Abstract
The surveillance and detection of zoonotic pathogens in animals is essential for predicting disease transmission pathways and the risks of spillover, but challenges include the costs, ethics and technical expertise required for vertebrate trapping, serum sampling and antibody or virus screening. Surveillance using haematophagous arthropods as a sampling tool offers a unique opportunity to obtain blood samples from a wide range of vertebrate species, allowing the study of host-mosquito associations, and host exposure to pathogens. We explored vertebrate diversity and potential Ross River virus (RRV) transmission pathways by analysing blood-fed mosquitoes collected in Brisbane, Australia. Host origins were identified using barcode sequencing, and host exposure to RRV was assessed using a modified plaque reduction neutralisation test. In total, 480 blood-fed mosquitoes were collected between February 2021 and May 2022. The host origins of 346 (72%) bloodmeals were identified, with humans (73%) and cattle (9%) comprising the dominant hosts. RRV seroprevalence was high in both vertebrate species with evidence of RRV exposure in 70% (21/30) of cattle and 52% (132/253) of humans. This is a novel, non-invasive method of estimating seroprevalence in vertebrate host populations. Our results highlight the potential of blood-fed mosquitoes to provide species-specific insights into pathogen transmission dynamics.
Similar content being viewed by others
Introduction
The emergence, and re-emergence of infectious diseases is difficult to predict and may have devastating economic and social consequences locally and globally. In recent decades we have witnessed the appearance of novel infectious diseases1 a shifting distribution of vectors and pathogens2, and the resurgence of mosquito-borne diseases, such as malaria in the USA3, yellow fever in Brazil4 and Japanese encephalitis in Australia5. This underscores an urgent and critical need for surveillance systems that can detect and monitor transmission across multiple vectors, pathogen reservoirs and environments. This challenge is complicated for mosquito-borne zoonoses, because extensive surveillance is required across time, space and multiple species. Understanding the transmission ecology of such multi-host pathogens is a major challenge6,7 and new, robust, scalable, and cost-effective approaches to surveillance are needed, for implementation across a variety of environments, vectors, reservoirs and pathogens8. Xeno-surveillance may offer a solution, using the blood-feeding behaviour of arthropods to determine vector-host relationships and to survey vertebrates for pathogen signals9 without having to sample vertebrates directly.
Haematophagous insects can yield information on local pathogen prevalence without being part of the transmission cycle. For example, collections of tsetse flies in Tanzania10 and Culex mosquitoes in the Brazilian Amazon11 yielded malaria parasites while urban Culex collections identified dengue, Zika and chikungunya viruses in Pernambuco, Brazil12.The presence of non-vector-borne pathogens, such as hepatitis C virus8 and H5N1 virus13 have also been noted in mosquito collections. Xeno-surveillance can also be used to detect vertebrate antibodies to pathogens. Proof-of-principle studies have demonstrated that pathogen-specific antibodies can be recovered from blood-engorged mosquitoes fed with vertebrate blood containing naturally-acquired antibodies in the laboratory (e.g. Toxoplasma gondii and SARS-CoV-2 antibodies14) and from wild mosquitos collected from disease endemic areas (e.g., dengue and Japanese encephalitis virus antibodies15). Antibodies to the pathogenic piroplasm Theileria sergenti have been recovered from ticks16. These proofs detect host-specific conjugated antibodies, and so cannot be adapted to determine exposure in bloodmeals derived from multiple hosts. This makes them unsuitable for investigating complex zoonoses (i.e., those with multiple vertebrate hosts such as Ross River virus (RRV)) where vertebrate blood sources are diverse or unknown.
The analysis of the vertebrate host preferences of haematophagous arthropods, using molecular identification of bloodmeals is common practice and a key part of investigations on potential transmission pathways17,18,19,20, but only recently have bloodmeals also provided an opportunity to determine pathogen exposure in the host21. Screening for live virus in large undifferentiated mosquito collections is onerous and time consuming, and usually only a low detection rate is achievable22. It does not indicate the vertebrate source of the virus nor the vector association. Simultaneous screening of pathogen-specific antibodies and host identification in bloodmeals from specific mosquito species yields information on vector-host interactions, vertebrate exposure to target pathogens and, therefore, potential virus transmission pathways.
Plaque reduction neutralisation tests (PRNTs) are the gold standard for quantifying the presence of neutralising antibodies in serum samples. Unlike other assays (e.g. ELISAs or antigen assays) they are not reliant on host-specific conjugated antibodies and can therefore be applied to serum from any source. This is of particular relevance for the investigation of complex zoonoses like RRV where potential hosts are myriad or unknown. The PRNT has been adapted for screening RRV antibodies from the small volumes of blood present in mosquito abdomens21 and can be used for all vertebrates23,24. In tandem with DNA barcoding of the vertebrate origin of the bloodmeal, the adapted PRNT can give a measure of vertebrate host exposure.
DNA barcoding is commonly applied to identify invertebrate bloodmeal sources using species specific fragments of cytochrome c oxidase subunit 1 (CO1) and/or cytochrome b (cyt b) genes for molecular identification18,19,25,26. This technique supports the use of haematophagous insects as “flying syringes”10 by representing a means of surveying vertebrate diversity by exploiting the host-seeking behaviours of mosquitoes. These may be effective sampling tools where mosquitoes are abundant and exhibit generalist, opportunistic feeding patterns.
The objective of this study was to demonstrate that blood-fed mosquitoes, collected as a part of routine surveillance exercises, can yield useful insights regarding vertebrate diversity, host preference, and virus exposure in the vertebrate supplying the bloodmeal. This can help identify potential disease transmission pathways. We focus on RRV as a case study, because it is endemic within our study area, a nationally notifiable disease of significant public health importance in Australia, and has the potential for global spread27. RRV is a complex mosquito-borne zoonosis, circulating between multiple vertebrate species and mosquito vectors, and regularly “spilling over” into humans28. We addressed three questions: (1) do existing trapping networks yield mosquitoes with bloodmeals of sufficient quality to yield data on host feeding patterns and RRV antibody prevalence? (2) how do mosquito feeding patterns reflect vertebrate diversity and relative host preference? (3) how does host antibody prevalence revealed by bloodmeals compare with published, conventional sero-surveys for RRV?
To answer these questions, we collected mosquitoes in CO2-baited light traps from four urban parks in Brisbane from February to May 2021 and 2022. We selected all of the blood fed mosquitoes from these collections, determined the vertebrate host origin of those blood meals by DNA barcoding, and screened for host exposure to RRV by using a modified PRNT. Conventional vertebrate surveys were conducted from November 2017 to March 2018 and from March 2020 and April 2021. That data was used to explore correlations between vertebrate diversity, host preference and RRV exposure in the study area.
Results
A total of 54,670 mosquitoes were collected during the trapping period of this study (February to May 2021 and 2022) as part of an established mosquito surveillance program implemented by Brisbane City Council (BCC), Queensland, Australia, and some additional traps deployed by Queensland Health, Australia. Of these, 480 mosquitoes (0.9% of the total catch) from 14 species were identified as being blood-fed (Supplementary Table S1). Five species accounted for > 85% of blood-feds collected: Culex annulirostris (68.7%), Cx. orbostiensis (9.4%), Aedes procax (3.9%), Cx. sitiens (3.7%), and Verrallina Marks sp. No. 52 (2.71%).
A single vertebrate origin was identified from 341 bloodmeals, while another five bloodmeals were of mixed origin (human and one other vertebrate), totalling 346 identified bloodmeals (Table 1 and Supplementary Table S2). A total of 26 vertebrate species were identified from these bloodmeals, 10 birds, nine placental mammals, three marsupials, two reptiles, one amphibian, and one semi-aquatic fish. Humans were the dominant source of bloodmeals, accounting for 73.1% of bloodmeals (253/346), followed by cattle and marsupials (8.7% and 6.9% of bloodmeals, respectively).
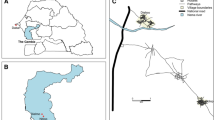
By comparison, conventional vertebrate surveys, conducted by human observers at the same sites, identified a much higher number of vertebrate species (85). Most of these species were birds (74), with 6 placental mammals, 2 marsupials, 2 amphibians and a reptile. The vertebrate survey did not observe cattle, deer, fox, sheep, or fish, all of which were fed on by mosquitoes. Human abundance was not quantified during conventional vertebrate surveys, but was estimated using population statistics from areas within the dispersal kernel of the key mosquito species sampled (Fig. 1).
All 480 bloodmeal samples collected in this study were screened for the presence of anti-RRV antibodies using an adapted micro-PRNT21 and more than half (n = 243, 51%) were seropositive (Table 1). RRV seroprevalence was 52% (131/253) in humans, 70% in cattle and eastern grey kangaroos (21/30 and 14/20, respectively), and ranged from 25 to 100% in samples from other animals. This is comparable with published data on conventional sero-surveys (Supplementary Table S3) that report seroprevalences of 7–79% in humans, 36–100% in eastern grey kangaroos.
Among the 14 blood-fed mosquito species, 12 were found to have fed on RRV seropositive hosts, with Ae. alternans (n = 1) and Uranotaenia nivipes (n = 1) being the exceptions.
Discussion
This is the first field demonstration that mosquito bloodmeals can be used as a resource for estimating vertebrate seroprevalence. Our results indicate varying seroprevalence of RRV antibodies across different host species, and some common combinations of vector, vertebrate and antibody combinations that highlight potential transmission pathways. RRV is thought to be maintained in the environment by multiple host and vector species, with little understanding about how this community shifts across environments, seasons, and the degree of spillover to the human population. High seroprevalence demonstrated in this blood meal study and in conventional surveys (Supplementary Table S3) was demonstrated among humans (ranging from 7 to 79%), horses (21 to 94%), cattle (6 to 100%), and kangaroos (36 to 100%), along with a very limited data-set on species presenting with RRV-induced viraemias28,29,30, suggest that their susceptibility to RRV infection and their role as amplifying or diluting RRV hosts needs to be re-examined; even for those species long thought of as ‘dead-end’ hosts (e.g. cattle).
Cattle are not considered important reservoir hosts for RRV, because under experimental conditions only one individual (of six) developed viraemia which was low and short-lived29. Yet, our results highlight that cattle are frequently exposed to RRV and may contribute to transmission dynamics. Notably, little corellas (Cactua sanguinea) and cattle develop near identical viraemic responses to infection in terms of peak and duration and the former was shown to infect 14% of susceptible Cx. annulirostris mosquitoes29. Additionally, varying seroprevalence in blood meals from other placental mammals, including deer, red foxes and some birds, underscores the catholic feeding behaviour of RRV vectors and the potential complexity of RRV’s host range. Our data emphasises the fact that, 60 years after the initial solation of RRV from a mosquito vector31 we still have little understanding of the reservoirs or their role in maintain transmission cycles in different habitats. For example, we show that deer are clearly exposed to RRV, but there are no experimental studies that assess the potential for confirm transmission.
Humans are potential amplifying hosts of RRV28,32,33. Their high RRV seroprevalence rates indicate high rates of exposure. The human seropositivity in this blood meal study (52%) agrees with previous, conventional sero-surveillance studies in QLD with antibody prevalence of 9–50% (Supplementary Table S3). It is notable that this study sampled bloodmeals from mosquitoes caught in urban parklands. Although the movement patterns of those humans is unknown, the high seropositivity rates suggest that at least some of those humans are being exposed in an urban transmission cycle rather than a rural one.
Culex annulirostris, a major vector of RRV and other medically-important arboviruses in Australia34,35, is a freshwater mosquito that is abundant across the country. They are considered some of the most prominent vectors of RRV due to (i) being a frequent sources of RRV isolates, with over 250 isolates36,37,38, (ii) their demonstrated ability to become infected with and transmit RRV under laboratory settings (e.g., from horses or little corellas29), and (iii) spatio-temporal associations between infected Cx. annulirostris and RRV disease36,39. They are generalist-opportunistic feeders and feed readily on humans18,40,41,42. Cx. annulirostris was the most abundant species in this study, as reported in other Queensland collections36,39. It also yielded the greatest number of bloodmeals for analysis and was found to have fed on six bird and 12 mammal species. This suggests a wide host range with potential for a variety of RRV transmission pathways. The species is likely a key means of spillover to humans. Its eclectic feeding habits also makes it a powerful sampling tool for sampling vertebrate diversity.
Further empirical research on viraemic response and transmission to mosquitoes is desirable for many of the potential hosts identified in this study. However, these experimental infection studies for complex zoonoses are ethically and logistically difficult. The micro-PRNT approach serves as a valuable, field-based but circumstantial alternative that can aid pathway incrimination and prioritise targets for further study.
The micro-PRNT method estimates pathogen exposure by screening mosquito bloodmeals for antibodies produced in response to viral infection in the vertebrate host. These antibodies can persist for the lifetime of the exposed vertebrate. This is in contrast to viraemias which are typically short. Antibodies therefore are a more reliable measure of exposure than the prevalence of live infections. Similarly, mosquitoes are often extremely abundant, have short lifespans and take bloodmeals at fixed intervals (the gonotrophic cycle). There are therefore limited opportunities for them to acquire virus, and “hit” rates during screening can be very low22. Moreover, the presence of virus in a mosquito does not necessarily relate to transmission. Mosquitoes can be incidental carriers of pathogens that are circulating in the environment10,11,12. There is no “smoking gun” in terms of transmission proofs from field-collections of vertebrates or mosquitoes.
The capacity to characterise host exposure to pathogens such as RRV, across multiple vertebrates, using mosquito blood meals is demonstrated for the first time in the field. This is an exciting development that can offer descriptive insights into potential virus reservoirs and transmission pathways. It is also a potentially powerful means of quantifying key parameters in deterministic compartmental estimates of disease transmission such as Susceptible-Infected-Recovered (SIR) models. These require measures of the proportion susceptible, infected and immune (recovered) individuals. For multi-host pathogens this can be highly challenging because estimates must be made for multiple populations30. This is traditionally done through vertebrate surveys and a great deal of guess work. Antibody screening in bloodmeals allows a direct measure of the S (susceptible) and R (immune) proportion of the reservoir population and could help build transmission models. The “infected” parameter in these models could be derived through an additional screen of the mosquito bloodmeals for (rare) virus signals.
Collection and analysis of blood-fed mosquitos offers an alternative to conventional vertebrate surveys. Excluding bird species, the number of vertebrate species represented by mosquito bloodmeals was greater than that observed during conventional faunal surveys. This is probably because the observations made during traditional surveys will be more likely to capture species that are present along the transects, least cryptic, locally abundant and most active. Traditional surveys may record greater bird diversity because observers use additional cues such as bird calls. Using mosquitoes as the sampling tool, we may have greater access to species that are cryptic, at low density, or not captured by traditional survey transects and short observation times. For example, cattle were not noted during surveys across transects, but they were a relatively common source of blood meals.
Humans made up the majority of bloodmeals across all locations, although they only represent a small proportion of vertebrate diversity. Our estimates of accessible humans were based on residential populations within the effective dispersal kernel of mosquitoes. Thus, we undoubtedly overestimated the human population available to the mosquito. The high proportion of human bloodmeals may reflect host availability in the study area. Humans were likely to represent a large proportion of the overall vertebrate biomass in the parks, particularly at dawn and dusk (i.e., peak times for commuting or recreation may coincide with the crepuscular rhythms of many mosquito species). This would undoubtedly influence mosquito feeding patterns. There are over 2,180 parks across Brisbane43, and they may be major foci for transmission and human spillover, the specific patterns of which will depend on vertebrate diversity, virus amplification and virus dilution.
The diversity of bloodmeal origins captured across a number of mosquito species, confirms that mosquitoes are a powerful, emerging tool for characterising vertebrate diversity. The technique has potential application to a range of ecological and conservation issues, such as monitoring rare or endangered species44, and measuring biodiversity45,46. It can also be used to understand host feeding patterns, which is a crucial component for assessing the risk of pathogen transmission pathways and human spillover. It is as important to identify non-reservoirs and the dilution of transmission as it is to identify potential virus sources. For example, some mosquito species will preferentially feed on reptiles, amphibians and even fish (particularly Uranotaenia spp.)47. An expansion to this study, involving greater numbers of bloodmeals, might explore whether some vertebrates are predictably “non-reactive” and whether some mosquito species show strong preference for those vertebrates.
Blood-fed mosquitoes can be difficult to collect in sufficient quantities for surveillance campaigns48. The baited, fan-assisted traps commonly used for mosquito surveillance in the field (i.e., BioGents Sentinel traps, CDC light traps or CDC gravid traps) target mosquitoes that are seeking hosts or an oviposition site. Blood-fed mosquitoes are not optimally attracted by these traps but prefer to rest while digesting bloodmeals and developing eggs49. In this study we demonstrate that an existing network of CO2 baited traps, typically used for routine surveillance was an efficient and convenient tool for trapping blood-fed mosquitoes at least for some species and in some habitats. The BCC network of traps, spread across four urban parks and operating over a six-month period, collected a small percentage (0.9%, n = 480) of blood-fed mosquitoes (largely Cx. annulirostris) but yielded a reasonable number of bloodmeal IDs (n = 346) to facilitate our investigations. The success of CO2 baited light trap networks in collecting blood-feds is dependent on locality, target species and the extent of the trap network. Flies et al.19 assessed historic captures by a routine survey in South Australia and found just 280 (0.06%) blood-feds in a collection of 350,000 mosquitoes made over a 10-year period (mostly Ae. camptorhynchus and Cx. pipiens spp). Conversely, in Queensland, Kay et al.40 caught 1,119 blood-fed mosquitoes over a seven-month period representing 3.7% of the catch (mostly Cx. annulirostris), showing its potential for efficiently trapping blood-fed mosquitoes.
A notable limitation of our study is that, due to resource constraints we sampled vertebrates during the same months as the vectors, but in different years. However, comparisons of survey results between 2017/18 and 2020/21 does demonstrate that diversity is similar. In Brisbane, in 2020/21, the SARS‑CoV‑2 pandemic had limited transient impacts on human movement characterised by very brief periods of “lockdown”50.
Conclusion
This study examined the utility of using mosquito blood meals to determine host-vector relationships, estimate vertebrate diversity, and measure pathogen exposure in the vertebrate population. No previous bloodmeal analyses have been used to derive information on vertebrate diversity, nor have they been used to screen for antibodies across a diverse set of vertebrates. Conventional seroprevalence studies (direct sampling of blood), especially in non-human vertebrates, are extremely challenging due to ethical and practical limitations. Using mosquitoes as “flying syringes” offers a novel, non-invasive approach to estimate pathogen exposure in vertebrates and linking that to vector-host networks. This can help determine which pathways may facilitate the transmission of mosquito-borne zoonoses to humans.
Methods
Study area
The study was undertaken in Brisbane (27° 28′ 12″ S and 153° 01′ 15″ E), the capital city of Queensland, which is the second largest state in Australia in terms of area. Brisbane is geographically the largest capital city in Australia and the third most populous city in the country, with approximately 2.5 million people. The city has a subtropical climate characterised by an average annual precipitation level of 1011.5 mm. The rainy season typically occurs from November to March, while the average monthly temperatures range from 10 °C to 22 °C in winter and 20 °C to 29 °C in summer51. Brisbane comprises diverse natural ecosystems, including freshwater and estuarine wetlands, mangroves, saltmarshes, bushlands, and rainforests52. These varied ecosystems provide a range of habitats suitable for both mosquito vector species and reservoir hosts. The presence of such ecological diversity, coupled with the persistently high rates of human notifications of RRV34, makes Brisbane an ideal location for investigating RRV dynamics.
Mosquito collection and identification
Mosquito collections were carried out by the BCC as part of their ongoing mosquito surveillance and control program from February to May 2021 and during the same period in 2022. Four sites were chosen based on historical detections of RRV in mosquito collections (Fig. 2 and Supplementary Table S4). These were all urban recreational parks within established residential areas, with human population density ranging from 7.75 to 21.56 people per hectare. To increase the number of blood-fed individuals, particularly those that had potentially fed on a host that had previously been exposed to RRV, QH conducted additional surveillance at sites where RRV had recently been detected in sentinel traps during the trapping period.
Traps were set once a week prior to dusk and then collected the following morning. We employed CDC-style light traps sourced from Pacific Biologics (Scarborough, Australia), with 1 kg of dry ice as a CO2 source and supplemented by 1-octen-3-ol.
After collection, mosquitoes were transported to the Mosquito Control Laboratory (MCL) for identification. Mosquitoes were identified to the species level using dichotomous keys53,54,55. Blood-engorged mosquitoes were stored at − 80 °C until analysis by the respective assays.
Sample preparation
The abdomens of individual blood-fed mosquitoes were dissected under a stereo microscope. The dissected abdomens were subsequently homogenised in 70 µL of tissue culture media RPMI-1640, supplemented with 1% Penicillin, Streptomycin, and L-glutamine (Sigma-Aldrich, USA). To prevent fungal contamination 0.4% amphotericin B (Sigma-Aldrich, USA) was added to the homogenisation media.
Following homogenisation, the samples were centrifuged at 10,000 x g for 10 min, and the resulting supernatant removed. A volume of 55 µL of the supernatant was transferred to a sterile 1.5 ml microtube for subsequent testing to determine the presence of neutralising antibodies against RRV. The remaining volume of the supernatant was retained in a separate tube for molecular identification of the host species from which the bloodmeal was obtained.
Nucleic acid extraction and DNA barcoding
The DNA barcoding was performed using different regions of the mitochondrial DNA: the subunit I of the cytochrome oxidase (COI)56,57 or cytochrome b (cyt b)25,58 genes. The sets of primers chosen (Supplementary Table S5) were tested and validated on known host species, including humans, sheep (Ovis aries), koalas (Phascolarctos cinereus), and birds (Australian magpie, Cracticus tibicen), using whole blood or serum samples.
DNA was extracted from up to 10 µL of the mosquito bloodmeal samples using DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. The extracted DNA was then amplified using a touchdown cycling sequence to minimize non-specific amplification. The PCR mixture consisted of 3 µL of template DNA, 2X Phusion High-Fidelity PCR Master Mix with HF Buffer (New England BioLabs, Ipswich MA), 0.5% DMSO, and 0.5 µM of each of a forward and reverse primer in a 30 µL reaction mix. Each sample was submitted to the four combinations of primers (Supplementary Table S5). Cycling conditions comprised a step at 98 °C for 30s, followed by 5 touchdown cycles of 98 °C 5s, 49.5 °C 30s, 72 °C 45s, 30 cycles of 98 °C 5s, 54 °C 30s, 72 °C 45s, and a final elongation step of 72 °C for 5 min. The PCR products were visualised on a 2% agarose gel.
Amplified target DNA bands were either purified directly from the PCR reaction or from the gel using Qiagen Gel kit or PCR purification kits. Samples were sequenced by the QIMRB DNA Sequencing Facility using the BigDye™ Terminator v3.1 Cycle Sequencing kit in an ABI-PRISM 3130 Genetic Analyser (Applied Biosystems, Foster City, CA). The obtained sequences were then compared to the NCBI nucleotide database using a Basic Local Alignment Search Tool (BLAST) search.
Sequences showing a ≥ 95% sequence identity with those in the NCBI database were considered as matches. If the sequence identity was below 95%, the samples were considered inconclusive, and additional genus-specific PCR amplification and sequencing were performed.
Micro-plaque reduction neutralisation test (micro-PRNT)
The presence of neutralising antibodies against RRV in mosquito bloodmeals was assessed using a micro-PRNT, developed by Gyawali et al.21. Briefly, African green monkey kidney (Vero) cells (ATCC, CCL-81) were cultivated as monolayers in 96-well tissue culture plates (Nunclon, Thermo Scientific, Australia). The bloodmeals from mosquitoes were mixed with a quantity of RRV virus (T48 prototype strain31), calibrated to form 30 plaques per well.
The bloodmeal-virus mixture was added to wells in duplicate. Wells containing only Vero cells with and without RRV served as positive and negative controls, respectively. After incubation at 37 °C for 2 h, the mixture was removed and 0.75% w/v carboxymethyl cellulose (CMC, Sigma-Aldrich) in RPMI-1640 medium was added to each well. The plates were incubated for a further 40 h at 37 °C in an atmosphere of 5% v/v CO2/air.
Following incubation, the media was decanted, and the wells stained with a solution of 0.05% w/v crystal violet in formaldehyde (1% v/v) and methanol (1% v/v). After 24 h, the plates were rinsed, air-dried, and the number of plaques per well was counted. Plaques were counted in duplicate wells, and the average number was determined. Bloodmeals that resulted in a ≥ 50% reduction in the number of viral plaques compared to the positive control (RRV alone) were considered RRV seropositive.
Conventional host abundance and diversity surveys
Methods for the vertebrate surveys were adapted from the Queensland Fauna Survey Guidelines59. In brief, we estimated the abundance of hosts using four-point surveys, six minutes in duration, performed at least 150 m apart across Brisbane at the same sites used to collect mosquitoes monthly from November 2017 to March 2018 and again from March 2020 and April 2021. Vertebrate surveys were conducted during times of peak activity (within 30 min of dawn for birds, and after sunset for mammals). During each survey we recorded the species, number of individuals, whether the observation was seen or heard, and the microhabitat the species was in (i.e. on the ground, in the canopy, in the bottom third of a tree). Equipment used to identify vertebrates included binoculars, handheld torches, field guides and the Birds of Australis mobile phone application60.
Human abundance in each area was calculated by multiplying the average number of people per household in each suburb (as per Census data61) by the number of households within 2 km2 of each vector trapping site. This distance was chosen based on typical RRV vector dispersal range (100–2000 m) and to capture a representative sample of the surrounding environment.
Data availability
Genome sequences obtained in this study have been deposited in GenBank under accession numbers PP563681 to PP563700 and PP566797 to PP566875. All other data generated and analysed during this study are included in this article and its Supplementary Information files.
References
Khalil, A. M., Martinez-sobrido, L. & Mostafa, A. Zoonosis and zooanthroponosis of emerging respiratory viruses. Front. Cell. Infect. Microbiol. 13, 1232772 (2024).
Gilbert, L. The impacts of climate change on ticks and tick-borne disease risk. Annu. Rev. Entomol. 66, 373–388 (2021).
Vasu, B. et al. Epidemiological shifts: The emergence of malaria in America. Bayl. Univ. Med. Cent. Proc. 36, 745–750 (2023).
Figueiredo, P. D. O. et al. Re-Emergence of yellow fever in Brazil during 2016–2019: Challenges, lessons learned, and perspectives. Viruses 12, 1233 (2020).
Yakob, L. et al. Japanese Encephalitis Emergence in Australia: the potential Population at Risk. Clin. Infect. Dis. 76, 335–337 (2023).
Webster, J. P., Borlase, A. & Rudge, J. W. Who acquires infection from whom and how? Disentangling multi-host and multimode transmission dynamics in the ‘elimination’ era. Philos. Trans. R Soc. B Biol. Sci. 372 (2017).
Woolhouse, M. E. J., Taylor, L. H. & Haydon, D. T. Population biology of multihost pathogens. Sci. (80-). 292, 1109–1112 (2001).
Fauver, J. R. et al. Xenosurveillance reflects traditional sampling techniques for the identification of human pathogens: A comparative study in West Africa. PLoS Negl. Trop. Dis. 12, e0006348 (2018).
Cameron, M. M. & Ramesh, A. The use of molecular xenomonitoring for surveillance of mosquito-borne diseases. Philos. Trans. R Soc. B Biol. Sci. 376, 20190816 (2021).
Mwakasungula, S. et al. Using haematophagous fly blood meals to study the diversity of blood-borne pathogens infecting wild mammals. Mol. Ecol. Resour. 22, 2915–2927 (2022).
Nascimento, J. et al. Use of anthropophilic culicid-based xenosurveillance as a proxy for Plasmodium Vivax malaria burden and transmission hotspots identification. PLoS Negl. Trop. Dis. 12, 1–16 (2018).
Krokovsky, L. et al. Arbovirus Surveillance in Field-Collected mosquitoes from Pernambuco-Brazil, during the Triple Dengue, Zika and Chikungunya Outbreak of 2015–2017. Front. Trop. Dis. 3, 33 (2022).
Barbazan, P. et al. Detection of H5N1 avian influenza virus from mosquitoes collected in an infected poultry farm in Thailand. Vector-Borne Zoonotic Dis. 8, 105–109 (2008).
Štefanić, S., Grimm, F., Mathis, A., Winiger, R. & Verhulst, N. O. Xenosurveillance proof-of-principle: detection of Toxoplasma Gondii and SARS-CoV-2 antibodies in mosquito blood meals by (pan)-specific ELISAs. Curr. Res. Parasitol. Vector-Borne Dis. 2, 100076 (2022).
Barbazan, P., Palabodeewat, S., Nitatpattana, N. & Gonzalez, J. P. Detection of host virus-reactive antibodies in blood meals of naturally engorged mosquitoes. Vector-Borne Zoonotic Dis. 9, 103–107 (2009).
Fujisaki, K., Kamio, T. & Kitaoka, S. Passage of host serum components, including antibodies specific for Theileria sergenti, across the digestive tract of argasid and ixodid ticks. Ann. Trop. Med. Parasitol. 78, 449–150 (1984).
Kilpatrick, A. M., Daszak, P., Jones, M. J., Marra, P. P. & Kramer, L. D. Host heterogeneity dominates West Nile virus transmission. Proc. R Soc. B Biol. Sci. 273, 2327–2333 (2006).
Jansen, C. C. et al. Blood sources of mosquitoes collected from urban and peri-urban environments in eastern Australia with species-specific molecular analysis of avian blood meals. Am. J. Trop. Med. Hyg. 81, 849–857 (2009).
Flies, E. J., Flies, A. S., Fricker, S. R., Weinstein, P. & Williams, C. R. Regional comparison of Mosquito bloodmeals in South Australia: Implications for Ross River Virus Ecology. J. Med. Entomol. 53, 902–910 (2016).
Biteye, B. et al. Host-feeding patterns of Aedes (Aedimorphus) vexans arabiensis, a Rift Valley Fever virus vector in the Ferlo pastoral ecosystem of Senegal. PLoS One 14, e0215194 (2019).
Gyawali, N., Murphy, A. K., Hugo, L. E. & Devine, G. J. A micro-PRNT for the detection of Ross River virus antibodies in mosquito blood meals: a useful tool for inferring transmission pathways. PLoS One 15, e0229314 (2020).
Ramírez, A. L., Van Den Hurk, A. F., Meyer, D. B. & Ritchie, S. A. Searching for the proverbial needle in a haystack: Advances in mosquito-borne arbovirus surveillance. Parasites and Vectors vol. 11 1–12 (2018).
Togami, E. et al. First evidence of concurrent enzootic and endemic transmission of Ross River virus in the absence of marsupial reservoirs in Fiji. Int. J. Infect. Dis. 96, 94–96 (2020).
Johnson, B. J. et al. The environmental and ecological determinants of elevated Ross River Virus exposure in koalas residing in urban coastal landscapes. Sci. Rep. 11, (2021).
Townzen, J. S., Brower, A. V. Z. & Judd, D. D. Identification of mosquito bloodmeals using mitochondrial cytochrome oxidase subunit I and cytochrome b gene sequences. Med. Vet. Entomol. 22, 386–393 (2008).
Gyawali, N. et al. Identification of the source of blood meals in mosquitoes collected from north-eastern Australia. Parasites Vectors 12, 198 (2019).
Shanks, G. D. Could Ross River Virus be the next Zika? J. Travel Med. 26, 1–2 (2019).
Kain, M. P., Skinner, E. B., van den Hurk, A. F., McCallum, H. & Mordecai, E. A. Physiology and ecology combine to determine host and vector importance for Ross River virus. Elife 10, 1–40 (2021).
Kay, B. H. et al. Experimental infection of vertebrates with Murray Valley encephalitis and Ross River viruses. Arbovirus Res. Aust. 4, 71–75 (1986).
Stephenson, E. B., Peel, A. J., Reid, S. A., Jansen, C. C. & McCallum, H. The non-human reservoirs of Ross River virus: A systematic review of the evidence. Parasit. Vectors 11, 188 (2018).
Doherty, R. L., Whitehead, R. H., Gorman, B. M. & O’Gower, A. K. The isolation of a third group a arbovirus in Australia, with preliminary observations on its relationship to epidemic polyarthritis. Aust J. Sci. 26, 183–184 (1963).
Tesh, R. B., McLean, R. G., Shroyer, D. A., Calisher, C. H. & Rosen, L. Ross River virus (Togaviridae: Alphavirus) infection (epidemic polyarthritis) in american Samoa. Trans. R Soc. Trop. Med. Hyg. 75, 426–431 (1981).
Lindsay, M. et al. The epidemiology of outbreaks of Ross River virus infection in Western Australia in 1991–1992. Arbovirus Res. Aust. 6, 72–76 (1992).
Knope, K. et al. Arboviral diseases and malaria in Australia, 2014–15: Annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. 43, (2019).
Ong, O. T. W., Skinner, E. B., Johnson, B. J. & Old, J. M. Mosquito-borne viruses and non-human vertebrates in Australia: A review. Viruses. 13, 265 (2021).
Webb, C. E. & Russell, P. R. C. Living with mosquitoes: In The Lower Hunter and Mid North Coast Region of NSW. (Department of Medical Entomology The University of Sydney & Westmead Hospital, (2009).
Doggett, S. et al. The New South Wales Arbovirus Surveillance and Mosquito Monitoring Program. 2009 – 2018 Annual Reports. (2018).
Harley, D., Sleigh, A. & Ritchie, S. Ross River virus transmission, infection, and disease: A cross-disciplinary review. Clin. Microbiol. Rev. 14, 909–932 (2001).
Jansen, C. C. et al. Epidemiologic, entomologic, and virologic factors of the 2014-15 Ross River virus outbreak, Queensland, Australia. Emerg. Infect. Dis. 25, 2243–2252 (2019).
Kay, B. H., Boyd, A. M., Ryan, P. A. & Hall, R. A. Mosquito feeding patterns and natural infection of vertebrates with Ross River and Barmah Forest viruses in Brisbane, Australia. Am. J. Trop. Med. Hyg. 76, 417–423 (2007).
Van Den Hurk, A. F. et al. Mosquito host-feeding patterns and implications for Japanese encephalitis virus transmission in northern Australia and Papua New Guinea. Med. Vet. Entomol. 17, 403–411 (2003).
Williams, C. R., Kokkinn, M. J. & Smith, B. P. Intraspecific variation in odor-mediated host preference of the mosquito Culex annulirostris. J. Chem. Ecol. 29, 1889–1903 (2003).
BCC, B. C. C. Brisbane Parks. Brisbane City Council (2023). www.brisbane.qld.gov.au/things-to-see-and-do/council-venues-and-precincts/parks#:~:text=Brisbane City Council maintains more,gardens and many bushland reserves.
Abrams, J. F. et al. Shifting up a gear with iDNA: from mammal detection events to standardised surveys. J. Appl. Ecol. 56, 1637–1648 (2019).
Saranholi, B. et al. Comparing iDNA from mosquitoes and flies to survey mammals in a semi-controlled area. Mol. Ecol. Resour. 23, 1790–1799 (2023).
Cutajar, T. P. & Rowley, J. J. L. Surveying frogs from the bellies of their parasites: invertebrate-derived DNA as a novel survey method for frogs. Glob Ecol. Conserv. 22, e00978 (2020).
Hawkes, F. M. & Hopkins, R. J. The mosquito: An introduction. in Mosquitopia: The Place of Pests in a Healthy World (eds. Hall, M. & Tamïr, D.) 16–31 (Routledge, 2021). https://doi.org/10.4324/9781003056034
Burkot, T. R. et al. Barrier screens: A method to sample blood-fed and host-seeking exophilic mosquitoes. Malar. J. 12, 49 (2013).
Williams, G. M. & Gingrich, J. B. Comparison of light traps, gravid traps, and resting boxes for West Nile virus surveillance. J. Vector Ecol. 32, 285–291 (2007).
Weiss, D. J. et al. Impacts on Human Movement in Australian Cities Related to the COVID-19 pandemic. Trop. Med. Infect. Dis. 8, 363 (2023).
BoM, B. of M. Climate statistics for Australian locations. Monthly climate statistics. Australian Government Bureau of Meteorology (2023). http://www.bom.gov.au/climate/averages/tables/cw_040913.shtml
Queensland Government. Land zones within Southeast Queensland bioregion. (2023). https://apps.des.qld.gov.au/regional-ecosystems/landzones/?bioregion=12
Russell, R. C. & Debenham, M. L. A Colour Photo Atlas of Mosquitoes of Southeastern Australia (R.C. Russell, 1996).
Marks, E. N. An Atlas of Common Queensland Mosquitoes (Queensland Institute of Medical Research, 1982).
Russell, R. C. Mosquitoes and mosquito-borne Disease in Southeastern Australia: A Guide to the Biology, Relation to Disease, Surveillance, Control and the Identification of Mosquitoes in Southeastern Australia (Westmead Hospital, Department of Medical Entomology, 1993).
Parodi, B. et al. Species identification and confirmation of human and animal cell lines: A PCR-based method. Biotechniques. 32, 432–440 (2002).
Reeves, L. E., Gillett-Kaufman, J. L., Kawahara, A. Y. & Kaufman, P. E. Barcoding blood meals: New vertebrate-specific primer sets for assigning taxonomic identities to host DNA from mosquito blood meals. PLoS Negl. Trop. Dis. 12, e0006767 (2018).
Cicero, C. & Johnson, N. K. Higher-level phylogeny of new world vireos (aves: vireonidae) based on sequences of multiple mitochondrial DNA genes. Mol. Phylogenet Evol. 20, 27–40 (2001).
Eyre, T. J. et al. Terrestrial Vertebrate Fauna Survey Assessment Guidelines for Queensland (Department of Environment and Science, 2018).
Morcombe, M. K. Field Guide to Australian Birds (Steve Parish Publishing, 2003).
Census Australian Bureau of Statistics & QuickStats Australian Bureau of Statistics (2021). https://www.abs.gov.au/census/find-census-data/quickstats/2021/3GBRI (2022).
Funding
C.J.S.P.V. was supported by a QIMRB International PhD Scholarship and QUT Tuition Fee Sponsorship.
Author information
Authors and Affiliations
Contributions
G.J.D. devised the project. G.L.W., A.F.v.d.H., F.D.F. and G.J.D. supervised the project. C.J.S.P.V., M.B.O., M.A.S., D.S., J.M.D. and E.B.S. coordinated field activities. C.J.S.P.V. and N.G. analysed mosquito sera collections. C.J.S.P.V. and E.B.S. analysed vertebrate faunal surveys. C.J.S.P.V. collated and analysed the data, drafted the manuscript and designed the figures. J.M.D., N.G., G.L.W., A.F.v.d.H., F.D.F., E.B.S. and G.J.D. contributed to the editing of the final manuscript. All authors edited and approved the version submitted for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vieira, C.J.S.P., Gyawali, N., Onn, M.B. et al. Mosquito bloodmeals can be used to determine vertebrate diversity, host preference, and pathogen exposure in humans and wildlife. Sci Rep 14, 23203 (2024). https://doi.org/10.1038/s41598-024-73820-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-73820-y