Abstract
Obesity has become a global public health problem, and its relationship with gastrointestinal diseases has become a major concern. The visceral adiposity index (VAI) is a novel index to assess the distribution and content of visceral fat, and this study aimed to investigate the association between VAI and bowel habits (chronic diarrhea, chronic constipation) and inflammatory bowel disease (IBD). The 2005–2010 National Health and Nutrition Examination Survey (NHANES) dataset was used for the cross-sectional survey. Bowel habits and IBD were defined by self-report. Multiple logistic regression models were used to test the linear association of VAI with bowel habits and IBD. Fitted smoothed curves and threshold effects analyses were used to characterize nonlinear relationships. This cross-sectional study included 10,391 adults (≥ 20 years). After adjusting for covariates, there was a significant negative association between VAI and chronic constipation (OR [95% CI]: 0.97 [0.95, 1.00]) but no significant association with IBD (OR [95% CI]: 0.97 [0.87, 1.07]). Additionally, there was a nonlinear association between VAI and chronic diarrhea with a breakpoint of 3.08, with a positive correlation between the two on the left side of the breakpoint and no statistical significance on the right side. Subgroup analyses and interaction tests showed that maintaining sleep health was associated with a low risk of chronic constipation. Elevated VAI levels were negatively associated with chronic constipation, and elevated levels were positively associated with chronic diarrhea at VAI < 3.08. This reminds us that maintaining moderate levels of visceral fat may prevent the onset of chronic constipation and circumvent the risk of chronic diarrhea. Notably, maintaining healthy sleep may play a positive role in reducing chronic constipation.
Similar content being viewed by others
Introduction
With the changes in people’s increasingly rich diets, a variety of gastrointestinal symptoms are on the rise. The prevalence of chronic diarrhea in the United States is estimated to be 11-30% of the total population, affecting 6.6% of the U.S. population1,2, and the combined global prevalence of chronic constipation is 14%, with little variation by geographic region3, and the two have become one of the most common gastrointestinal disorders worldwide. IBD is an idiopathic inflammatory disease of the gastrointestinal tract, including Crohn’s disease (CD) and ulcerative colitis (UC), in which chronic diarrhea is the most prevalent symptom4. It has been reported that more than 3 million people in the United States and Europe suffer from IBD, with the prevalence increasing on all continents and incurring high healthcare costs5,6.
Obesity is associated with a variety of gastrointestinal disorders and is thought to be a risk factor for a variety of gastrointestinal symptoms, but the association between obesity and lower gastrointestinal symptoms remains unclear. Although some studies have shown that the risk of developing gastrointestinal symptoms increases with increasing obesity7,8,9, there have also been some conflicting findings10,11,12. The World Obesity Federation released the “2023 World Obesity Atlas” still uses the body mass index (BMI) to assess the degree of “obesity”, but in October of the same year, the journal Nature pointed out that the BMI is a somewhat crude indicator to determine the health risks, and can not be used to measure the degree of health of the body, and it does not measure the body fat, which is the body fat is not to be ignored in the part of the obesity13.
VAI is a novel gender-specific index based on waist circumference (WC), BMI, triglycerides (TGs), and high-density lipoprotein cholesterol (HDL-C) that indirectly expresses visceral adiposity function and has been proposed as a marker of visceral adipose tissue accumulation and dysfunction14. Many prospective studies have shown that excess visceral adiposity is often associated with increased risk and mortality from diseases such as diabetes, hyperlipidemia, and hypertension, as well as coronary, cerebrovascular, and peripheral vascular atherosclerosis15,16,17,18. To the best of our knowledge, no study has comprehensively examined the relationship between VAI and chronic diarrhea, chronic constipation, and IBD. Therefore, we conducted a population-based cross-sectional study aimed at assessing whether VAI is associated with the risk of these lower GI symptoms.
Materials and methods
Survey description
Cross-sectional data were obtained from the NHANES program conducted by the National Center for Health Statistics (NCHS). The program assesses the health and nutritional status of children and adults in the United States through screening, laboratory studies, and interviews. Each study participant in NHANES signed an informed consent form that was approved by the NCHS Research Ethics Review Board. All detailed NHANES study designs and data are publicly available at www.cdc.gov/nchs/nhanes/. The study was conducted in accordance with relevant guidelines and legislation.
Study population
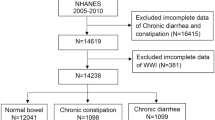
The samples used were from three cross-sectional surveys (2005–2006, 2007–2008, and 2009–2010) from NHANES with data on “bowel health”. A total of 31,034 adult participants (age ≥ 20 years) were enrolled in the study, and after excluding patients with missing bowel habits (n = 16,415) and missing VAI (n = 4,228), we ultimately enrolled 10,391 eligible subjects, including those with normal bowel habits (n = 8,792), chronic diarrhea (n = 780), and chronic constipation (n = 819). Because the NCHS only surveyed questions related to IBD in 2009–2010, we only analyzed IBD in 4535 subjects who were eligible out of 10,537 participants in 2009–2010 (Fig. 1).
Definition of VAI and bowel habits
Assessment of VAI
VAI, the exposure variable in this study, is a new index used to assess the level of visceral adiposity. The calculation of VAI includes WC, TG, HDL-C, and BMI. VAI is calculated in two ways according to gender, male: VAI = WC/(39.68+(1.88*BMI)*(TG/1.03)*(1.31/HDL-C); female: VAI = WC/(36.58+(1.89*BMI))*(TG/0.81)*(1.52/HDL-C). TGs and HDL-C are measured in mmoL/L, and WC is measured in cm. Higher levels of VAI mean higher levels of visceral fat.
Assessment of bowel habits
Bowel habits were determined by the results of the Bristol Stool Form Scale (BSFS; Type 1-Type 7). Participants were shown a colorful picture and the BSFS and asked “Please look at this card and tell me the number that corresponds to your usual or most common type of stool”. According to previous studies chronic constipation was defined as BSFS type 1 (hard lumps alone, such as nuts) or type 2 (sausage-like but lumpy), and chronic diarrhea was defined as BSFS type 6 (fluffy lumps with rough edges, pasty fecal matter) or type 7 (watery, no solid debris). Type 3,4,5 were defined as having normal bowel habits1,19,20,21. Subjects were defined as having IBD if they answered “yes” to the question “Has a doctor or other health professional ever told you that you had ulcerative colitis/Crohn’s Disease? “.
Selection of covariates
To make the association between VAI and defecation habits and IBD more robust, we adjusted for the following covariates: age, gender, race, education level, Socioeconomic status, smoking, drinking, mental status, sleep status, and diabetes. Races include non-Hispanic white, non-Hispanic black, Mexican American, and others. Education levels include less than high school, high school, or more than high school. Socioeconomic status includes Low income (PIR < 1.3), Middle income (1.3 ≤ PIR < 3.5), and High income (PIR ≥ 3.5). Smoking status (Smoked at least 100 cigarettes in life? ). Drinking status (Had at least 12 alcohol drinks/lifetime? ). Sleeping status (Ever told doctor had trouble sleeping? ). Mental status included Not Depressed (PHQ-9 score < 10), and Depressed (PHQ-9 score ≥ 10).
Statistical analysis
All statistical analyses were performed using EmpowerStats (2.0) and R (4.1.3). NHANES sampling weights were applied to every analysis. Baseline characteristics were described using means SD for continuous variables and percentages for categorical variables based on bowel habits and IBD. Using multivariate logistic regression analysis, we were able to determine the odds ratios (ORs) and 95% confidence intervals for the following relationships: VAI and chronic diarrhea, chronic constipation, and IBD. Three models were used to construct multivariate regression analyses: model 1 was unadjusted for variables, model 2 was adjusted for age, gender, and race, and model 3 was adjusted for all covariates. To evaluate the robustness of VAI, we divided it into thirds for additional sensitivity analysis. To handle nonlinearities, smooth curve fitting was utilized, and thresholds and breakpoints (K) were found using a threshold effects analysis model. Subgroup analyses and interaction effect tests were then run to determine whether the study’s findings were heterogeneous. At bilateral P < 0.05, the results were deemed statistically significant.
Results
Baseline characteristics of participants
A total of 10,391 subjects were enrolled in the “Bowel Habits” survey, of whom 8,792 had normal stools, 780 had chronic diarrhea, and 819 had chronic constipation. The mean age of the patients with chronic diarrhea and constipation was 50.13 ± 15.82 and 45.17 ± 17.47, respectively. Women outnumbered men in both chronic diarrhea and constipation. The highest proportion of non-Hispanic whites was found among the participating races. The mean VAI values for chronic diarrhea and constipation were 6.34 ± 2.72 and 6.62 ± 3.29, respectively. Regarding the investigation of IBD, we included a total of 4535 subjects from 2009 to 2010, of which a total of 58 patients with IBD, including 49 patients with UC and 9 patients with CD. The mean age of the patients with IBD was 50.55 ± 13.09 years. Females comprised 59.33% and males 40.67%. Non-Hispanic whites comprised 78.20% of the patients. The mean VAI was 6.80 ± 2.27.
Supplementary Materials 1 shows all the basic characteristics of the study population. We found that the differences in age, gender, education level, socioeconomic status, smoking status, sleeping status, and mental status were statistically significant in chronic diarrhea and constipation (p < 0.05). Whereas, the differences in age, education level, sleep status, and mental status were statistically significant in IBD patients (p < 0.05).
The association between VAI and chronic diarrhea
Table 1 shows the association between VAI and chronic diarrhea. We found no significant association between the continuous variable VAI and chronic diarrhea. Then, when VAI was transformed into a categorical variable (tertiles) for sensitivity analysis, we found that there was still no significant association between the two in the Minimally adjusted model and Fully adjusted model, and a positive correlation between the two was observed only in the Crude model (OR [95% CI]: 1.20 [1.01, 1.44]).
In addition, we performed a smoothed curve-fitting test and found that the relationship between VAI and chronic diarrhea exhibited a nonlinear relationship (Fig. 2), with a calculated breakpoint (K) of 3.08. On the left side of the breakpoint, the risk of chronic diarrhea increased 1.4-fold for each 1-unit increase in VAI (OR [95% CI]: 2.40 [1.15, 5.01]), whereas no statistically significant results were found on the right side (OR [95% CI]: 0.97 [0.95, 1.00]) (Table 2).
The association between VAI and chronic constipation
Table 3 shows the association between VAI and chronic constipation. We found a positive association between the continuous variable VAI in the Crude model and chronic constipation, but a negative association in the Minimally adjusted model and Fully adjusted model. In the Fully adjusted model, for every 1-unit increase in VAI score, subjects had a 3% lower risk of developing chronic constipation (OR [95% CI]: 0.97 [0.95, 1.00]). When VAI was categorized into tertiles, the highest VAI (tertile 3) in the Fully adjusted model had a 19% less risk of developing chronic constipation (OR [95% CI]: 0.81 [0.67, 1.00]) than the lowest VAI (tertile 1).
Smooth curve fitting showed a nonlinear relationship between VAI and chronic constipation (Fig. 3), which was subsequently verified by threshold analysis and found that this nonlinear relationship was not statistically significant (Log-likelihood ratio = 0.094) (Table 4).
The association between VAI and IBD
Table 5 shows the association between VAI and IBD. We found no significant association with IBD either in the VAI continuous or VAI categorical variables (p = 0.5427; p = 0.2964). In addition, we performed a smoothed curve-fitting test and did not find a nonlinear relationship (Fig. 4).
Subgroup analysis
To assess whether the association between VAI and chronic diarrhea, chronic constipation and IBD was influenced by a particular baseline characteristic. We performed subgroup analyses and interaction tests stratified by sex, age, race, socioeconomic status, sleep status, mental status, and diabetes (Supplementary Materials 2). Interaction tests showed that VAI and chronic constipation were negatively associated in subjects without sleep difficulties, with a 5% reduction in the likelihood of chronic constipation for every 1-unit increase in VAI. None of the other covariates had a significant effect on the association between VAI and chronic diarrhea, chronic constipation, and IBD (all P for interaction > 0.05).
Discussion
Based on our cross-sectional study of 10,391 subjects from 2005 to 2010 and 4,535 subjects from 2009 to 2010, we found a negative association between VAI and chronic constipation. There was a nonlinear relationship between VAI and chronic diarrhea, with a calculated breakpoint of 3.08, where the two were positively correlated on the left side of the breakpoint and not statistically significant on the right side. In addition, no significant association was found between VAI and IBD.
To our knowledge, this is the first study to assess the relationship between VAI and bowel habits and IBD. In previous studies, the relationship between bowel symptoms and obesity has been controversial. A cross-sectional study of Chinese participants showed that high BMI was associated with functional diarrhea22, and another study of U.S. participants also showed that high BMI was associated with a higher prevalence of chronic diarrhea23. However, some studies have come up with different results, suggesting that high BMI is associated with an increased prevalence of constipation rather than diarrhea8,24.
Visceral obesity is a marker of adipose tissue dysfunction and a risk factor for gastrointestinal diseases that is gaining attention. In order to better reflect the association between obesity and disease, we chose a more comprehensive and precise indicator, VAI, as a criterion for evaluating obesity. VAI is an indicator of visceral fat levels and provides a better assessment of fat distribution and visceral fat content in the body than indicators such as BMI and WC.
After adjusting for known confounders as much as possible19,25,26, our study found a negative correlation between a 3% reduction in the risk of developing chronic constipation for every 1-unit increase in VAI. As for the association with chronic diarrhea, we found that the elevation of VAI was positively correlated with chronic diarrhea when VAI < 3.08, while the association was no longer significant when VAI > 3.08. Our findings suggest that elevated levels of obesity appear to be associated with a reduced risk of chronic constipation and an increased risk of chronic diarrhea.
Several studies have found accelerated small intestinal transit and distal colonic transit times in obese patients, which may lead to bile acid malabsorption and thus diarrhea in patients27,28. Visceral fat has also been found to increase the release of pro-inflammatory cytokines, which in turn alters intestinal permeability, leading to loose stools and increased stool frequency29. This may be the mechanism behind the association between high obesity levels and low risk of constipation and high risk of diarrhea.
Furthermore, we did not find a significant relationship between VAI and IBD. The results of past studies on the association between the two are controversial. It has been shown that obesity as measured by BMI is not significantly associated with the development of IBD12. It has also been shown that the risk of CD in patients with abdominal obesity is positively associated with WC30. Although our study did not find a significant relationship between the two, a number of studies have now reported suggesting that there may be a plausible biological mechanism between visceral obesity and IBD. It has been suggested that excess adipose tissue, especially visceral adipose tissue, has pro-inflammatory, immunomodulatory, and endocrine activity functions and participates in the immune response to the gastrointestinal microbiota, producing pro-inflammatory cytokines and adipokines, leading to a pro-inflammatory state of the gut, which ultimately predisposes to IBD or influences the course of IBD31,32,33,34.
Finally, we performed subgroup analyses and interaction tests. The results showed that participants with healthy sleep were less likely to experience chronic constipation and were a protective factor of VAI against chronic constipation, which is consistent with known past findings35,36. This suggests that maintaining sleep health has important implications for chronic constipation. In addition, confounders such as gender, age, race, socioeconomic status, mental status, and diabetes did not significantly influence the associations between VAI and bowel habits and IBD.
There are various advantages to our study. To increase the reliability of the results, we first included a sizable sample size and corrected for known confounding variables. Second, to make the intestinal symptoms extremely comprehensive and to better understand the role of VAI in the assessment of intestinal health, we examined chronic diarrhea, chronic constipation, and IBD individually. There are some restrictions on this study, though. Initially, the cross-sectional design of this study limits causal inferences between VAI and gut health. Second, we were unable to completely rule out the impact of additional potential confounding variables, even after adjusting for a large number of significant covariates. Third, chronic constipation was defined through participants’ self-reports, and the information collected may have been influenced by participants’ subjective awareness. Fourth, because of the small number of IBD patients in the NHANES database and the relatively inadequate sample size included, caution should be exercised in interpreting IBD results, and larger sample sizes are needed for further validation.
Conclusion
In conclusion, elevated VAI levels are negatively associated with chronic constipation. And within a specific range, elevated VAI was positively associated with chronic diarrhea. This reminds us that maintaining moderate levels of visceral fat may prevent the onset of chronic constipation and circumvent the risk of chronic diarrhea. It is worth noting that maintaining healthy sleep may play a positive role in reducing constipation.
Data availability
Publicly available datasets were analyzed in this study. These data can be found at: www.cdc.gov/nchs/nhanes/.
References
Singh, P. et al. Demographic and dietary associations of chronic diarrhea in a representative sample of adults in the United States. Am. J. Gastroenterol.113, 593–600. https://doi.org/10.1038/ajg.2018.24 (2018).
Bharucha, A. E. et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and kidney diseases (NIDDK) workshop. Am. J. Gastroenterol.110, 127–136. https://doi.org/10.1038/ajg.2014.396 (2015).
Black, C. J. & Ford, A. C. Chronic idiopathic constipation in adults: epidemiology, pathophysiology, diagnosis and clinical management. Med. J. Australia. 209, 86–91. https://doi.org/10.5694/mja18.00241 (2018).
Hodson, R. Inflammatory bowel disease. Nature. 540https://doi.org/10.1038/540S97a (2016).
Weisman, M. H. et al. Inflammatory bowel disease prevalence: Surveillance data from the U.S. National Health and Nutrition Examination Survey. Prev. Med. Rep.33, 102173. https://doi.org/10.1016/j.pmedr.2023.102173 (2023).
Kaplan, G. G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol.12, 720–727. https://doi.org/10.1038/nrgastro.2015.150 (2015).
Camilleri, M., Malhi, H. & Acosta, A. Gastrointestinal complications of obesity. Gastroenterology. 152, 1656–1670. https://doi.org/10.1053/j.gastro.2016.12.052 (2017).
Silveira, E. A. et al. Prevalence of constipation in adults with obesity class II and III and associated factors. BMC Gastroenterol.21, 217. https://doi.org/10.1186/s12876-021-01806-5 (2021).
Steed, H., Walsh, S. & Reynolds, N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes. Facts. 2, 370–372. https://doi.org/10.1159/000262276 (2009).
Koppen, I. J., Velasco-Benítez, C. A., Benninga, M. A., Di Lorenzo, C. & Saps, M. Is there an association between functional constipation and excessive bodyweight in children? J. Pediatr.171, 178–182. https://doi.org/10.1016/j.jpeds.2015.12.033 (2016).
Singh, S., Dulai, P. S., Zarrinpar, A., Ramamoorthy, S. & Sandborn, W. J. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol.14, 110–121. https://doi.org/10.1038/nrgastro.2016.181 (2017).
Chan, S. S. et al. Body mass index and the risk for Crohn’s disease and ulcerative colitis: data from a European prospective cohort study (the IBD in EPIC Study). Am. J. Gastroenterol.108, 575–582. https://doi.org/10.1038/ajg.2012.453 (2013).
Prillaman, M. Why BMI is flawed - and how to redefine obesity. Nature. 622, 232–233. https://doi.org/10.1038/d41586-023-03143-x (2023).
Amato, M. C. et al. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes care. 33, 920–922. https://doi.org/10.2337/dc09-1825 (2010).
Nusrianto, R., Tahapary, D. L. & Soewondo, P. Visceral adiposity index as a predictor for type 2 diabetes mellitus in Asian population: A systematic review. Diabetes Metabolic Syndrome. 13, 1231–1235. https://doi.org/10.1016/j.dsx.2019.01.056 (2019).
Sun, J. et al. Higher visceral adiposity index and lipid accumulation product in relation to increased risk of atherosclerotic burden in community-dwelling older adults. Exp. Gerontol.174, 112115. https://doi.org/10.1016/j.exger.2023.112115 (2023).
Xu, C. et al. Visceral adiposity index and the risk of heart failure, late-life cardiac structure, and function in ARIC study. Eur. J. Prev. Cardiol.30, 1182–1192. https://doi.org/10.1093/eurjpc/zwad099 (2023).
Zhang, H. et al. Associations of Chinese visceral adiposity index and new-onset stroke in middle-aged and older Chinese adults: An observational study. Lipids Health Dis.22, 74. https://doi.org/10.1186/s12944-023-01843-x (2023).
Ballou, S. et al. Chronic diarrhea and constipation are more common in depressed individuals. Clin. Gastroenterol. Hepatology: Official Clin. Pract. J. Am. Gastroenterological Association. 17, 2696–2703. https://doi.org/10.1016/j.cgh.2019.03.046 (2019).
Markland, A. D. et al. Association of low dietary intake of fiber and liquids with constipation: Evidence from the National Health and Nutrition Examination Survey. Am. J. Gastroenterol.108, 796–803. https://doi.org/10.1038/ajg.2013.73 (2013).
Sommers, T. et al. Prevalence of chronic constipation and chronic Diarrhea in Diabetic individuals in the United States. Am. J. Gastroenterol.114, 135–142. https://doi.org/10.1038/s41395-018-0418-8 (2019).
Zhao, Y. F. et al. Epidemiology of functional diarrhea and comparison with diarrhea-predominant irritable bowel syndrome: A population-based survey in China. PloS One. 7, e43749. https://doi.org/10.1371/journal.pone.0043749 (2012).
Ballou, S. et al. Obesity is associated with significantly increased risk for diarrhoea after controlling for demographic, dietary and medical factors: A cross-sectional analysis of the 2009–2010 National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther.50, 1019–1024. https://doi.org/10.1111/apt.15500 (2019).
Wang, G. N., Zhang, K., Xiong, Y. Y. & Liu, S. The relationship between functional constipation and overweight/obesity in children: A systematic review and meta-analysis. Pediatr. Res.94, 1878–1886. https://doi.org/10.1038/s41390-023-02711-1 (2023).
Zhang, J. et al. Associations of chronic diarrheal symptoms and inflammatory bowel disease with sleep quality: A secondary analysis of NHANES 2005–2010. Front. Neurol.13, 858439. https://doi.org/10.3389/fneur.2022.858439 (2022).
Du, Y. T., Rayner, C. K., Jones, K. L., Talley, N. J. & Horowitz, M. Gastrointestinal symptoms in diabetes: Prevalence, assessment, pathogenesis, and management. Diabetes care. 41, 627–637. https://doi.org/10.2337/dc17-1536 (2018).
Xing, J. & Chen, J. D. Alterations of gastrointestinal motility in obesity. Obes. Res.12, 1723–1732. https://doi.org/10.1038/oby.2004.213 (2004).
Fu, X. Y. et al. Effects of gastrointestinal motility on obesity. Nutr. Metabolism. 11https://doi.org/10.1186/1743-7075-11-3 (2014).
Lee, C. G. et al. Visceral abdominal obesity is associated with an increased risk of irritable bowel syndrome. Am. J. Gastroenterol.110, 310–319. https://doi.org/10.1038/ajg.2014.422 (2015).
Je, Y. et al. Association of Waist Circumference with the risk of inflammatory bowel disease: A Nationwide Cohort Study of 10 million individuals in Korea. J. Crohn’s Colitis. 17, 681–692. https://doi.org/10.1093/ecco-jcc/jjac193 (2023).
Dandona, P., Aljada, A. & Bandyopadhyay, A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol.25, 4–7. https://doi.org/10.1016/j.it.2003.10.013 (2004).
Gummesson, A. et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obes. (Silver Spring Md). 19, 2280–2282. https://doi.org/10.1038/oby.2011.251 (2011).
Ouchi, N., Parker, J. L., Lugus, J. J. & Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol.11, 85–97. https://doi.org/10.1038/nri2921 (2011).
Karaskova, E. et al. Role of adipose tissue in inflammatory bowel disease. Int. J. Mol. Sci.22https://doi.org/10.3390/ijms22084226 (2021).
Yang, S. et al. Association of sleep duration with chronic constipation among adult men and women: Findings from the National Health and Nutrition Examination Survey (2005–2010). Front. Neurol.13, 903273. https://doi.org/10.3389/fneur.2022.903273 (2022).
Nakagawa, H. et al. Poor sleep quality as a risk factor for Constipation among Community-Dwelling older adults in Japan. Cureus. 15, e46175. https://doi.org/10.7759/cureus.46175 (2023).
Acknowledgements
Thanks to all the authors for their contributions.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Xiaoxian Yang and Manli Wang conceptualized the study. Statistical analysis was done by Xiaoxian Yang and Kinyu Shon. The manuscript was reviewed by Lang Ren, Guoliang Cui, Yiyao Cheng, Zhiguang Sun and Xiaohong Wang. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This is an observational study. The studies involving human participants were reviewed and approved by the NCHS Ethics Review Board. The participants provided their written informed consent to participate in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, X., Wang, M., Ren, L. et al. Association between visceral adiposity index and bowel habits and inflammatory bowel disease: a cross-sectional study. Sci Rep 14, 23923 (2024). https://doi.org/10.1038/s41598-024-73864-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-73864-0
Keywords
This article is cited by
-
Association Between Chronic Diarrhea and Relative Fat Mass: A Cross-Sectional Study Based on NHANES
Digestive Diseases and Sciences (2026)
-
Association of the body roundness index with chronic diarrhea and chronic constipation: findings based on the National Health and Nutrition Examination Survey 2005–2010 data
Lipids in Health and Disease (2025)
-
Association between atherogenic index of plasma and chronic diarrhea: a cross-sectional study of the NHANES 2005–2010
BMC Gastroenterology (2025)
-
Association between the Zhejiang University index and chronic diarrhea: a cross-sectional study
European Journal of Medical Research (2025)
-
The relationship of cardiometabolic index with bowel movement frequency: an NHANES-based cross-sectional analysis
Lipids in Health and Disease (2025)