Fiber expression analysis: the Image-Pro Plus 6.0 image analysis system (Media Cybernetics Company, USA) was used to determine the fibrous tissue expression area (Area) in the collected pictures. Compared with mice in the CON group, mice in the SHAM group showed glomerular mesangial proliferation, fibrosis, renal tubular degeneration and necrosis, and inflammatory cell infiltration, while the above pathological characteristics of the mice in the SG group were significantly alleviated. Orange arrows indicate renal tubular degeneration and necrosis, green arrows indicate glomerular mesangial proliferation, yellow arrows indicate lymphocyte proliferation, black arrows indicate glycogen deposition, and blue arrows indicate fiber expression. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01 CON vs. SHAM; #P < 0.05, ##P < 0.01 SHAM vs. SG. CON, control group; SHAM, sham group; SG, sleeve gastrectomy group.
SG activates the AMPK/Nrf2 pathway and modulates urate transporters and inflammatory markers of hyperuricemic nephropathy mice
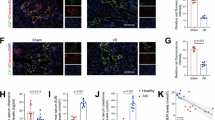
Western blot was employed to assess the levels of p-AMPK and p-Nrf2 in mouse kidney tissues. The analysis revealed increased levels of p-AMPK and p-Nrf2 in the kidneys of mice in the SG group compared to the SHAM group. These findings indicate activation of the AMPK/Nrf2 pathway following SG surgery (Fig. 4A). RT-qPCR demonstrated that the expression of TNF-α, IL-1β, IL-6 in the SG group were lower than that in the SHAM group (Fig. 4B). Furthermore, the expression of UA transporters in the SHAM group and SG group changed significantly (Fig. 4C). Western blot analysis evaluated the expression of key urate transporters in the kidney. Figure 4D illustrates that the SG group exhibited a significantly increased expression of the ABCG2 compared to the SHAM group, while the levels of OAT1, OAT3, URAT1, and GLUT9 remained relatively unchanged. Immunohistochemical analysis revealed enhanced ABCG2 expression in the SG group relative to the SHAM group (Fig. 4E).
Effects of SG on AMPK/Nrf2 pathway, inflammatory markers and urate transporters. (A) Western blot analysis of AMPK/Nrf2 in renal tissue: The levels of p-AMPK and p-Nrf2 in kidney tissues were significantly increased. (B) Renal expression levels of pro-inflammatory cytokines, with reduced TNF-α, IL-1β, and IL-6 in the SG group compared to the SHAM group. (C) RT-qPCR analysis of the expression of urate transporters responsible for urate secretion and reabsorption in renal tissue. After SG surgery, the expression of ABCG2 was significantly different from that in the SHAM group. (D) Western blot analysis and relative protein expression of ABCG2, OAT1, OAT3, GLUT9 and URAT1 protein expression in kidney tissues. The expression level of ABCG2 in the SG group changed significantly, while the expression levels of OAT1, OAT3, GLUT9 and URAT1 had no significant changes. (E) Immunohistochemical staining of ABCG2 in renal tissue from obese mice with HN (original magnification, ×400; scale bars, 40 μm), demonstrating increased ABCG2 expression in the SG group relative to the SHAM group. Original blots are presented in Supplementary Fig. 1. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01 CON vs. SHAM; #P < 0.05, ##P < 0.01 SHAM vs. SG. CON, control group; SHAM, sham group; SG, sleeve gastrectomy group.
Activation of AMPK/Nrf2 pathway enhances ABCG2 expression in HK-2 cells
In wild-type HK-2 cells, ABCG2 expression was assessed following treatment with the AMPK activator A-769,662, which resulted in a significant upregulation (Fig. 5A-B). Next, we evaluated the ABCG2 expression in HK-2 cells with reduced Nrf2 expression post-lentiviral transfection. According to the results of CCK-8, A-769,662 at a concentration of 300 µM for 24 h can maintain cell viability (Supplementary Fig. 2). We then quantified ABCG2 expression in the Nrf2 knockdown HK-2 cell line following A-769,662 treatment. This revealed a markedly decreased expression of ABCG2 in the Nrf2 knockdown cells compared to the wild-type HK-2 cells (Fig. 5C-D). Furthermore, similar results were observed in the immunofluorescence assay (Fig. 5E-F).
The increased activity of ABCG2 is mediated by Nrf2 upregulation following. (A-D) Western Blot detects AMPK and Nrf2 phosphorylation levels and ABCG2 expression analysis in HK-2 cells. After the application of A-769,662, the activity of ABCG2 in the KD group was lower than that in the Vector group, while the levels of ABCG2 in other groups increased significantly after the application of A-769,662; (E-F) Immunofluorescence staining and fluorescence intensity analysis of ABCG2 in HK-2 cells (original magnification ×400, scale bars = 20 μm). Compared with the PBS group, UA group, DMSO group and Vector group, the fluorescence intensity of ABCG2 in the A-769,662 group increased significantly, and the fluorescence intensity of ABCG2 in the KD group decreased significantly. Original blots are presented in Supplementary Fig. 3. DAPI-stained cell nuclei are blue, and positive expression of ABCG2 is green. Data were expressed as the mean ± SEM. *P < 0.05 UA vs. A-769,662 group, **P < 0.01 UA vs. A-769,662 group, #P < 0.05 A-769,662 vs. DMSO group, ##P < 0.01 A-769,662 vs. DMSO group, @P < 0.05 KD vs. A-769,662 group, @@P < 0.01 KD vs. A-769,662 group, $P < 0.05 Vector vs. KD group, $$P < 0.01 Vector vs. KD group.
PBS group, control cells incubated in DMEM supplemented with PBS; UA group, cells treated with UA at a concentration of 4 mg/dL in DMEM; DMSO group, control cells treated with DMSO in DMEM containing UA at a concentration of 4 mg/dL; A-769,662 group, cells incubated in DMEM with UA (4 mg/dL) and the AMPK activator A-769,662 at a concentration of 300 µM; Vector group, cells incubated in DMEM with UA (4 mg/dL) and A-769,662 (300 µM) and transfected with an empty control vector; KD group, cells incubated in DMEM with UA (4 mg/dL), A-769,662 (300 µM), and infected with a lentivirus targeting Nrf2.
Nrf2 binding to ABCG2 promoter region
To further investigate the potential interaction between Nrf2 and ABCG2, we conducted ChIP analysis in renal cancer cells (786-0). Utilizing the UCSC Genome Browser database, we identified three putative Nrf2 binding sites within the ABCG2 gene locus, and accordingly, designed the corresponding detection primers: ABCG2-P1 (F1/R1), ABCG2-P2 (F2/R2), and ABCG2-P3 (F3/R3). Additionally, as reported in the literature32,33, a primer pair F4/R4 was designed to target the − 431 to -420 bp Antioxidant Response Element (ARE) within the ABCG2 promoter region, which includes the Nrf2 binding motif (Table 2). The detection regions of the four pairs of primers all contain the ARE cis-acting element of the Nrf2 protein bZIP core binding site. As depicted in Fig. 6B-C, the ChIP-PCR data suggest that Nrf2 binds to ABCG2 promoter region, with a significantly higher signal enrichment observed in the Nrf2-specific group compared to the negative control IgG group (Fig. 6).
Nrf2 binds to the ABCG2 promoter region. (A) Schematic of the consensus motif. (B) Representation of the primer detection regions, with the red segments highlighting the predicted Nrf2 binding sites. (C,D) ChIP assays demonstrate Nrf2’s interaction with the ABCG2 in 786-0 cells. Data are presented as the mean ± SEM. *P < 0.05 Nrf2 vs. IgG, **P < 0.01 Nrf2 vs. IgG.
Discussion
This study aimed to investigate the effects and underlying mechanisms of SG on renal injury induced by HN and to ascertain the regulation of kidney-related urate transporter expression by SG, elucidating the specific mechanism by which bariatric surgery improves HN in obese mice. Our findings show that SG improved the renal histological changes and biochemical indicators — such as serum uric acid, serum creatinine, and blood urea nitrogen in obese mice with HN, concurrent with the activation of the AMPK/Nrf2 pathway and upregulation of ABCG2 expression. Interestingly, the expression of other urate transporters (such as OAT1, OAT3, URAT1, and GLU9) did not show significant expression differences after SG surgery. In addition, the downregulation of inflammatory markers also suggests a positive effect of bariatric surgery. In vitro experiments, we constructed a high uric acid environment and employed AMPK activator A-769,662 to verify the upregulatory effect of AMPK/Nrf2 on ABCG2. Through ChIP-PCR, we confirmed that NF-E2-related factor 2 (Nrf2) binds to the promoter region of ABCG2, which may promote the transcription of ABCG2 and thus increase its expression level, further verified that the activation of AMPK leads to increased Nrf2 activity and upregulation ABCG2 expression.
Studies have reported that obesity is related to a variety of metabolic diseases, including HUA. Thus, managing body weight and optimizing metabolic profiles are crucial for preventing and treating a range of chronic diseases, including HUA34. Bariatric surgery, uniquely capable of sustaining weight loss and ameliorating comorbidities in obese individuals35. The association between bariatric surgery and the AMP-activated protein kinase (AMPK) pathway has been well-established by many researchers. Bariatric surgery indirectly regulates the expression of downstream targets such as sirtuin-1 (SIRT1), protein kinase C-zeta (PKC-ζ), and nuclear receptor subfamily 4 group A member 1 (NR4A1) by activating the AMPK pathway. This activation can lead to enhanced fatty acid oxidation, improved glucose metabolism, and augmented mitochondrial function36,37. Furthermore, research has demonstrated that AMPK can mitigate ferroptosis through the regulation of downstream Nrf2, thereby improving renal tubular apoptosis, reducing insulin resistance, and ameliorating diabetic cardiomyopathy38,39,40. In our prior research, we observed that Roux-en-Y gastric bypass effectively controlled long-term blood pressure in obese hypertensive rats and prevented renal damage by decreasing renal inflammation, a response that was mediated by alterations in the NLRP3-ASC/IL-1β pathway41. Building on these findings, we hypothesize that the renal protective effects of SG in HN may also be attributed to the downregulation of inflammatory markers. SG exerts a mitigating effect on HN-related renal injury, potentially through dual mechanisms.
HUA affects renal function in various ways, such as urate crystal accumulation, interstitial nephritis, and reduced glomerular filtration rate, and is an indicator of prognosis in CKD and end-stage renal disease42,43. HUA pathogenesis is a multifactorial process involving the complex interplay of numerous regulatory pathways. The complex etiology of the disease is one factor contributing to the challenges in its treatment. The AMPK pathway is pivotal in regulating cellular energy metabolism and maintaining metabolic balance44. Research indicates that the AMPK pathway can modulate SUA levels by regulating SIRT145. Urate transporters play a critical role in the renal handling and excretion of uric acid, significantly influencing SUA levels. ABCG2, also known as BCRP (Baeast Cancer Resistance Protein), is a membrane protein belonging to the ATP-binding cassette (ABC) transporter family. The ABC transporter family comprises transmembrane proteins found across diverse species, harnessing energy from ATP hydrolysis to facilitate the translocation of substances across cellular membranes or within the membrane itself46. ABCG2 is predominantly expressed in the brush border membrane of the renal proximal tubules, where it actively transports uric acid out of the cells, thus modulating uric acid concentrations47. Our research identified the modulatory impact of SG on renal ABCG2 expression, highlighting its potential role in the prevention and management of HUA and renal impairment. Furthermore, we also found that ABCG2 activity after bariatric surgery is closely related to the AMPK/Nrf2 pathway, and the development of novel drugs based on this pathway may be significant for regulating urate excretion and thus improving HN.
It is important to acknowledge the limitations of our study. Firstly, while our study focused on renal ABCG2 expression due to its high levels in the kidney47, it should be noted that ABCG2, albeit in smaller quantities, is also expressed in the intestine and contributes to uric acid homeostasis48. Considering this, future research will encompass a broader range of extrarenal tissues to provide a more systematic and comprehensive assessment of the effects of bariatric surgery on urate excretion. In addition, many other signaling pathways may also play a role in improving metabolism by regulating the expression of ABCG2, such as the PI3K-Akt signaling pathway, the Wnt/β-catenin signaling pathway, etc. Whether they can play a role in improving HN after bariatric surgery requires further discussion in future studies. Furthermore, in this study, we used mice as one of the experimental models for research, however, it must be acknowledged that there are certain species differences between mice and humans, which may lead to differences in their responses to SG, and these differences may affect the applicability and interpretation of the research results in clinical practice. Finally, the precise mechanism through which Nrf2 enhances ABCG2 expression remains to be fully elucidated. We hypothesize that Nrf2 may upregulate ABCG2 by augmenting the transcriptional activity of the ABCG2 promoter; however, further experimental evidence is required to confirm this.
Conclusion
As shown in Fig. 7, our study demonstrates bariatric surgery’s potential to ameliorate HN in murine model, possibly through the upregulation of ABCG2 expression in the kidneys, thereby enhancing uric acid excretion and ameliorating renal injury. Additionally, our findings indicate that bariatric surgery enhances the phosphorylation of AMPK and Nrf2, subsequently activating ABCG2 by facilitating Nrf2’s binding to ABCG2 promoter region. Our findings offer novel insights into the mechanisms underlying the renoprotective effects of bariatric surgery and shed light on potential targeted therapies for HUA. Future research will explore additional mechanisms that may contribute to the beneficial effects of bariatric surgery on HN patients.
Data availability
The data that support the findings of this study are available from the corresponding author, Yixing Ren, upon reasonable request.
References
Perdomo, C. M. et al. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet401(10382), 1116–1130. https://doi.org/10.1016/s0140-6736(22)02403-5 (2023).
Ran, Z. et al. Decrease in serum urate level is associated with loss of visceral fat in male gout patients. Front. Endocrinol. (Lausanne) 12, 724822. https://doi.org/10.3389/fendo.2021.724822 (2021).
Dalbeth, N. et al. Gout. Lancet397(10287), 1843–1855. https://doi.org/10.1016/s0140-6736(21)00569-9 (2021).
Shi, J. et al. Prevalence and correlates of metabolic syndrome and its components in Chinese children and adolescents aged 7–17: The China National Nutrition and Health Survey of Children and Lactating mothers from 2016–2017. Nutrients14(16). https://doi.org/10.3390/nu14163348 (2022).
Song, S. et al. Alteration of gut microbiome and correlated amino acid metabolism contribute to hyperuricemia and Th17-driven inflammation in Uox-KO mice. Front. Immunol.13, 804306. https://doi.org/10.3389/fimmu.2022.804306 (2022).
Yanai, H. et al. Molecular Biological and Clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int. J. Mol. Sci.22(17). https://doi.org/10.3390/ijms22179221 (2021).
Braga, T. T., Foresto-Neto, O. & Camara, N. O. S. The role of uric acid in inflammasome-mediated kidney injury. Curr. Opin. Nephrol. Hypertens.29(4), 423–431. https://doi.org/10.1097/mnh.0000000000000619 (2020).
Hyndman, D., Liu, S. & Miner, J. N. Urate Handling in the human body. Curr. Rheumatol. Rep.18 (6), 34. https://doi.org/10.1007/s11926-016-0587-7 (2016).
Suliman, M. E. et al. J-shaped mortality relationship for uric acid in CKD. Am. J. Kidney Dis.48(5), 761–771. https://doi.org/10.1053/j.ajkd.2006.08.019 (2006).
Zhu, Y., Zhang, Y. & Choi, H. K. The serum urate-lowering impact of weight loss among men with a high cardiovascular risk profile: The multiple risk factor intervention trial. Rheumatology (Oxford) 49(12), 2391–2399. https://doi.org/10.1093/rheumatology/keq256 (2010).
Dessein, P. H. et al. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann. Rheum. Dis.59(7), 539–543. https://doi.org/10.1136/ard.59.7.539 (2000).
Gliozzi, M. et al. The treatment of hyperuricemia. Int. J. Cardiol. 213, 23 – 72. https://doi.org/10.1016/j.ijcard.2015.08.087 (2015).
Wadden, T. A. et al. End-of-trial health outcomes in look AHEAD participants who elected to have bariatric surgery. Obesity (Silver Spring) 27(4), 581–590. https://doi.org/10.1002/oby.22411 (2019).
Arterburn, D. E. & Courcoulas, A. P. Bariatric surgery for obesity and metabolic conditions in adults. BMJ349, g3961. https://doi.org/10.1136/bmj.g3961 (2014).
Zhang, X. et al. Gender difference in the relationship between serum uric acid reduction and improvement in body fat distribution after laparoscopic sleeve gastrectomy in Chinese obese patients: A 6-month follow-up. Lipids Health Dis.17(1), 288. https://doi.org/10.1186/s12944-018-0934-y (2018).
Lu, J. et al. Effects of bariatric surgery on serum uric acid in people with obesity with or without hyperuricaemia and gout: a retrospective analysis. Rheumatology (Oxford) 60(8), 3628–3634. https://doi.org/10.1093/rheumatology/keaa822 (2021).
Song, K. et al. Benefits of uric acid-lowering medication after bariatric surgery in patients with gout. BMC Surg.24(1), 186. https://doi.org/10.1186/s12893-024-02472-6 (2024).
Choi, H. K. & Zhang, Y. Bariatric surgery as urate-lowering therapy in severe obesity. Ann. Rheum. Dis.73(5), 791–793. https://doi.org/10.1136/annrheumdis-2013-204861 (2014).
Davoudi, Z. et al. Impact of sleeve gastrectomy on uric acid levels in patients with obesity: A comparative study. Obes. Surg.34(7), 2704–2710. https://doi.org/10.1007/s11695-024-07356-5 (2024).
McAdams-DeMarco, M. A. et al. A urate gene-by-diuretic interaction and gout risk in participants with hypertension: results from the ARIC study. Ann. Rheum. Dis.72(5), 701–706. https://doi.org/10.1136/annrheumdis-2011-201186 (2013).
Toyoda, Y. et al. Functional characterization of clinically-relevant rare variants in ABCG2 identified in a gout and hyperuricemia cohort. Cells 8(4). https://doi.org/10.3390/cells8040363 (2019).
Enomoto, A. & Endou, H. Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clin. Exp. Nephrol.9(3), 195–205. https://doi.org/10.1007/s10157-005-0368-5 (2005).
Eraly, S. A. et al. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol. Genomics33(2), 180–192. https://doi.org/10.1152/physiolgenomics.00207.2007 (2008).
Habu, Y. et al. Decreased activity of basolateral organic ion transports in hyperuricemic rat kidney: roles of organic ion transporters, rOAT1, rOAT3 and rOCT2. Biochem. Pharmacol. 66(6), 1107–1114. https://doi.org/10.1016/s0006-2952(03)00466-0 (2003).
Preitner, F. et al. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc. Natl. Acad. Sci. U S A. 106 (36), 15501–15506. https://doi.org/10.1073/pnas.0904411106 (2009).
So, A. & Thorens, B. Uric acid transport and disease. J. Clin. Invest.120 (6), 1791–1799. https://doi.org/10.1172/jci42344 (2010).
Wu, X. H. et al. Riparoside B and timosaponin J, two steroidal glycosides from Smilax Riparia, resist to hyperuricemia based on URAT1 in hyperuricemic mice. Phytomedicine 21(10), 1196–1201. https://doi.org/10.1016/j.phymed.2014.03.009 (2014).
Wang, M. X. et al. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur. J. Pharmacol.747, 59–70. https://doi.org/10.1016/j.ejphar.2014.11.035 (2015).
Wang, M. et al. Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomed. Pharmacother.83, 975–988. https://doi.org/10.1016/j.biopha.2016.07.025 (2016).
Chen, G. et al. Green tea polyphenols decreases uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J. Ethnopharmacol.175, 14–20. https://doi.org/10.1016/j.jep.2015.08.043 (2015).
Ayer, A. et al. Techniques of sleeve gastrectomy and modified Roux-en-Y Gastric bypass in mice. J. Vis. Exp. (121). https://doi.org/10.3791/54905 (2017).
Zhan, M. et al. Variants in oxidative stress-related genes affect the chemosensitivity through Nrf2-mediated signaling pathway in biliary tract cancer. EBioMedicine48, 143–160. https://doi.org/10.1016/j.ebiom.2019.08.037 (2019).
Singh, A. et al. Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotype. Mol. Cancer Ther.9(8), 2365–2376. https://doi.org/10.1158/1535-7163.Mct-10-0108 (2010).
Obermayr, R. P. et al. Elevated uric acid increases the risk for kidney disease. J. Am. Soc. Nephrol.19(12), 2407–2413. https://doi.org/10.1681/asn.2008010080 (2008).
Nielsen, S. M. et al. Weight loss for overweight and obese individuals with gout: a systematic review of longitudinal studies. Ann. Rheum. Dis.76(11), 1870–1882. https://doi.org/10.1136/annrheumdis-2017-211472 (2017).
Li, S. et al. Sleeve gastrectomy-induced AMPK activation attenuates diabetic cardiomyopathy by maintaining mitochondrial homeostasis via NR4A1 suppression in rats. Front. Physiol., 13, 837798. https://doi.org/10.3389/fphys.2022.837798 (2022).
Peng, Y. et al. Does LKB1 mediate activation of hepatic AMP-protein kinase (AMPK) and sirtuin1 (SIRT1) after Roux-en-Y gastric bypass in obese rats? J. Gastrointest. Surg.14 (2), 221–228. https://doi.org/10.1007/s11605-009-1102-5 (2010).
Zhang, Y. et al. Sulforaphane alleviates high fat diet-induced insulin resistance via AMPK/Nrf2/GPx4 axis. Biomed. Pharmacother. 152, 113273. https://doi.org/10.1016/j.biopha.2022.113273 ( ).
Wang, X. et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm. Sin. B12(2), 708–722. https://doi.org/10.1016/j.apsb.2021.10.005 (2022).
Lu, Q. et al. Empagliflozin attenuates the renal tubular ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway. Free Radic. Biol. Med.195, 89–102. https://doi.org/10.1016/j.freeradbiomed.2022.12.088 (2023).
Zhang, Y. et al. Long-term protective effects and mechanisms of gastric bypass surgery on the kidneys in hypertensive obese rat. Obes. Surg. https://doi.org/10.1007/s11695-024-07108-5 (2024).
Xu, X. et al. Uric acid transporters hiding in the intestine. Pharm. Biol.54(12), 3151–3155. https://doi.org/10.1080/13880209.2016.1195847 (2016).
Iseki, K. et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am. J. Kidney Dis.44(4), 642–650 (2004).
Herzig, S. & Shaw, R. J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol.19(2), 121–135. https://doi.org/10.1038/nrm.2017.95 (2018).
Chen, L. & Lan, Z. Polydatin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation by inhibiting NF-κB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food Funct.8(5), 1785–1792. https://doi.org/10.1039/c6fo01561a (2017).
Locher, K. P. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol.23(6), 487–493. https://doi.org/10.1038/nsmb.3216 (2016).
Eckenstaler, R. & Benndorf, R. A. The role of ABCG2 in the pathogenesis of primary hyperuricemia and Gout-An update. Int. J. Mol. Sci.22(13). https://doi.org/10.3390/ijms22136678 (2021).
Yin, H., Liu, N. & Chen, J. The role of the intestine in the development of hyperuricemia. Front. Immunol.13, 845684. https://doi.org/10.3389/fimmu.2022.845684 (2022).
Funding
This study was supported by the National Natural Science Foundation of China (82070535,82370601), Natural Science Foundation of Sichuan Province (2022NSFSC0706), Science and Technology Program of the Sichuan Provincial Health and Wellness Commission(23LCYJ031), Bureau of Science and Technology Nanchong City (22SXQT0126, 22YYJCYJ0054) and North Sichuan Medical College Clinical School·Affiliated Hospital Scientific Research Development Plan Project (2024-1KJ002).
Author information
Authors and Affiliations
Contributions
Ke Song, Xiangxin Kong, Zhongyang Zhang and Yin Xian contributed equally to this work and should be considered as co-first authors. Conceptualization, K.S. and Y.X.R.; methodology, K.S., X.X.K., M.H. and Y.X.; investigation, K.S. and Z.Y.Z.; resources, X.X.L., Z.Y.H. and Y.Z.; data curation, A.J.K. and D.Q.X.; writing—original draft preparation, K.S.; writing—review and editing, K.S. and Y.X.; funding acquisition, M.H., Y.X. and Y.X.R. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures were approved by the local animal ethics committee (West China Hospital Experimental Animal Ethics Committee, license number: 20240422010). All methods were carried out in accordance with relevant guidelines and regulations, and all animal studies complied with relevant ethical regulations on animal testing and research and followed by the ARRIVE guidelines (https://arriveguidelines.org).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, K., Kong, X., Zhang, Z. et al. Sleeve gastrectomy ameliorates renal injury in obesity-combined hyperuricemic nephropathy mice by modulating the AMPK/Nrf2/ABCG2 pathway. Sci Rep 14, 22834 (2024). https://doi.org/10.1038/s41598-024-73807-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73807-9
Keywords
This article is cited by
-
Obesity: pathophysiology and therapeutic interventions
Molecular Biomedicine (2025)
-
The effect of laparoscopic sleeve gastrectomy on serum uric acid levels: a meta-analysis
European Surgery (2025)