Abstract
Induction of autophagy represents an effective survival strategy for nutrient-deprived or stressed cancer cells. Autophagy contributes to the modulation of communication within the tumor microenvironment. Here, we conducted a study of the metabolic and signaling implications associated with autophagy induced by glutamine (Gln) and serum starvation and PI3K/mTOR inhibitor and autophagy inducer NVP-BEZ235 (BEZ) in the head and neck squamous cell carcinoma (HNSCC) cell line FaDu. We compared the effect of these different types of autophagy induction on ATP production, lipid peroxidation, mitophagy, RNA cargo of extracellular vesicles (EVs), and EVs-associated cytokine secretome of cancer cells. Both BEZ and starvation resulted in a decline in ATP production. Simultaneously, Gln starvation enhanced oxidative damage of cancer cells by lipid peroxidation. In starved cells, there was a discernible fragmentation of the mitochondrial network coupled with an increase in the presence of tumor susceptibility gene 101 (TSG101) on the mitochondrial membrane, indicative of the sorting of mitochondrial cargo into EVs. Consequently, the abundance of mitochondrial RNAs (mtRNAs) in EVs released by FaDu cells was enhanced. Notably, mtRNAs were also detectable in EVs isolated from the serum of both HNSCC patients and healthy controls. Starvation and BEZ reduced the production of EVs by cancer cells, yet the characteristic molecular profile of these EVs remained unchanged. We also found that alterations in the release of inflammatory cytokines constitute a principal response to autophagy induction. Importantly, the specific mechanism driving autophagy induction significantly influenced the composition of the EVs-associated cytokine secretome.
Similar content being viewed by others
Introduction
Low amino acid availability can be lethal to cancer cells, so starving cancer cells initiate adaptive responses to nutrient deprivation that go beyond angiogenesis. Starving cells could promote the reorganization of tumor microenvironment (TME) by producing cytokines and extracellular vesicles (EVs) with signaling functions1,2. A primary response to amino acid starvation is the inhibition of protein synthesis, induction of autophagy, and protein degradation by the ubiquitin-proteasome system. These processes are connected by the mammalian target of rapamycin complex 1 (mTORC1), which is a central regulator of cell growth and metabolism. mTORC1 inhibition contributes to the downregulation of protein synthesis and the induction of autophagy3. Glutamine (Gln) alone was shown to be sufficient to restore mTORC1 activity during prolonged amino acid starvation4and Gln deprivation could induce autophagy5. Nevertheless, the different forms of autophagy induction can strongly influence the resulting adaptive processes in the cell. For example, mTOR-dependent 4E-BP hyperphosphorylation (allowing eIF4E-dependent translation) was strongly inhibited by the PI3K/mTOR inhibitor and autophagy inducer NVP-BEZ235 (BEZ) throughout the 24-hour treatment period, but Gln starvation showed no such significant effect. Therefore, eIF4E-mediated translation can be partially maintained in the presence of Gln starvation, but probably not in the presence of BEZ6. Furthermore, NVP-BEZ235 is a dual PI3K/ mTOR inhibitor. On the other hand, mTOR inhibition or Gln starvation can, under some circumstances, induce the PI3K/Akt pathway7,8.
The composition of EVs has been shown to reflect the metabolic state of the cell9and some studies suggest that EVs release is regulated by mTORC1 in response to changes in nutrient and growth factor conditions10. Starvation also induces mitochondrial stress, and mitochondrial biogenesis and/or turnover of damaged mitochondria by mitophagy may both be part of the adaptive response11, as well as changes in the level of oxidative phosphorylation (OXPHOS)12. However, the link between cell stress, autophagy, cellular energy metabolism, and content of EVs remains somewhat elusive.
In this study, we focused on the consequences of BEZ- and Gln and FBS starvation-induced autophagy on the RNA cargo of EVs and the EVs-associated cytokine secretome (cytokines can exist internally in EVs and also bound to their membrane13). We also compared the effect of these different modes of autophagy induction on ATP production, lipid peroxidation, and mitophagy. We found that Gln and FBS starvation increased the abundance of mitochondrial RNAs in FaDu-derived EVs. This suggests that metabolic reprogramming in head and neck squamous cell carcinoma (HNSCC) cells may be reflected in EVs. As metabolic reprogramming is a key hallmark of malignancy14, we also tested how these findings could be translated to the whole body level.
Materials and methods
Model cell line and treatments
The cell line FaDu (HTB-43TM, RRID: CVCL_1218) derived from a squamous cell carcinoma (SCC) of the hypopharynx was used in this study. The authenticated cell line was purchased from the American Type Culture Collection (ATCC; Manassas, Virginia, USA) within the last three years. The cell lines were grown in a Dulbecco’s Modified Eagle’s medium/Nutrient Mixture F-12 Ham (DMEM/F12, Biosera) supplemented with 10% FBS (Biosera) in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Two passages before the experiment, cell harvest, or EVs isolation, the cells were washed with PBS and grown in a medium supplemented with Exosome-depleted FBS (Gibco, A2720801); hereafter referred to as exofree medium. The passages of FaDu cells ranged from 5 to 15. All experiments were performed with mycoplasma-free cells. Mycoplasma was detected by PCR (primers MYCO_A: GGCGAATGGGTGAGTAACACG and MYCO_B: CGGATAACGCTTGCGACCTATG). For autophagy modulation, FaDu cells were treated for 24 h with 30 nM of NVP-BEZ235 (MedChemExpress). To induce starvation, cells were cultured in DMEM F12 without glutamine and without FBS. Modulation of autophagy did not reduce the viability of FaDu cells (the viability ranged from 98.1 to 99.9% across treatments).
Conditioned media preparation
15 ml of conditioned medium (CM) was collected from 90 to 95% confluent FaDu cells grown in 75 cm2 cell culture flasks after 24 h of the selected treatment. The collected media were first subjected to a centrifugation step of 400 g for 10 min at room temperature (RT) to pellet and remove cells. All following centrifugation steps were performed at 4 °C. Next, the supernatant was centrifuged at 2,000 g for 20 min to remove debris and apoptotic bodies. Then, to remove large EVs, the supernatant was centrifuged at 10,000 g for 40 min. To remove any remaining large EVs, the supernatant from the first 10,000 g step was passed through a 0.22 μm pore PES filter (MF-Millipore TM Membrane Filter, GSWP01300). To remove the remaining exogenous treatments, we concentrated the 15 ml volume to a 1 ml volume using Amicon Ultra-15 Centrifugal Filter Unit (Merc, UCF9100, 100 kDa MWCO) at 2000 g for 10 min. Such CM should not contain large EVs (up to 220 nm), apoptotic bodies, or free cytokines.
Extracellular vesicle isolation
During processing, the conditioned media and patient sera were kept on ice. EVs were isolated from the pre-cleared concentrated medium (CM) according to the manufacturer’s instructions using the MagCapture Exosome Isolation Kit PS Ver. 2 (FUJIFILM Wako Pure Chemical Corporation, Japan). All isolated EVs were then placed in 1.5 ml Protein LoBind Tubes (Sigma, EP0030108116) and immediately used for further analyzes, or stored in the manufacturers’ elution buffer at -80 °C.
Nanoparticle tracking analyzis
Prior to measurement, EVs suspensions were diluted with sterile ice-cold filtered PBS. The concentration of EVs was determined using a Malvern Panalytical NanoSight LM10 nanoparticle tracking system. This instrument was equipped with a 532 nm laser diode, an sCMOS camera and a 20× optical objective. The EVs suspension was dosed into the NanoSight cell for analyzis. Videos of the nanoparticles were recorded at a constant temperature of 25 °C and a frame rate of 30 frames per second using NTA 3.4 software. The software was then used to track the recorded videos under constant parameters, providing data on the size distribution and concentration of EVs per milliliter using Finite Track Length Adjustment (FTLA). These measurements were repeated four times to generate error bars for the data.
Negative stain and Cryo TEM
For negative stain transmission electron microscopy (TEM) EVs samples (concentration 1.48–6.56 × 109 EVs/ml) were prepared by absorption at freshly plasma-cleaned Carbon coated copper grids for 30 s at RT. This was accomplished by pipetting 4 µl of the EVs containing elution buffer on the grid. Following sample adsorption, grids were quickly and gently blotted on filter paper and immediately washed with 2% uranyl acetate, which was also immediately and gently blotted on filter paper. Following the washing step, the sample was fixed for 1 min by 2% uranyl acetate which was subsequently blotted on a filter paper. EVs were examined at 200 kV with a Talos F200C transmission electron microscope (ThermoScientific) equipped with a Ceta-D scintillator-based camera (ThermoScientific). In all cases, TEM was performed on a fresh sample of EVs that had not been subjected to freezing temperatures at any step of purification or processing.
For cryo-electron microscopy, 4 µl of the EVs sample was applied to freshly plasma-cleaned TEM grids (Quantifoil, Cu, 300mesh, R2/1) and vitrified into liquid ethane using ThermoScientific Vitrobot Mark IV (4 °C, 100% rel. humidity, 30 s waiting time, 5 s blotting time). The grids were subsequently mounted into the Autogrid cartridges and loaded to Talos Arctica or Glacios (ThermoScientific) transmission electron microscope for imaging. The microscope was operated at 200 kV. The EVs cryo-TEM micrographs were collected on K2 or Falcon4 direct electron detection camera at the 8500× and 92,000× nominal magnification with the under focus in the range 2–4 μm and the overall dose of < 20 e/Å2.
Cytokine array
After 24 h of the selected treatment, Human Cytokine Antibody Array (ab133997, Abcam) was used for the simultaneous detection of 42 cytokines in each CM sample according to the manufacturer’s instructions. Experiments were performed in duplicates. The array membranes were incubated for 30 min at room temperature in a blocking buffer. CM samples were then incubated on the membranes overnight at 4 °C. Following one large volume wash in Wash buffer I for an extended time of 30 min, three washes in Wash buffer I and two washes in Wash buffer II, membranes were incubated in Biotin-Conjugated Anti-Cytokines overnight at 4 °C. After washing as described above, membranes were incubated in HRP-Conjugated Streptavidin overnight at 4 °C. Washed arrays were finally incubated with Chemiluminescence Detection reagents and images were captured using the Azure c400 Imager (Azure Biosystems). Pixel density (signal density) of each spot on the membrane was quantified using ImageJ - Array Analyzis plugin15.
Cell lysis and immunoblot analyzes
For protein extraction from cells, these were washed twice with ice-cold 1× PBS and lysed for 20 min on ice in Tris-EDTA-SDS lysis buffer (pH 8, 1 M Tris-HCl, 0.5 M EDTA, 10% SDS), supplemented with protease (Roche, 04693132001) and phosphatase inhibitors (MedChemExpress, HY-K0021). Protein concentration was determined using the Pierce BCA protein assay (Thermo Scientific, 23225). Equal amounts of total protein (15 µg) were separated by SDS–PAGE, transferred to a PVDF (GE, GE10600023) or nitrocellulose (Bio-Rad, 1620112) membrane and analyzed by using the following antibodies (in a dilution range of 1:500–1:5000): Annexin V (#8555), BNIP3 (D7U1T) (#44060), Caveolin-1 (D46G3) (#3267), CD9 (D8O1A) (#13174), EpCAM (D1B3) (#2626), Flotillin-1 (D2V7Z) (#18634), HSP-70 (D69) (#4876), ULK1 (D8H5) (#8054), Phospho-ULK1 (Ser757) (D7O6U) (#14202), Phospho-ULK1 (Ser556) (D1H4) (#5869), from Cell Signaling; ACTB (ab16039), GPX-4 (ab125066), GABARAP/GABARAPL1/GABARAPL2 (ab109364), SQSTM1/p62 (ab56416) and TSG101 (ab125011) from Abcam; Atg7 (B-9) (sc-376212), Atg5 (C-1) (sc-133158) and MAPLC3B (G-9) (sc-376404) from Santa Cruz.
Secondary antibodies (diluted 1:1000): HRP-linked Anti-Rabbit and Anti-Mouse IgG (H + L) from Promega (W4011 and W402B). For detection, the Clarity Western-ECL substrate (Bio-Rad) was used. Blot luminescence was measured using Azure c400 Imager (Azure Biosystems), and subsequent densitometry was performed using Fiji gel analyzer tools.
For protein extraction from isolated extracellular vesicles, the total yields from all methods used for EVs isolation (including magnetic beads) were lysed in RIPA buffer on ice for 20 min.
Proteins were separated by PAGE under nonreducing conditions, sample loading buffer (5x SDS Sample Buffer) was used without mercaptoethanol addition and transferred to a PVDF membrane (GE, GE10600023) and processed as described above.
Confocal microscopy
Fixed cells
First, the cells were seeded on 18 mm coverslips coated with 0.5% gelatin and cultured under standard conditions. After reaching 70% confluence, the cells were exposed to the desired conditions for 24 h. Prior to fixation, cells were incubated with 150 nM MitoTracker Red CMXRos (Thermo Fisher Scientific, Waltham, Ma, USA) for 45 min and washed three times with a pre-warmed medium. Then, the cells were fixed in a pre-warmed fix solution containing 4% paraformaldehyde, 3% sucrose, PBS, and water for 20 min at 37 °C and washed three times with PBS. Right after, the cells were permeabilized using 0,2% Triton X-100 for 10 min. Permeabilization was followed by the blocking of non-specific Ab-blocking sites in PBS-BSA 0,1% for 60 min. Such prepared cells were incubated with primary antibodies (SQSTM1/p62 1:200, MAPLC3 1:200, BNIP3 1:200, TSG101 1:200) for 90 min at 37 °C. Then, the cells were washed three times with PBS 0.05% Tween 20 (PBS-T) and incubated with compatible secondary antibodies (Alexa Fluor 488, 1:750; Alexa Fluor 647, 1:750) for 60 min in the dark at RT. After secondary antibody incubation, the cells were washed two times with PBS-T and then incubated with 7 µl/50 ml DAPI. Prior to the mounting, cells were washed two times with PBS-T and once with PBS. Coverslips were mounted on glass slides using ProLong Gold Antifade Mountant (Thermo Fisher Scientific, Waltham, Ma, USA).
Live cells
For live cell imaging, cells were cultured in Ibidi µ-Slide VI 0.4 (ibiTreat, Cat. No. 80606). After reaching 70% confluence, cells were washed twice with PBS and exposed to the treatment for 24 h. For lipid-peroxidation assay Image-iT, a Lipid Peroxidation kit (Thermo Scientific) was used, following the manufacturer’s protocol. The Image-iT kit enables the detection of lipid peroxidation in live cells through oxidation of BODIPY 581/591 C11 reagent.
Acquisition
Confocal microscopy images of fixed cells were acquired using Laser scanning confocal microscope Zeiss LSM 880 with AiryscanFast module (Carl Zeiss Inc.) using a Plan-Apochromat 63× / 1.40 OIL objective. DAPI was excited with 405 nm solid state laser, and emitted light was detected at 433 nm. Alexa Fluor™ 488 was excited with 488 nm Argon laser, and emitted light was detected at 516 nm. MitoTracker Red CMXRos was excited with 561 nm solid state laser, and emitted light was detected at 579 nm. Alexa Fluor™ 647 was excited with HeNe 633 nm laser and emitted light was detected at 654 nm. Live cells images were acquired using a Plan-Apochromat C-Apochromat 63× / 1.20 W objective. Oxidized Bodipy was excited with 488 nm Argon laser, and emitted light was detected at 541 nm and reduced Bodipy was excited with 561 nm solid state laser, and emitted light was detected at 640 nm. AiryScan images were acquired using 1.8× magnification and processed using Zen Black software (Carl Zeiss Inc.). Colocalization data were evaluated using Coloc tool using the Zen Blue Software (Carl Zeiss Inc.).
Image analysis of lipid peroxidation was performed using a custom Python script in a semiautomatic manner. For analysis, images were preprocessed using a median filter (7 × 7) and Gaussian filter (sigma 1). Cells were segmented using thresholding of image created as the sum of both fluorescence channels and morphological operations (closing and filtration of small objects) were applied to resulting masks, with additional manual spiling of individual cells and manual correction of segmentation masks. Then, the median intensity of individual cells was extracted for oxBODIPY/BODIPY fluorescence channels. To assess lipid peroxidation, 12 fields of view were evaluated for all treatments. The numbers of cells evaluated were as follows: 159 for control, 153 for BEZ and 88 for starvation. Five fields of view were evaluated for the colocalization experiments. In total, approximately 30 cells were analyzed.
RNA extraction and characterization
Total EVs RNA was extracted from pre-cleared conditioned cultivation medium (CM) and pre-cleared serum using a commercial column-based exoRNeasy Maxi Kit (Qiagen, 77164). Total amounts and integrity of RNA were assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA, USA). For NGS, RNA samples from each treatment were prepared in tetraplicates.
Next-generation sequencing and data analysis
High-throughput RNA-Seq data were prepared using NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (New England Biolabs, USA) with polyA selection and sequenced on Illumina sequencer (run length 1 × 75 nt); in cooperation with Core Facility Genomics (CEITEC MU, CZ). Bcl files were converted to Fastq format using bcl2fastq v. 2.20.0.422 Illumina software for basecalling. Quality check of raw single-end fastq reads was carried out by FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc). The adapters and quality trimming of raw fastq reads was performed using Trimmomatic v0.3916 with settings CROP:250 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:5 MINLEN:35. Trimmed RNA-Seq reads were mapped against the human genome (hs38) and Ensembl GRCh38-p10 annotation using STAR v2.7.3a17 as splice-aware short read aligner and default parameters except --outFilterMismatchNoverLmax 0.4 and --twopassMode Basic. Quality control after alignment concerning the number and percentage of uniquely- and multi-mapped reads, rRNA contamination, mapped regions, read coverage distribution, strand specificity, gene biotypes and PCR duplication was performed using several tools namely RSeQC v4.0.018, Picard toolkit v2.25.6 (https://broadinstitute.github.io/picard/; Broad Institute), Qualimap v.2.2.219.
The differential gene expression analysis was calculated based on the gene counts produced using featureCounts from Subread package v2.020 and further analyzed by Bioconductor package DESeq2 v1.34.021. Data generated by DESeq2 with independent filtering were selected for the differential gene expression analysis due to its conservative features and to avoid potential false positive results. Genes were considered as differentially expressed based on a cut-off of adjusted p-value < 0.05 and log2(fold-change) ≥ 1 or ≤-1. Differential expressed genese were visualized by the packages ggplot2, and tidyverse in R22. The functional roles of the significantly differentially expressed genes were performed according to the Gene Ontology (GO) enrichment analysis database on gene sets by applying the clusterProfiler package v3.18.123. Clustered heatmaps were generated from selected top differentially regulated genes using R package pheatmap v1.0.12 (https://cran.r-project.org/web/packages/pheatmap/index.html), volcano plots were produced using ggplot2 v3.3.5 package24 and MA plots were genereted using ggpubr v0.4.0 package (https://cran.r-project.org/web/packages/ggpubr/index.html).
Serum isolation
Human serum was obtained with informed consent from 41 patients with histologically confirmed HNSCC, and 20 healthy non-fasting adult volunteers. All experiments were performed in accordance with relevant guidelines and regulations. The experimental protocols were approved by the ethical committee of Motol University Hospital, Prague, Czech Republic. Patients were completely clinically examined, and serum samples were taken from patients with verified HNSCC; the inclusion criteria were as follows: histologically confirmed squamous cell carcinoma and no previous oncologic treatment in the last 5 years. Blood was drawn through a S-Monovette needle collection set (Sarstedt AG & Co.) and processed according to manufacturer’s instructions. After that, samples were rested upright for 30 min at room temperature (RT) to allow erythrocytes to clot. The clot was subsequently pelleted by centrifugation at 2000 g for 10 min at 4 °C and then upper phase (serum) was retrieved to DNA LowBind tubes (Eppendorf, Cat. No. 0030122208). Serum samples (2–3 ml) were stored at − 80 °C prior to analysis.
RealTime-PCR
Blood sera of HNSCC patients and controls were pre-filtred through a 0.45 μm pore PES filter (MF-Millipore Membrane Filter, HAWP04700) followed by isolation of exosomal RNA using exoRNeasy Maxi Kit (Qiagen, 77164). The exosomal RNA was transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA), which was used according to the manufacturer’s instructions. qRT-PCR was performed using TaqMan gene expression assays with the LightCycler 480 II System (Roche, Bazel, Switzerland) and the amplified cDNA was analyzed by the comparative Ct method using PSMB2 and ACTB as housekeeping genes. Primer and probe set for PSMB2 (Hs01009704m1), ACTB (Hs99999903m1), MT-RNR1 (Hs02596859g1), MT-RNR2 (Hs02596860g1), MT-CO2 (Hs02596865g1), and MT-CO3 (Hs02596866g1) were selected from the TaqMan gene expression assays (Thermo Fisher Scientific, Waltham, MA, USA). qRT-PCR was performed under the following amplification conditions: total volume 19,7 µl,
initial incubation at 50 °C/2 min followed by denaturation at 95 °C/10 min, then 15 cycles of preamplification (at 95 °C/15 s and 60 °C/1 min) and cooling down to 40 °C/30 s at the end. Subsequently, incubation at 50 °C/2 min followed by denaturation at 95 °C/10 min, 45 cycles of amplification (at 95 °C/15 s and 60 °C/1 min) and cooling down to 40 °C/30 s were done. qRT-PCR experiments were performed in duplicates.
Results
Autophagy induction by glutamine and FBS starvation or NVP-BEZ235 reduces ATP production in cancer cells but only gln starvation enhances oxidative damage
To verify the effect of selected treatments (NVP-BEZ235 and Gln + FBS starvation; further Gln starvation) on autophagy induction, we evaluated the expression of proteins involved in the autophagy machinery such as ULK1 and its phosphorylated forms pULK1Ser-556 and pULK1Ser-757 (phosphorylation of ULK1 at Ser-556 stimulates autophagy, whereas phosphorylation at Ser-757 is inhibitory), p62, GABARAP, and LC3 (see Fig. 1a and Supplementary Fig.S1). Both, NVP-BEZ235 and Gln starvation increased LC3II/I, GABARAPII/I ratio, and decreased Ser-757/ULK1 ratio indicating ULK1 activation and autophagy induction. The predominance of activatory ULK1 phosphorylation over inhibitory ULK1 phosphorylation in the form of an increased Ser-556/Ser-757 ratio was clearly observable in FaDu cells after the 12 h treatment with NVP-BEZ235 (hereafter BEZ). Gln starvation showed the effect on Ser-556/Ser-757 ratio later (after 24 h) (see Fig. 1b). These results suggest that if the content of EVs is to be affected by Gln starvation, their isolation must take place after at least 24 h of Gln starvation.
To assess the metabolic effects of Gln starvation and BEZ, we measured ATP production from glycolysis and OXPHOS based on the Seahorse (Agilent) parameters OCR (oxygen consumption rate) and ECAR (extracellular acidification rate). Both BEZ and Gln starvation decreased total ATP production. This observation supports the requirement of active mTOR for glycolysis and OXPHOS (see Fig. 1c). Furthermore, decreased proton leak was observable under both treatments. Gln starving cells had slightly increased coupling efficiency as compared to control cells (see Fig. 1c). Coupling efficiency is the proportion of the oxygen consumed to drive ATP synthesis compared with that driving proton leak.
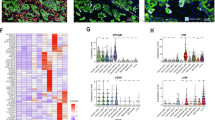
ATP production in FaDu cells and expression of proteins involved in the autophagy machinery. (a) Expression of autophagy-related proteins ULK1 and its phosphorylated forms p-ULK1 (Ser 757) and p-ULK1 (Ser 556), ATG5, ATG7, SQSTM1, GABARAP, and LC3; p indicates phosphorylation. Uncropped western blots for this figure are shown in supplementary Fig. S1. (b) Densitometric analyzes: the ratio of ULK1 phosphorylation on Ser 757 to ULK1 expression; the ratio of ULK1 phosphorylation on Ser 556 to ULK1 expression; the ratio of ULK1 phosphorylation on Ser 556/ Ser 757; the ratio of GABARAP II/I; the ratio of LC3 II/I. (c) l ATP production based on Seahorse parameters OCR (oxygen consumption rate) and ECAR (extracellular acidification rate). OM = oligomycin; FCCP = carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; rot = rotenone; AA = antimycin A. P-values from group comparisons based on the t-test are shown. Asterisks represent statistical significance (* p < 0.05; ** p < 0.01; *** p < 0.001).
We also observed that Gln-starvation leads to an increase in oxidative damage of cancer cells, particularly lipid peroxidation accompanied by depletion of the antioxidant defense protein GPX4 (see Fig. 2a and b). An increase in lipid peroxidation was indicated by a red to green fluorescence shift upon oxidation of BODIPY 581/591 C11 by lipid hydroperoxides. The mitochondrial network underwent significant remodeling during Gln deprivation, such as extensive fragmentation and swelling (see Fig. 2c). Furthermore, the levels of the mitophagy-related protein BNIP3 were increased after BEZ treatment and decreased by Gln starvation (see Fig. 2b), which could be a consequence of its degradation during mitophagy, where it is degraded together with damaged mitochondria. However, lower BNIP3 levels can be also associated with higher resistance to BNIP3-induced cell death. Therefore, we decided to perform confocal microscopy and evaluate the colocalization of mitochondria with BNIP3 and LC3 (a protein involved in autophagy substrate selection and autophagosome biogenesis).
An increase in lipid peroxidation accompanied by depletion of GPX4 and changes in the mitochondria after Gln-starvation. (a) Lipid peroxidation assessed by the Image-iT Lipid Peroxidation Kit; shift from red to green fluorescence is observable upon oxidation by lipid hydroperoxides. Scale bar: 10 μm, detail 20 × 20 μm. (b) Protein expression of antioxidant defense protein GPX4 and mitophagy-associated protein BNIP3.Uncropped western blots for this figure are shown in supplementary Fig. S1. (c) Changes in the mitochondrial network structure. MitoTracker Red stained mitochondria undergo significant remodeling during Gln deprivation such as extensive fragmentation and swelling. Scale bar:1 μm.
Glutamine starvation fails to elevate mitophagy markers but increases colocalization of TSG101 with mitochondria. Autophagy induction alters the spectrum of EV-associated cytokines
Since the mitochondrial network underwent significant remodeling during Gln deprivation, we further investigated whether mitophagy was involved in this process. Gln starvation or BEZ did not increase the abundance of the mitophagy markers LC3, p62 and BNIP3 on the mitochondrial membrane (see Fig. 3a, b and c, and Supplementary Fig. S2). This means that mitochondria damaged by Gln starvation are unlikely to be removed by mitophagy and may contribute to increased oxidative stress in cancer cells. Nevertheless, the colocalization of BNIP3 with mitochondria was high in all tested cells (control, BEZ treatment and Gln starvation). Nevertheless, BNIP3 does not colocalize with LC3 (see Fig. 3c). The colocalization of TSG101 with mitochondria was significantly increased by Gln starvation (see Fig. 3c and Fig. S3) indicating sorting of mitochondrial cargo into EVs.
Starving cells could promote TME reorganization by producing cytokines. Some of these cytokines can be associated with EVs (internally in EVs or membrane-bound). To assess small EVs-associated cytokines, conditioned medium (CM) without large EVs (up to 220 nm), apoptotic bodies, or free cytokines was prepared (see Fig. 4a and the Material and Methods section). We identified that the release of EVs-associated inflammatory cytokines is a primary response to autophagy induction and that the mechanism of this induction is important and significantly influences the composition of the EVs-associated cytokine secretome. We observed the up-regulation of IFNγ, IL-13, IL-1α, IL-15, I-309, IL-5, GM-CSF, TARC, RANTES, and IL-6 in response to Gln-starvation and BEZ compared to control cells. On the other hand, EVs-associated EGF and SDF-1 were down-regulated. Opposite effects of Gln-starvation and BEZ was also observed. IL-8, GRO-α, and IL-2 levels were decreased by starvation and increased by BEZ. On the other hand, thrombopoietin, oncostatin M, VEGF, and angiogenin levels were increased by starvation and decreased by BEZ. BEZ also enhanced EVs-associated levels of TNF-α and IL-1β (see Fig. 4b).
Immunofluorescence images of FaDu cells under glutamine starvation or treated with NVP-BEZ235. MitoTracker Red, autophagy-related proteins, BNIP3 and TSG101. (a) Confocal immunofluorescence images of MitoTracker Red/BNIP3/LC3 and their mutual colocalization. Scale bar: 5 μm (detail 1 μm) (b) Confocal immunofluorescence images of MitoTracker Red and TSG101 colocalization, and MitoTracker Red and p62 colocalization. Scale bar: 5 μm (c) Pearson correlations depicting the rate of colocalization of BNIP3, LC3 p62, and TSG101 with mitochondria.
Glutamine starvation and NVP-BEZ235 decrease the production of EVs by cancer cells but do not change their characteristic EVs molecules
After NVP-BEZ235 treatment and/or Gln starvation, we have isolated small EVs produced by FaDu cells using the MagCapture Exosome Isolation Kit PS Ver 2. (FUJIFILM). Cryo-electron microscopy of non-collapsed extracellular vesicles and negative staining transmission electron microscopy demonstrated the native character of isolated vesicles, with an intact double membrane and cup-shaped morphology (see Fig. 4c). Their size and concentration were assessed by NanoSight nanoparticle analyzer. The mode size of EVs produced during starvation was 138.7 nm (82.88 × 109 particles/ml). 137.1 nm for control (367.36 × 109 particles/ml), and 149.9 nm (246.96 × 109 particles/ml) for BEZ (see Fig. 4d). The mean size of EVs produced during starvation was 161.9 nm, 149.5 for control, and 156.1 for BEZ. Thus, Gln starvation and NVP-BEZ235 significantly decreased the production of EVs by cancer cells. For deeper characterization of isolated EVs, we performed WB. EVs after all treatments were positive for EpCAM, CD9, Cav-1, flotillin-1, annexin V, and TSG101 (see Fig. 4e). Lower expression of these proteins after Gln starvation is due to a lower concentration of EVs after starvation not because of reduced EpCAM expression in starved FaDu cells (see Fig. 4f). Due to the low protein concentration in the lysates from the isolated EVs, the concentrations of the samples loaded on the gel were not equilibrated. The aim was only to demonstrate sample positivity for selected markers of EVs, not to compare samples with each other.
Characterization of cytokines and extracellular vesicles produced by FaDu cells after NVP-BEZ235 treatment or glutamine starvation. (a) Conditioned media (CMs) preparation scheme. Large EVs (larger than 220 nm), apoptotic bodies, or free cytokines were removed during CM preparation. These CMs were used for the cytokine array.(b) Heatmap of normalized cytokine levels in CM after NVP-BEZ235 treatment or glutamine starvation. This CM should not contain large EVs (larger than 220 nm) apoptotic bodies or free cytokines. (c) (left column) Cryo-electron microscopy of non-collapsed extracellular vesicles demonstrating the native character of isolated vesicles with an apparently intact double membrane. Scale bar equals 50 nm. Negative staining transmission electron micrograph of cup-shaped extracellular vesicles. Shown is a heterogeneous population of vesicles consisting of a range of sizes (30–200 nm) with low densities typical for exosomes. Scale bar equals 1000 nm (the middle column) and 100 nm (the right column). TEM microscopy was performed on a fresh sample of EVs not subjected to freezing temperatures.(d) Size and concentration of EVs assessed by NanoSight nanoparticle analyzer. The sample was diluted 65x before measurement Numbers indicate peak size.(e) Characterization of isolated EVs. Protein expression of EpCAM CD9 Cav-1 flotillin-1 annexin V and TSG101. (f) Protein expression of EpCAM in FaDu cells. Uncropped western blots for this figure are shown in supplementary Fig. S1.
Glutamine starvation alters the abundance of mitochondrial RNAs in EVs
In further experiments, the content of RNAs in EVs produced by FaDu cells was characterized under Gln starvation and NVP-BEZ-235 treatment. RNAs encoding proteins were the most abundant type of RNAs in EVs produced by FaDu cells. No statistically significant changes in the representation of main RNA biotypes in EVs produced by FaDu cells were found. Weak peaks of ribosomal RNA and miscellaneous RNA were also detected, but this was seen only in EVs produced by cells after Gln starvation (see Fig. 5a). RNA content of EVs was significantly affected by Gln starvation. The effect of PI3K/mTOR inhibitor NVP-BEZ-235 was nonsignificant (see Fig. 5b and supplementary Fig. S4). It implies that Gln starvation exerts a higher pressure to alter vesicular communication than induction of autophagy by BEZ. Gln starvation significantly affected the abundance of mitochondrial RNA (e.g., RNAs for NADH-ubiquinone oxidoreductase chains 1 to 6, for cytochrome B, for mitochondrially encoded cytochrome C oxidases I, II, and III, for mitochondrially encoded ATP synthase 6 and 8, or mt-RNR1 and mt-RNR2); see Fig. 5b. According to the Gene Ontology (GO) pathway analyzis, the RNAs changed due to Gln starvation are involved in biological processes such as aerobic respiration, OXPHOS, purine nucleotide biosynthesis, or energy derivation by oxidation of organic compounds (see Fig. 5c).
The RNA content of EVs produced by FaDu cells and its changes after autophagy modulation. (a) Representation of different RNA biotypes in EVs produced by FaDu cells. Sequencing of each treatment variant was performed in tetraplex. (b) Volcano plot of changes in RNA content of FaDu-derived EVs due to Gln starvation and NVP-BEZ-235. Significant changes in expression are marked in red (upregulated; HC_log2FC > 1 and HC_padj < = 0.05) or blue (downregulated; HC_log2FC < 1 and HC_padj < = 0.05). FC = fold change, MT = mitochondrial.(c) Categories of biological processes from the GO database. Changes in biological processes which can be influenced by RNA cargo changes caused by Gln starvation.
Mitochondrial RNAs are detectable in EVs isolate from serum of HNSCC patients and healthy controls
A total of 41 patients with head and neck squamous cell cancers (age; median 61; interquartile range 52 to 72) and 20 healthy controls (age; median 55.5; interquartile range 50 to 61) were used in the study. The healthy cohort included men without a cancer diagnosis, diabetes, and/or chronic inflammatory diseases. The HNSCC patient cohort was defined as follows: squamous cell carcinoma histology, curative therapeutic intention using surgery +/− radio or radio/chemotherapy. Tumors with the following locations were included: oropharynx (N = 29), larynx (N = 6), hypopharynx (N = 1), tongue (N = 4), and the floor of the mouth (N = 1). The p16 status was analyzed in all subjects. TNM8 system was used. The description of HNSCC patients is shown in Table 1. Exosomal RNA was isolated from blood serum of HNSCC patients and controls by exoRNeasy Maxi Kit (Qiagen). Expression of mtRNR1, mtRNR2, mt-CO2, and mt-CO3 was detected in both types of EVs, HNSCC patients-derived and healthy controls-derived. There was no statistically significant difference in the expression of the studied mtRNAs between patients and controls. The expression of studied mtRNAs was not associated with age, stage, or grade of HNSCC patients but was higher in patients with oropharyngeal cancer compared to other localizations; (see Fig. 6a and b).
Expression of mitochondrial RNAs in EVs isolated from sera of HNSCC patients and healthy controls. (a) Differential expression in HNSCC patients (N = 41) and controls (N = 20) did not reveal a significant difference in the expression of any of the analyzed genes. (b) Heatmap showing relationship with clinicopathological indicators. Shown as log 2-fold change (FC) relative to the mean of the controls. All selected genes show higher levels in the oropharyngeal location (vs. all other locations). See supplementary Table S1 for details.
Discussion
Induction of autophagy can be a very efficient survival mechanism for starved or stressed cancer cells and other cells in the TME. Induction of autophagy can also alter signaling between cells in the TME25,26and can cause TME reorganization27. It has been shown that different types of autophagy modulation can have different effects on signaling molecules produced by cancer cells6. Here, we studied metabolic and signaling consequences of glutamine (Gln) starvation-induced and NVP-BEZ235-induced autophagy in cancer cells. Both NVP-BEZ235 and Gln starvation decreased ATP production from glycolysis and OXPHOS. This observation supports the requirement of active mTOR for glycolysis28,29. It was shown that inhibition of the PI3K/mTOR pathway effectively suppressed glycolysis by preventing membrane localization of glucose transporter 1 (GLUT1)28. mTORC1 also controls mitochondrial activity and biogenesis30. Autophagy induction by Gln starvation or NVP-BEZ235 reduces ATP production in cancer cells, but only Gln starvation enhances oxidative damage of cancer cells, particularly lipid peroxidation accompanied by depletion of the antioxidant defense protein GPX4 (GPX4 maintains mitochondrial membrane potential under oxidative stress)31and changes in the mitochondrial network structure. Tne mitochondrial network in Gln-starved cells became fragmented, which may be the result of increased oxidative stress32,33. Prasad et al. showed that Gln deficiency can promote stemness and chemoresistance in ovarian cancer cells through DRP1-induced mitochondrial fragmentation34. On the other hand, Abdullah et al. observed mitochondrial hyper-fusion in 4T1 breast cancer cells facing amino acid starvation35. This suggests that the effect of glutamine depletion is cancer type specific. FaDu cells express high levels of the NFE2L2mRNA, which encodes the Nrf2 protein36. Nrf2 induces the high expression of the cystine/glutamate antiporter SLC7A11 and promotes SLC7A11 membrane transfer37,38. Consequently, FaDu cells express high amount of SLC7A1139which facilitates the transport of cystine, supporting glutathione biosynthesis and preserving redox balance, thereby shielding cells from ferroptosis. Nonetheless, cancer cells exhibiting high SLC7A11 levels seem to be vulnerable to cell death induced by glucose or glutamine deprivation40. It is consistent with our findings that Gln starvation significantly increases oxidative damage in FaDu cells.
One of the strategies to get rid of damaged mitochondria that produce reactive oxidative species is mitophagy41. The colocalization of the mitophagy-related protein BNIP3 with mitochondria was high in all tested samples (control, BEZ treatment and Gln starvation). BNIP3 was shown to cause mitochondrial fission42and an increase in mitochondrial protease activity promoting degradation of mitochondrial proteins43. The protein levels of BNIP3 were increased after BEZ treatment and decreased by Gln starvation, which may be a consequence of its degradation during mitophagy, where it can be degraded together with damaged mitochondria41 or it can reflect lower susceptibility of Gln starving cells to cell death induced by BNIP344. Nevertheless, colocalization of BNIP3 with LC3 was low and Gln starvation or BEZ did not increase the abundance of the mitophagy markers LC3, p62 or BNIP3 on the mitochondrial membrane. This means that mitochondria damaged by Gln starvation are unlikely to be removed by mitophagy and may contribute to increased oxidative stress in cancer cells. However, the colocalization of TSG101 with mitochondria was significantly increased by Gln starvation (but not by BEZ) indicating the sorting of mitochondrial cargo into EVs45,46. Interestingly, we found an increase in mitochondrial RNAs in EVs produced by Gln-starved cells. In our previous study, we found that Gln starvation also increased mitochondrial protein levels in cancer cell-derived EVs6. This suggests that if damaged mitochondria and mitochondrial proteins are not sufficiently removed by mitophagy or autophagy, a compensatory mechanism in the form of increased exocytosis of these mitochondrial components (proteins, RNAs) by EVs may be involved47. TSG101 also binds to PARP1 and is required for PARP1 and NF-κB activation48. The level of mitochondrial RNAs in EVs was significantly affected by Gln starvation. The effect of PI3K/mTOR inhibitor NVP-BEZ-235 was nonsignificant. It implies that Gln starvation exerts a higher pressure to alter vesicular communication in the TME than simple PI3K/mTOR inhibition.
In addition to the reorganization of metabolism, we identified the release of EVs-associated inflammatory cytokines as a primary response to autophagy induction by BEZ or Gln starvation. This is consistent with the observation that starvation and antimetabolic therapy promote cytokine release and recruitment of immune cells49. For example, higher EVs-associated levels of IL-6, IFNγ, IL-13, IL-1α, TARC, and RANTES (CCL5) were observed in response to Gln-starvation and BEZ treatment. IL-6 plays an important role in angiogenesis in the TME50. Moreover, IL-651, IL-1352, or TARC53can trigger proliferation of cancer cells. Signaling involving CCL5 and its receptor CCR5 can also promote tumor cell proliferation, induce collagen degradation by activating matrix metalloproteinases in macrophages and fibroblasts and enhance tumor cell migration and invasion by activating integrins and actin polarisation54. CCL5-CCR5 interaction can provide anti-apoptotic signals55. IL-1α can stimulate proliferation of cancer-associated fibroblasts (CAFs). CAFs then secrete CCL7, CXCL1, and IL-8 cytokines facilitating cancer progression and invasion56. Opposite effects of Gln-starvation and BEZ on cytokine production were also observed indicating that the mechanism of the autophagy induction is important and significantly influences the composition of the EVs-associated cytokine secretome. IL-8, GRO-α, and IL-2 levels were decreased by Gln starvation and increased by BEZ. BEZ also enhanced EVs-associated levels of TNF-α and IL-1β. The increased production of these cytokines may be related to the diarrhoea and mucositis reported following treatment with NVP-BEZ235 (dactolisib)57as IL-8, TNF-α and IL-1β were associated with post-radiation mucositis in cancer patients58,59,60.
Glutamine starvation and NVP-BEZ235 treatment decreased the production of EVs by cancer cells but did not change their characteristic molecules. Fader et al. showed that the initiation of autophagy or the heightened expression of LC3 impeded the release of exosomes. Under circumstances promoting autophagy, the multivesicular bodies (MVBs) were directed toward fusion with autophagic vacuoles, leading to the subsequent inhibition of exosome release61. In further experiments, the content of RNAs in EVs produced by FaDu cells was characterized. RNAs encoding proteins were the most abundant type of RNAs in EVs produced by FaDu cells. A weak peak of ribosomal RNA was also detected, but this was only in EVs produced by cells after Gln starvation. RNA profiling performed by Crescitelli et al. showed that ribosomal RNA (rRNA) was primarily detectable in apoptotic bodies. Smaller RNAs without prominent peaks of rRNA were detectable in exosomes. In contrast, microvesicles contained little or no RNA62. Based on these facts and the molecules that were detected on the EVs that we isolated, such as EpCAM, CD9, Cav-1, flotillin, annexin V, and TSG101, we can conclude that they are exosomes.
We found that Gln starvation enhances the abundance of mitochondrial RNAs in FaDu-derived EVs. The study by Dohl et al. showed that Gln depletion can disrupt mitochondrial integrity63. Mitochondrial damage may be more pronounced in FaDu cells because they are highly dependent on Gln64. Mitochondrial RNA or DNA from stressed mitochondria can be packaged into EVs and serve as an important signaling factor in the TME65. For example, packaging and transfer of mitochondrial DNA by EVs regulates the escape from dormancy in breast cancer66. Our results suggest that mitochondrial stress and reprogramming of cellular metabolism may be reflected in the molecular content of EVs. Head and neck squamous cell carcinomas (HNSCC) appear to be glutamine-dependent tumor types as HNSCC patients with low expression of glutaminase 1 (GLS1) have better disease-free survival after cancer treatment64. Since stress induced by glutamine deficiency is reflected in the abundance of mtRNAs in EVs, it can be concluded that these mtRNAs may be suitable markers of response to HNSCC therapy targeting the metabolic weaknesses of these tumors67. Here, we determined the expression of mitochondrial RNAs in EVs isolated from the serum of HNSCC patients and healthy controls. Expression of mtRNR1, mtRNR2, mt-CO2, and mt-CO3 was detected in both types of EVs, HNSCC patients-derived and healthy controls-derived, but there was no statistically significant difference in the expression of studied mt-RNAs between patients and controls. The expression of studied mt-RNAs was not associated with age, stage, or grade of HNSCC patients but was higher in patients with oropharyngeal cancer compared to other localizations.
Data availability
We have submitted all relevant data of our experiments to the EV-TRACK knowledgebase (EV-TRACK ID: EV231021)63. Confocal microscopy data are stored in the Zenodo depository, https://zenodo.org/records/10479096, DOI 10.5281/zenodo.10479096.
References
Briukhovetska, D. et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer. 21, 481–499 (2021).
Raudenska, M., Balvan, J. & Masarik, M. Crosstalk between autophagy inhibitors and endosome-related secretory pathways: a challenge for autophagy-based treatment of solid cancers. Mol. Cancer. 20, 140 (2021).
Zhao, J., Zhai, B., Gygi, S. P. & Goldberg, A. L. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proceedings of the National Academy of Sciences 112, 15790–15797 (2015).
Tan, H. W. S., Sim, A. Y. L. & Long, Y. C. Glutamine metabolism regulates autophagy-dependent mTORC1 reactivation during amino acid starvation. Nat. Commun.8, 338 (2017).
Zhu, Y. et al. L-Glutamine deprivation induces autophagy and alters the mTOR and MAPK signaling pathways in porcine intestinal epithelial cells. Amino Acids. 47, 2185–2197 (2015).
Hanelova, K. et al. Autophagy modulators influence the content of important signalling molecules in PS-positive extracellular vesicles. Cell Communication Signaling: CCS21, (2023).
Li, N. & Neu, J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J. Nutr.139, 710–714 (2009).
Shin, S. et al. mTOR inhibition reprograms cellular proteostasis by regulating eIF3D-mediated selective mRNA translation and promotes cell phenotype switching. Cell. Rep.42, 112868 (2023).
Kucharzewska, P. et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. U S A. 110, 7312–7317 (2013).
Zou, W. et al. Exosome Release is regulated by mTORC1. Adv. Sci.6, 1801313 (2019).
Palikaras, K., Lionaki, E. & Tavernarakis, N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell. Death Differ.22, 1399–1401 (2015).
Marini, C. et al. Divergent targets of glycolysis and oxidative phosphorylation result in additive effects of metformin and starvation in colon and breast cancer. Sci. Rep.6, 19569 (2016).
Lima, L. G. et al. Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution. Nat. Commun.12, 3543 (2021).
Hönigova, K. et al. Metabolic tricks of cancer cells. Biochim. Biophys. Acta Rev. Cancer. 1877, 188705 (2022).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9, 676–682 (2012).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30, 2114–2120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 29, 15–21 (2013).
Wang, L., Wang, S. & Li, W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 28, 2184–2185 (2012).
Okonechnikov, K., Conesa, A. & García-Alcalde, F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics. 32, 292–294 (2016).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15, 550 (2014).
Wickham, H. et al. Welcome to the Tidyverse. J. Open. Source Softw.4, 1686 (2019).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innov. (Camb). 2, 100141 (2021).
Wickham, H. ggplot2. WIREs Computational Statistics 3, 180–185 (2011).
O’Donovan, T. R., O’Sullivan, G. C. & McKenna, S. L. Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. Autophagy. 7, 509–524 (2011).
Jena, B. C., Rout, L., Dey, A. & Mandal, M. Active autophagy in cancer-associated fibroblasts: recent advances in understanding the novel mechanism of tumor progression and therapeutic response. J. Cell. Physiol.236, 7887–7902 (2021).
Bustos, S. O., Antunes, F., Rangel, M. C. & Chammas, R. Emerging autophagy functions shape the Tumor Microenvironment and play a role in Cancer Progression - implications for Cancer Therapy. Front. Oncol.10, 606436 (2020).
Makinoshima, H. et al. Signaling through the phosphatidylinositol 3-Kinase (PI3K)/Mammalian target of Rapamycin (mTOR) Axis is responsible for aerobic glycolysis mediated by glucose transporter in Epidermal Growth Factor Receptor (EGFR)-mutated lung adenocarcinoma. J. Biol. Chem.290, 17495–17504 (2015).
Yecies, J. L. & Manning, B. D. Transcriptional Control of Cellular Metabolism by mTOR Signaling. Cancer Res.71, 2815–2820 (2011).
Morita, M. et al. mTORC1 controls mitochondrial activity and Biogenesis through 4E-BP-Dependent translational regulation. Cell Metabol.18, 698–711 (2013).
Liang, H. et al. Gpx4 protects mitochondrial ATP generation against oxidative damage. Biochem. Biophys. Res. Commun.356, 893–898 (2007).
Wu, S., Zhou, F., Zhang, Z. & Xing, D. Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission–fusion proteins. FEBS J.278, 941–954 (2011).
Gwangwa, M. V., Joubert, A. M. & Visagie, M. H. Effects of glutamine deprivation on oxidative stress and cell survival in breast cell lines. Biol. Res.52, 15 (2019).
Prasad, P., Ghosh, S. & Roy, S. S. Glutamine deficiency promotes stemness and chemoresistance in tumor cells through DRP1-induced mitochondrial fragmentation. Cell. Mol. Life Sci.78, 4821–4845 (2021).
Abdullah, M. O. et al. Mitochondrial hyperfusion via metabolic sensing of regulatory amino acids. Cell. Rep.40, 111198 (2022).
Peltanová, B. et al. mRNA subtype of Cancer-Associated fibroblasts significantly affects key characteristics of Head and Neck Cancer cells. Cancers (Basel). 14, 2286 (2022).
Feng, L. et al. SLC7A11 regulated by NRF2 modulates esophageal squamous cell carcinoma radiosensitivity by inhibiting ferroptosis. J. Transl Med.19, 367 (2021).
Liu, J. et al. Nrf2 and its dependent autophagy activation cooperatively counteract ferroptosis to alleviate acute liver injury. Pharmacol. Res.187, 106563 (2023).
Chintala, S. et al. Downregulation of Cystine Transporter xc– by Irinotecan in Human Head and Neck Cancer FaDu xenografts. Chemotherapy. 56, 223–233 (2010).
Zhang, C. et al. Crosstalk between ferroptosis and stress—implications in cancer therapeutic responses. Cancer Innov.1, 92–113 (2022).
Field, J. T. & Gordon, J. W. BNIP3 and Nix: atypical regulators of cell fate. Biochimica et Biophys. Acta (BBA) - Molecular Cell. Research. 1869, 119325 (2022).
Landes, T. et al. The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO Rep.11, 459–465 (2010).
Rikka, S. et al. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell. Death Differ.18, 721–731 (2011).
Ma, Z. et al. BNIP3 induces apoptosis and protective autophagy under hypoxia in esophageal squamous cell carcinoma cell lines: BNIP3 regulates cell death. Dis. Esophagus. 30, 1–8 (2017).
Garrus, J. E. et al. Tsg101 and the Vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 107, 55–65 (2001).
Malerød, L. et al. Cargo-Dependent degradation of ESCRT-I as a feedback mechanism to modulate endosomal sorting. Traffic. 12, 1211–1226 (2011).
Liang, W. et al. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat. Commun.14, 5031 (2023).
Tufan, A. B. et al. TSG101 associates with PARP1 and is essential for PARylation and DNA damage-induced NF‐κB activation. EMBO J.41, e110372 (2022).
Püschel, F. et al. Starvation and antimetabolic therapy promote cytokine release and recruitment of immune cells. Proc. Natl. Acad. Sci.117, 9932–9941 (2020).
Nagasaki, T. et al. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour–stroma interaction. Br. J. Cancer. 110, 469–478 (2014).
Wang, Y., van Boxel-Dezaire, A. H. H., Cheon, H., Yang, J. & Stark, G. R. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc. Natl. Acad. Sci.110, 16975–16980 (2013).
Matsui, S. et al. Interleukin-13 and its signaling pathway is associated with obesity-related colorectal tumorigenesis. Cancer Sci.110, 2156–2165 (2019).
Goldie, S. J. et al. Loss of GRHL3 leads to TARC/CCL17-mediated keratinocyte proliferation in the epidermis. Cell. Death Dis.9, 1–12 (2018).
Aldinucci, D., Borghese, C. & Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers (Basel). 12, 1765 (2020).
Tyner, J. W. et al. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat. Med.11, 1180–1187 (2005).
Bae, J. Y. et al. Reciprocal interaction between carcinoma-associated fibroblasts and squamous carcinoma cells through interleukin-1α induces cancer progression. Neoplasia. 16, 928–938 (2014).
Pongas, G. & Fojo, T. BEZ235: when Promising Science meets clinical reality. Oncologist. 21, 1033–1034 (2016).
Principe, S., Zapater-Latorre, E., Arribas, L., Garcia-Miragall, E. & Bagan, J. Salivary IL-8 as a putative predictive biomarker of radiotherapy response in head and neck cancer patients. Clin. Oral Investig. 26, 437–448 (2022).
Sonis, S. T. Oral Mucositis in Head and Neck Cancer: Risk, Biology, and Management. Am Soc Clin Oncol Educ Book e236–e240 doi: (2013). https://doi.org/10.14694/EdBook_AM.2013.33.e236
Thornton, C. P., Kozachik, S. & Ruble, K. Study protocol to evaluate influences of stress and inflammation on Mucositis in adolescents and Young adults with Cancer. Nurs. Res.71, 404–410 (2022).
Fader, C. M., Sánchez, D., Furlán, M. & Colombo, M. I. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic. 9, 230–250 (2008).
Crescitelli, R. et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles. 2, 20677 (2013).
Dohl, J. et al. Glutamine depletion disrupts mitochondrial integrity and impairs C2C12 myoblast proliferation, differentiation, and the heat-shock response. Nutr. Res.84, 42–52 (2020).
Yang, J. et al. Targeting cellular metabolism to reduce head and neck cancer growth. Sci. Rep.9, 4995 (2019).
Puhm, F. et al. Mitochondria are a subset of Extracellular vesicles released by activated monocytes and induce type I IFN and TNF responses in endothelial cells. Circul. Res.125, 43–52 (2019).
Sansone, P. et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proceedings of the National Academy of Sciences 114, E9066–E9075 (2017).
Lin, X., Zhou, W., Liu, Z., Cao, W. & Lin, C. Targeting cellular metabolism in head and neck cancer precision medicine era: a promising strategy to overcome therapy resistance. Oral Dis.29, 3101–3120 (2023).
Van Deun, J. et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods. 14, 228–232 (2017).
Acknowledgements
We acknowledge the core facility CELLIM supported by MEYS CR (LM2018129 Czech-BioImaging). We acknowledge the support of the Cryo-electron Microscopy and Tomography Core Facility of CEITEC MU CIISB, Instruct-CZ Centre supported by the Ministry of Education of the Czech Republic (LM2018127). We acknowledge the support of the Core Facility Genomics (CEITEC MU, CZ). We acknowledge the support of the CzechNanoLab project at the CEITEC Nano Research Infrastructure Meys CR (LM2023051).
Funding
This work was supported by the Ministry of Health of the Czech Republic (NU20J-08-00018 and NU21-03-00223) and by the Czech Science Foundation (GACR-21–06873 S).
Author information
Authors and Affiliations
Contributions
J.Ba., M.R. and M.M. designed the experimental plan; D.K., M.G., J. Be, J.P. collected serum samples and performed clinical characterization of HNSCC patients; K.H., J.N., M.B., S.H., and J.Ba. performed the biological experiments; J.G., T.V. and F.P. evaluated the NGS data and confocal microscopy data, performed statistical analyzis, and created figures. M.R., K.H., J.Ba. and M.B. evaluated the data and interpreted the results; M.R. and M.B. wrote the main text of the manuscript. J.Ba. supervised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bugajova, M., Raudenska, M., Hanelova, K. et al. Glutamine and serum starvation alters the ATP production, oxidative stress, and abundance of mitochondrial RNAs in extracellular vesicles produced by cancer cells. Sci Rep 14, 25815 (2024). https://doi.org/10.1038/s41598-024-73943-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73943-2