Abstract
The safety and efficacy of coronavirus disease 2019 (COVID-19) vaccination in patients diagnosed with Moyamoya disease (MMD) have not been established. Using National Health Insurance Service data, this study analyzed the occurrence of stroke-related events and mortality following COVID-19 vaccination among patients diagnosed with MMD from 2008 to 2020. Among 10,297 MMD patients, 296 (2.9%) experienced events and 175 (1.7%) died in 2021. Significant risk factors for events included ages 50–59 (Odds Ratio [OR] 3.29; P = 0.022) and 60 or above (OR 5.20; P = 0.001) (reference group: age in 20s), low BMI (OR 2.00; P = 0.011), previous stroke (OR 1.96; P < 0.001), and COVID-19 infection (OR 2.28; P = 0.034). Female (OR 0.64 [95% CI 0.50–0.82]; p = 0.011), revascularization surgery (RS) (OR 0.38 [95% CI 0.21–0.70]; p < 0.001), and vaccination (OR 0.17 [95% CI 0.13–0.22]; p < 0.001) were associated with reduced odds of stroke-related events. For mortality, significant risks were age over 60 (OR 7.09; P = 0.008), low BMI (OR 3.87; P = 0.001), and prior stroke (OR 1.74; p = 0.004), while being female, RS (OR 0.41; P = 0.022), and vaccination (OR 0.12; P < 0.001) were associated with a lower frequency of mortality. mRNA vaccines were associated with a significantly lower incidence of events, mortality, and COVID-19 infections compared to vector vaccines. COVID-19 vaccination is linked to reduced stroke-related events and mortality in MMD patients, with mRNA vaccines showing a significantly lower incidence compared to vector vaccines. COVID-19 infection raises the risk of events, underscoring the benefit of vaccination.
Similar content being viewed by others
Introduction
Numerous studies have underscored cerebral venous sinus thrombosis (CVST) due to vaccine-induced thrombotic thrombocytopenia (VITT) as a critical, albeit rare, adverse effect of coronavirus disease 2019 (COVID-19) vaccination. It is reported that nearly half of the patients who experience strokes related to CVST endure severe outcomes1,2. Despite these concerns, the literature suggests that the benefits of proceeding with COVID-19 vaccination outweigh the risks associated with complications from a COVID-19 infection3,4,5,6. However, there remains a pronounced scarcity of data concerning the risks tied to the adverse effects of COVID-19 vaccination in patients with moyamoya disease (MMD) despite isolated case reports documenting cerebral hemorrhages post-vaccination in individuals with pre-existing vascular conditions7.
MMD, a rare condition with a notably high prevalence in Korea, Japan, Taiwan, and China8,9,10,11, features bilateral constriction or closure at the bifurcation of the distal internal carotid artery, along with the emergence of compensatory arterial collateral formations at the base of the brain12. Initial manifestations of hemorrhagic and ischemic strokes in MMD cohorts are quantified at 17% and 33%, respectively. Longitudinal observations further elucidate that recurrent events escalate to 30–60% for hemorrhagic strokes and 65–82% for ischemic strokes. Notably, even in a hemodynamically compensated state, patients with symptomatic MMD encounter substantial risks for subsequent cerebrovascular accidents, with a 5-year and 10-year stroke risk of 15% and 40%, respectively13,14,15,16,17. There have been reports of instances where patients diagnosed with MMD suffered cerebral hemorrhages subsequent to COVID-19 vaccination, resulting in either fatality or significant disability18,19. These incidents have highlighted the urgent need for research into the impact of vaccination on patients with MMD.
The rarity of MMD introduces challenges in the precise evaluation of stroke incidence and risk subsequent to COVID-19 vaccination. However, the National Health Insurance Service (NHIS) in Korea, through its comprehensive compilation of medical records covering 98% of the population and the classification of MMD as a specially noted condition, provides a distinct and valuable dataset. This resource enables the undertaking of extensive observational studies, thereby significantly improving the robustness and reliability of the resultant data.
Therefore, this study is designed to examine the effects of COVID-19 vaccination on the incidence of stroke in patients with MMD, utilizing data sourced from the NHIS and focusing on vaccinations administered during the year 2021.
Methods
Data source
This study utilized data sourced from the NHIS, comprising medical insurance billing records. Notably, approximately 97% of the Korean population is registered with the NHIS, enabling comprehensive documentation of all insurance-based medical interventions. The NHIS database encompasses a wide array of records, including hospital admissions, outpatient visits, pharmaceutical prescriptions, and national health examination outcomes. Korean adults are mandated to partake in biannual health screenings conducted by the NHIS, which assess vital statistics such as height, weight, and blood pressure (BP), in addition to conducting urine and blood laboratory tests. Participants are also required to complete questionnaires detailing their medical history, familial health background, and lifestyle habits. Significantly, the registration of serious or rare diseases within the NHIS registry adheres to stringent criteria, rendering the database an invaluable resource for conducting epidemiological research on large cohorts with respect to grave or uncommon ailments. The reliability of the NHIS data has been corroborated through numerous studies, affirming its utility in the field of medical research20,21,22,23,24. Using the International Classification of Diseases, 10th Revision (ICD-10) diagnostic codes, we collected data from patients with primary outcomes or medical histories.
Statement of ethics
The current study was approved by the Institutional Review Board and Ethical Committee of Seoul National University Bundang Hospital (X-1910-572-903), and the requirement for obtaining individual patient consent was waived owing to the retrospective nature of the study. Our research was conducted in accordance with the ethical standards of the aforesaid ethics committee and the tenets of the 1964 Declaration of Helsinki and its later amendments.
Study population and cohort design
To enhance the reliability of the study, data were meticulously gathered from tertiary referral general hospitals, general hospitals, and semi-general hospitals exclusively. This research focused on patients diagnosed with MMD for the first time between 2008 and 2020. Only those with the diagnostic code ICD-10 V128 recorded, and who underwent a transfemoral cerebral angiograph (TFCA) at the time of diagnosis were considered definitively diagnosed with MMD. Data from patients diagnosed with MMD in the preceding five years were excluded to ensure the purity of the study cohort. From a pool of 16,546 individuals first diagnosed with MMD between 2008 and 2020, only those who underwent health examinations in 2020 and 2021 were selected, culminating in a final cohort of 10,297 patients for this study.
Through the analysis of NHIS claims data, variables such as age, sex, the performance of revascularization surgery (RS), COVID-19 vaccination status, vaccination date, and type of vaccines (Astrazeneca; Pfizer; Moderna; Janssen; Novavax; ) were ascertained. Additionally, COVID-19 infection status was determined. The health examination database facilitated the examination of variables including body mass index (BMI; kg/m²), BP (mmHg), fasting blood sugar (FBS; mg/dl), and history of previous stroke, enriching the dataset with crucial health indicators.
Primary endpoint
The primary endpoint was established as stroke-related hospital admissions, determined by the incidence of an event. This encompasses admissions due to cerebral infarction (ICD-10 code I63) or intracranial hemorrhage (ICD-10 code I60, I61), necessitating a hospital stay exceeding 5 days in departments of neurosurgery, neurology, or rehabilitative medicine, or resulting in mortality within 5 days, irrespective of the duration of hospitalization with brain computed tomography, magnetic reasonance image, or TFCA20,21,22,23,24,25. To mitigate potential confounders, records indicating admission for revascularization procedures, identified by the billing of RS codes (S4661; S4662), were systematically excluded. Secondary endpoints were delineated as occurrences of mortality within the year 2021 among patients who had previously experienced the primary endpoint. For subjects who underwent vaccination, only post-vaccination events and mortality were meticulously documented.
Statistical analysis
Data manipulation, extraction, and statistical analysis were performed using the SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). Differences between patients with and without event or mortality were compared by the Student’s t test for continuous variables and the chi-square test for categorical variables. The logistic regression analysis was performed to test which variables were associated with event and mortality. Clinical variables that achieved a p-value < 0.05 in the univariable analysis were included in the multivariable analysis. Backward elimination was performed, that is, multivariable logistic regression that removes the variables one by one in a backwards fashion. For significant variables identified through multivariable logistic regression, retrospective statistical power analysis was additionally performed using two-sample t-test allowing unequal variance. Data are reported as odds ratios (ORs), 95% confidence intervals (CIs), and two-sided p-values. A p-value < 0.05 was defined as statistically significant. The interval between the date of the last vaccination before the events and the date of the events was expressed as the mean ± standard deviation (range). To categorize events into short-term and long-term groups, K-means clustering was employed. The optimal number of clusters was determined to be two. This determination was made by iteratively adjusting the distribution of the intervals and validating the cut-off point through k-fold cross-validation and bootstrap sampling to ensure reliability.
Results
Baseline characteristics
Between 2008 and 2020, out of 10,297 patients diagnosed with MMD, 296 patients (2.9%) experienced an event in 2021, and 175 patients (1.7%) died. When comparing the non-event group to the event group, the proportion of individuals aged 60 and over was significantly higher in the event group (non-event group vs. event group, 31.1% vs. 48.0%; p < 0.001), as was the proportion of males (33.4% vs. 42.2%; p = 0.002). The percentage of patients with a BMI < 18.5 (2.9% vs. 6.1%; p = 0.004) and those with a history of previous stroke (57.9% vs. 77.7%; p < 0.001) were also significantly higher in the event group. Conversely, the rates of undergoing RS (8.8% vs. 3.4%; p < 0.001) and receiving a COVID-19 vaccination (91.3% vs. 63.5%; p < 0.001) were significantly lower in the event group, whereas the incidence of COVID-19 infection (1.1% vs. 2.7%; p = 0.013) was significantly higher in the event group (Table 1).
When comparing the non-mortality group to the mortality group, the proportions of individuals aged 60 and over (32.0% vs. 58.9%; p < 0.001), males (33.5% vs. 44.6%; p = 0.002), those with a BMI < 18.5 (2.8% vs. 9.1%; p < 0.001), and those with a history of previous stroke (58.1% vs. 78.9%; p < 0.001) were significantly higher in the mortality group. Additionally, the incidence of COVID-19 infection was higher in the mortality group (1.2% vs. 2.9%; p = 0.037). Conversely, the rates of undergoing RS (8.8% vs. 3.4%; p = 0.005) and receiving a COVID-19 vaccination (91.2% vs. 54.9%; p < 0.001) were significantly lower in the mortality group (Table 1).
Risk factors evaluation for occurrence of events
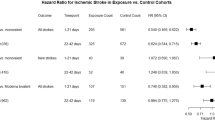
In the evaluation of risk factors for event occurrence through univariable analysis, being over the age of 40, having a BMI below 18.5, a history of previous stroke, and COVID-19 infection were identified as significant risk factors. Conversely, undergoing RS and receiving vaccinations emerged as significant preventive factors. Subsequent multivariable analysis, incorporating variables significant in the univariable analysis, revealed that ages 50–59 (OR 3.29 [95% CI 1.18–9.12]; p = 0.022) and 60 or above (OR 5.20 [95% CI 1.89–14.29]; p = 0.001), a BMI below 18.5 (OR 2.00 [95% CI 1.18–3.41]; p = 0.011), a history of previous stroke (OR 1.96 [95% CI 1.47–2.60]; p < 0.001), and COVID-19 infection (OR 2.28 [95% CI 1.07–4.87]; p = 0.034) were significant risk factors. Being female (OR 0.64 [95% CI 0.50–0.82]; p = 0.011), undergoing RS (OR 0.38 [95% CI 0.21–0.70]; p < 0.001), and vaccination (OR 0.17 [95% CI 0.13–0.22]; p < 0.001) were significant preventive factors against event occurrence (Table 2).
Risk factors evaluation for occurrence of mortality
In the univariable analysis, factors identified as significant included being aged 50 or above, having a BMI below 18.5, a FBS level of 126 or higher, a history of previous stroke, COVID-19 infection, undergoing RS, and receiving vaccinations. The multivariable analysis further identified being aged 60 or above (OR 7.09 [95% CI 1.69–29.81]; p = 0.008), having a BMI below 18.5 (OR 3.87 [95% CI 1.79–8.38]; p = 0.001), and a history of previous stroke (OR 1.74 [95% CI 1.19–2.54]; p = 0.004) as independent risk factors for mortality. Conversely, being female (OR 0.52 [95% CI 0.38–0.72]; p < 0.001), undergoing RS (OR 0.41 [95% CI 0.24–0.89]; p = 0.022), and vaccination (OR 0.12 [95% CI 0.09–0.16]; p < 0.001) were independently preventive factors against the occurrence of mortality (Table 3).
Risk and effectiveness evaluation according to types of vaccines
The impact of different vaccine types on the occurrence of events, mortality, and COVID-19 infections was analyzed. The interval between the date of the last vaccination prior to the events and the date of the events was 137.10 ± 91.70 days (range: 1-312 days). Based on K-means clustering analysis, a cut-off value of 17 days was determined. Accordingly, the subjects were divided into two groups. The short-term group consisted of 30 (0.32%) individuals of 9322 vaccinated patients with an interval of 7.23 ± 4.26 days (range: 1–13 days). In contrast, the long-term group included 158 (1.69%) individuals with an interval of 161.75 ± 78.60 days (range: 19–312 days). It was found that the Pfizer (OR 0.42 [95% CI] 0.31–0.59]; p < 0.001) and Moderna vaccines (OR 0.45 [95% CI 0.30–0.67]; p < 0.001) had a significantly higher preventive effect against the occurrence of events compared to the AstraZeneca vaccine. Similarly, for the prevention of mortality, the Pfizer (OR 0.34 [95% CI 0.22–0.52]; p < 0.001) and Moderna vaccines (OR 0.30 [95% CI 0.17–0.55]; p < 0.001) were significantly more effective compared to the AstraZeneca vaccine. Likewise, regarding the prevention of COVID-19 infections, the Pfizer (OR 0.62 [95% CI 0.41–0.96]; p = 0.032) and Moderna vaccines (OR 0.33 [95% CI 0.18–0.36]; p = 0.001) were found to have significantly higher efficacy compared to the AstraZeneca vaccine (Table 4).
Discussion
Review of stroke-related adverse effects following COVID-19 vaccination
Although strokes associated with COVID-19 vaccination are extremely rare, they can be fatal if misdiagnosed or improperly treated. Vaccine-induced thrombotic thrombocytopenia (VITT) is considered one of the significant mechanisms behind stroke complications following COVID-19 vaccination, though its pathophysiology has not been fully elucidated. However, VITT is highly similar to autoimmune heparin-induced thrombocytopenia (HIT), an immune-mediated disorder triggered by Immunoglobulin G (IgG) antibodies against the platelet factor 4 complexed with heparin. These antibodies activate platelets and create platelet microparticles by binding to platelet FcRIIA receptors, leading to blood clot formation and consequent thrombotic thrombocytopenia due to platelet consumption. Thrombotic thrombocytopenia post-COVID-19 vaccination occurs independently of heparin use and can be triggered by interactions between PF4 and specific COVID-19 vaccine components, microtrauma and microbleeding at the injection site, or vaccine-induced inflammation1,2,26,27. Vaccine-induced inflammation may cause vascular endothelial dysfunction, potentially leading to large-vessel strokes. CVST, as one of the ischemic stroke types post-vaccination, can be more severe and have a poorer prognosis when caused by VITT2. Such cases may lead to refractory increased intracranial pressure, cerebral infarction, and intracerebral hemorrhage (ICH). The occurrence of ICH or subarachnoid hemorrhage following vaccination is significantly influenced by primary or secondary venous thrombosis and may also be associated with vaccine-induced hypertension. Arterial hypertension, regardless of vaccination, can itself be a cause of ICH27.
Impact of COVID-19 vaccination on patients with MMD
Nannoni et al. carried out a meta-analysis on strokes associated with COVID-19 infection, reporting an overall pooled incidence of acute cerebrovascular disease (CVD) at 1.4%6. This incidence is comparable to the 1-year incidence rates of hemorrhagic stroke (0.046%) and ischemic stroke (0.16%) in the general population according to a nationwide study21,22. Furthermore, the meta-analysis identified severe infection (OR 5.10 [95% CI 2.72–9.54]) as a significant risk factor for CVD in patients with COVID-196. In our study, COVID-19 infection was recognized as a risk factor for stroke-related events (OR 2.28 [95% CI 1.07–4.87]; p = 0.034), whereas COVID-19 vaccination was found to serve as a protective measure against both stroke-related events (OR 0.17 [95% CI 0.13–0.22]; p < 0.001) and mortality (OR 0.12 [95% CI 0.09–0.16]; p < 0.001). In contrast, within the vaccinated cohort, a cut-off value of 17 days was derived based on the statistically significant difference in the mean interval from vaccination to the occurrence of events. A total of 30 individuals (0.32%) experienced events within 17 days post-vaccination, suggesting a potential association with vaccine-related adverse effects. This observation aligns with previous reports in the literature indicating that strokes are more likely to occur within two weeks following vaccination28. Nevertheless, combining these findings with several studies indicating that COVID-19 vaccination reduces infection rates and alleviates symptoms29,30,31, it can be hypothesized that, although it is difficult to prove the exact mechanism, the benefits of preventing COVID-19 infection or mitigating its symptoms through vaccination may outweigh the risks of serious adverse effects, particularly in reducing the occurrence of strokes among patients with MMD. The precise mechanisms of acute stroke in COVID-19 patients remain largely unknown. Proposed mechanisms for acute ischemic stroke include a hypercoagulation state promoting venous thromboembolism, a hyperinflammatory state, hypoxemia from respiratory dysfunction leading to hypoperfusion, endothelial dysfunction resulting in vasoconstriction and increased blood-brain barrier (BBB) permeability, and myocardial injury. Potential mechanisms for acute hemorrhagic stroke involve a hyperinflammatory state and endothelial dysfunction leading to increased vasoconstriction, elevated BP, enhanced BBB permeability, hypoxic microvascular injury, and consumption coagulopathy related to fibrinogen depletion6,32. Moreover, strokes related to COVID-19 infection predominantly displayed patterns of large vessel occlusion, thrombosis and/or thromboembolism resulting in multi-territory infarctions, with in-hospital mortality rates reaching as high as 36.4%3,4,5,6,33.
Risk and effectiveness evaluation according to types of vaccines
Venous thrombosis, including CVST, has been reported to occur primarily following viral vector-based vaccination, with most cases occurring after Astrazeneca (ChAdOx1 nCoV-19) vaccination and being more common than after mRNA vaccination, where the associated mortality was also higher. In addition, ICH was more frequently reported after Janssen vaccination, and VITT occurred more commonly following viral vector-based vaccination than mRNA vaccination, with more severe clinical manifestations reported in the former1,2,26,27. This aligns with the findings of our study, which demonstrated that mRNA vaccines were more effective than vector vaccines in preventing events, reducing mortality, and protecting against COVID-19 infections.
Nevertheless, it is important to recognize the potential for concurrent COVID-19 infection following vaccination with either of the two vaccines, as it increases the risk of both ischemic and hemorrhagic strokes34.
Modifiable risk factors for stroke in MMD patients
In this study, a low BMI of less than 18.5 was identified as a modifiable risk factor for stroke-related events (OR 2.00 [95% CI 1.18–3.41]) and mortality (OR 3.87 [95% CI 1.79–8.38]). Current evidence suggests a correlation between MMD and lipid metabolism, with high BMI identified as a contributing risk factor35,36,37. Though the impact of low BMI on stroke risk in MMD patients remains unexplored, several studies on stroke unrelated to MMD support our findings. A large-scale prospective cohort study demonstrated that low BMI was associated with an increased risk of hemorrhagic stroke (Hazard Ratio 5.10 [95%CI, 1.80–14.50])38. Additional studies have similarly identified low BMI as a significant risk factor for hemorrhagic stroke, comparable to the risk posed by obesity, though the underlying mechanisms are not yet fully understood39,40. Despite this, the lack of specific data on MMD-related stroke risks underscores the need for further research to clarify this potential risk factor. Moreover, our research indicates that RS significantly reduces the risk of stroke-related events (OR 0.38 [95% CI 0.21–0.70]) and mortality (OR 0.41 [95% CI 0.24–0.89]), as numerous studies have demonstrated that RS is beneficial in preventing strokes in patients with MMD41,42,43. These findings suggest that maintaining a normal BMI and considering RS as a preventive measure against further stroke, irrespective of COVID-19 considerations.
Limitations
This study has several limitations inherent to the use of NHIS data. Notably, while the analysis encompassed MMD patients vaccinated in 2021 who subsequently experienced stroke-related events within the same year, a comprehensive longitudinal analysis was not achievable. Challenges included difficulties in accurately capturing the number of vaccinations and accounting for repeated vaccinations after events. Moreover, inconsistent use of diagnostic codes for post-vaccination stroke necessitated the indirect extraction of data through operational definitions, categorizing post-vaccination strokes as admissions due to stroke-related events and stroke-related mortality following vaccination. This approach complicates the ability to definitively ascertain whether post-vaccination strokes are attributable to vaccination-related adverse events or a manifestation of MMD’s progression, and whether post-vaccination deaths, though rare, are due to causes unrelated to stroke. Additionally, due to the characteristics of the NHIS data, other stroke risk factors with many missing values, such as smoking history, alcohol consumption, physical activity, family history, and genetic factors like RNF213, were excluded from the analysis. This exclusion may limit the ability to assess interaction effects comprehensively. Furthermore, we could not determine the exact mechanism of the impact of COVID-19 vaccination on MMD patients, and the absence of a control group consisting of healthy individuals limits our ability to fully understand MMD’s influence on the outcomes of COVID-19 infection and vaccination side effects. Despite these constraints, the findings significantly underscore the clinical importance, from a statistical perspective, of the effects of COVID-19 vaccination and infection on the incidence of stroke in patients with this rare condition.
Conclusions
COVID-19 vaccination is significantly associated with a reduced occurrence of stroke-related events and mortality among patients with MMD, while COVID-19 infection is significantly associated with an increased risk of stroke-related events. Therefore, vaccination is beneficial for patients with MMD, with mRNA vaccines being associated with a significantly lower incidence of these outcomes compared to vector vaccines.
Data availability
Due to the nature of NHIS data, the raw dataset cannot be disclosed publicly. However, detailed statistical results from the analyses conducted in this study are available from the corresponding author upon reasonable request.
References
Kolahchi, Z., Khanmirzaei, M. & Mowla, A. Acute ischemic stroke and vaccine-induced immune thrombotic thrombocytopenia post COVID-19 vaccination; A systematic review. J. Neurol. Sci. 439, 120327. https://doi.org/10.1016/j.jns.2022.120327 (2022).
Palaiodimou, L. et al. Cerebral venous sinus thrombosis in the setting of COVID-19 vaccination: A systematic review and meta-analysis. J. Neurol. 269, 3413–3419. https://doi.org/10.1007/s00415-022-11101-2 (2022).
Jabbour, P. et al. Characteristics of a COVID-19 cohort with large vessel occlusion: A multicenter international study. Neurosurgery 90, 725–733. https://doi.org/10.1227/neu.0000000000001902 (2022).
Li, Y. et al. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc. Neurol. 5, 279–284. https://doi.org/10.1136/svn-2020-000431 (2020).
Majidi, S. et al. Emergent large vessel occlusion stroke during New York City’s COVID-19 outbreak: Clinical characteristics and paraclinical findings. Stroke 51, 2656–2663. https://doi.org/10.1161/STROKEAHA.120.030397 (2020).
Nannoni, S., de Groot, R., Bell, S. & Markus, H. S. Stroke in COVID-19: A systematic review and meta-analysis. Int. J. Stroke 16, 137–149. https://doi.org/10.1177/1747493020972922 (2021).
Kim, B. H. & Yoo, M. C. Intracranial hemorrhage due to potential rupture of an arteriovenous malformation after BNT162b2 COVID-19 mRNA vaccination in a young korean woman: Case report. Vaccines (Basel) https://doi.org/10.3390/vaccines10030362 (2022).
Ahn, I. M. et al. Incidence, prevalence, and survival of moyamoya disease in Korea: A nationwide, population-based study. Stroke 45, 1090–1095. https://doi.org/10.1161/STROKEAHA.113.004273 (2014).
Huang, S., Guo, Z. N., Shi, M., Yang, Y. & Rao, M. Etiology and pathogenesis of Moyamoya disease: An update on disease prevalence. Int. J. Stroke 12, 246–253. https://doi.org/10.1177/1747493017694393 (2017).
Kuriyama, S. et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: Findings from a nationwide epidemiological survey. Stroke 39, 42–47. https://doi.org/10.1161/STROKEAHA.107.490714 (2008).
Zhang, H., Zheng, L. & Feng, L. Epidemiology, diagnosis and treatment of moyamoya disease. Exp. Ther. Med. 17, 1977–1984. https://doi.org/10.3892/etm.2019.7198 (2019).
Suzuki, J. & Takaku, A. Cerebrovascular, “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 20, 288–299. https://doi.org/10.1001/archneur.1969.00480090076012 (1969).
Cho, W. S. et al. The natural clinical course of hemodynamically stable adult moyamoya disease. J. Neurosurg. 122, 82–89. https://doi.org/10.3171/2014.9.JNS132281 (2015).
Hallemeier, C. L. et al. Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke 37, 1490–1496. https://doi.org/10.1161/01.STR.0000221787.70503.ca (2006).
Kobayashi, E., Saeki, N., Oishi, H., Hirai, S. & Yamaura, A. Long-term natural history of hemorrhagic moyamoya disease in 42 patients. J. Neurosurg. 93, 976–980. https://doi.org/10.3171/jns.2000.93.6.0976 (2000).
Miyamoto, S. et al. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: Results of the Japan Adult Moyamoya Trial. Stroke 45, 1415–1421. https://doi.org/10.1161/STROKEAHA.113.004386 (2014).
Morioka, M. et al. High-risk age for rebleeding in patients with hemorrhagic moyamoya disease: long-term follow-up study. Neurosurgery52, 1049–1054; discussion 1054–1045 (2003).
Choi, J. K. et al. Intracerebral hemorrhage due to thrombosis with thrombocytopenia syndrome after vaccination against COVID-19: the first fatal case in Korea. J. Korean Med. Sci. 36, e223. https://doi.org/10.3346/jkms.2021.36.e223 (2021).
Takeyama, R. et al. Intracerebral hemorrhage due to vasculitis following COVID-19 vaccination: A case report. Acta Neurochir. (Wien) 164, 543–547. https://doi.org/10.1007/s00701-021-05038-0 (2022).
Lee, S. H. et al. Clinical outcomes of clipping and coiling in elderly patients with unruptured cerebral aneurysms: A national cohort study in Korea. J. Korean Med. Sci. 36, e178. https://doi.org/10.3346/jkms.2021.36.e178 (2021).
Lee, S. U. et al. Trends in the incidence and treatment of cerebrovascular diseases in Korea: Part I. Intracranial aneurysm, intracerebral hemorrhage, and arteriovenous malformation. J. Korean Neurosurg. Soc. 63, 56–68. https://doi.org/10.3340/jkns.2018.0179 (2020).
Lee, S. U. et al. Trends in the incidence and treatment of cerebrovascular diseases in Korea : Part II. Cerebral infarction, cerebral arterial stenosis, and moyamoya disease. J. Korean Neurosurg. Soc. 63, 69–79. https://doi.org/10.3340/jkns.2018.0182 (2020).
Ryu, B. G. et al. Clinical outcomes of coil embolization for unruptured intracranial aneurysms categorized by region and hospital size: A nationwide cohort study in Korea. J. Korean Neurosurg. Soc. 66, 690–702. https://doi.org/10.3340/jkns.2023.0033 (2023).
Shim, H. S. et al. Optimal target blood pressure for the primary prevention of hemorrhagic stroke: A nationwide observational study. Front. Neurol. 14, 1268542. https://doi.org/10.3389/fneur.2023.1268542 (2023).
Ryu, S. I. et al. Optimal blood pressure for stroke prevention in healthy adults below 65 years: A nationwide 10-year observational study. J. Clin. Neurosci. 122, 44–52. https://doi.org/10.1016/j.jocn.2024.03.004 (2024).
Stefanou, M. I. et al. Acute arterial ischemic stroke following COVID-19 vaccination: A systematic review and meta-analysis. Neurology 99, e1465–e1474. https://doi.org/10.1212/WNL.0000000000200996 (2022).
Kakovan, M., Ghorbani Shirkouhi, S., Zarei, M. & Andalib, S. Stroke associated with COVID-19 vaccines. J. Stroke Cerebrovasc. Dis. 31, 106440. https://doi.org/10.1016/j.jstrokecerebrovasdis.2022.106440 (2022).
Liu, J., Cao, F., Luo, C., Guo, Y. & Yan, J. stroke following coronavirus disease 2019 vaccination: Evidence based on different designs of real-world studies. J. Infect. Dis. 228, 1336–1346. https://doi.org/10.1093/infdis/jiad306 (2023).
Mohammed, I. et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccin. Immunother. 18, 2027160. https://doi.org/10.1080/21645515.2022.2027160 (2022).
Rahmani, K. et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front. Public Health 10, 873596. https://doi.org/10.3389/fpubh.2022.873596 (2022).
Wu, N. et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 11, 439–452. https://doi.org/10.1016/S2213-2600(23)00015-2 (2023).
Fan, H., Tang, X., Song, Y., Liu, P. & Chen, Y. Influence of COVID-19 on cerebrovascular disease and its possible mechanism. Neuropsychiatr. Dis. Treat. 16, 1359–1367. https://doi.org/10.2147/NDT.S251173 (2020).
Sweid, A. et al. Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int. J. Stroke 15, 733–742. https://doi.org/10.1177/1747493020937189 (2020).
Nahab, F. et al. Factors associated with stroke after COVID-19 vaccination: A statewide analysis. Front. Neurol. 14, 1199745. https://doi.org/10.3389/fneur.2023.1199745 (2023).
Hirano, Y. et al. Association between the onset pattern of adult Moyamoya disease and risk factors for stroke. Stroke 51, 3124–3128. https://doi.org/10.1161/STROKEAHA.120.030653 (2020).
Ge, P. et al. Modifiable Risk Factors Associated With Moyamoya Disease: A Case-Control Study. Stroke 51, 2472–2479. https://doi.org/10.1161/STROKEAHA.120.030027 (2020).
Church, E. W. et al. Clinical course of unilateral moyamoya disease. Neurosurgery 87, 1262–1268. https://doi.org/10.1093/neuros/nyaa284 (2020).
Gu, H. et al. Age- and sex-associated impacts of body mass index on stroke type risk: A 27-year prospective cohort study in a low-income population in China. Front. Neurol. 10, 456. https://doi.org/10.3389/fneur.2019.00456 (2019).
Kurth, T. et al. Body mass index and the risk of stroke in men. Arch Intern. Med. 162, 2557–2562. https://doi.org/10.1001/archinte.162.22.2557 (2002).
Shiozawa, M. et al. Association of body mass index with ischemic and hemorrhagic stroke. Nutrients https://doi.org/10.3390/nu13072343 (2021).
Jeon, J. P. et al. Meta-analysis of the surgical outcomes of symptomatic moyamoya disease in adults. J. Neurosurg. 128, 793–799. https://doi.org/10.3171/2016.11.JNS161688 (2018).
Kim, T. et al. Stroke prevention by direct revascularization for patients with adult-onset moyamoya disease presenting with ischemia. J. Neurosurg. 124, 1788–1793. https://doi.org/10.3171/2015.6.JNS151105 (2016).
Park, H. et al. Association of bypass surgery and mortality in moyamoya disease. J. Am. Heart Assoc. 12, e030834. https://doi.org/10.1161/JAHA.123.030834 (2023).
Funding
This research was funded by a grant from Seoul National University Bundang Hospital (14-2022-0028).
Author information
Authors and Affiliations
Contributions
Conception and design: SU.L., YD.W.; Acquisition of data: SU.L., YD.W., Y.K.; Analysis and interpretation of data: SH.L., TW.C.; Drafting the article: SU.L., HS.B.; Critically revising the article: SU.L., HS.B., SP.B.; Reviewed submitted version of manuscript: JS.B., O.K., CW.O.; Approved the final version of the manuscript on behalf of all author: SU.L., HS.B.; Study supervision: SU.L., YD.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Byoun, H., Lee, S., Won, Y. et al. Nationwide cohort observational study on the safety and efficacy of COVID-19 vaccination in patients with moyamoya disease. Sci Rep 14, 24400 (2024). https://doi.org/10.1038/s41598-024-73940-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73940-5