Abstract
This study aimed to evaluate the impact of Fabry disease (FD) on retinal microvasculature using optical coherence tomography angiography (OCTA), arterial stiffness, and the resistive index (RI) of the central retinal artery (CRA) in early disease stages. Twenty-nine genetically confirmed FD patients and twenty-six healthy controls were enrolled. Vessel density (VD) values of the superficial, deep, and choriocapillaris plexuses (SCP, DCP, and CC) were measured via OCTA. CRA RI was studied using color Doppler and grayscale sonography, and aortic pulse wave velocity (PWV) was assessed with the Complior method. CRA RI was significantly lower in the control group compared to the Fabry group (p < 0.001). Central VD was found to be significantly higher in the control group compared to the Fabry group in all the retinal layers (SCP (p < 0.001), DCP (p < 0.005), CC (p < 0.001)). PWV was significantly higher in the Fabry group than in the control group (p = 0.03). Fabry disease patients demonstrate elevated arterial stiffness, increased CRA RI, and diminished retinal microvascular density compared to healthy controls, indicating early ocular damage. Continuous monitoring and targeted screening for organ impairment are crucial in FD management. Identifying biomarkers for assessing ocular vascular involvement and treatment response is imperative. Further research is needed.
Similar content being viewed by others
Introduction
Fabry disease (FD) is an X-linked lysosomal storage disease caused by pathogenic variants in the galactosidase alpha (GLA) gene1,2. It leads to the cumulative deposition of unmetabolized glycosphingolipids within the lysosomes of various tissues3,4. This scenario is thought to cause inflammation in several organs, including the eyes5,6. Multiorgan involvement may complicate the clinical picture, creating diagnostic delays and worsening the morbidity and mortality associated with this disease. Indeed, it is critical for FD patients to have a precise diagnosis, followed by quick and successful therapy before permanent end-organ damage develops7,8.
Ocular evidence of FD, such as cornea verticillata, subcapsular cataract development, and vascular tortuosity of the conjunctival and retinal vessels, can be noticed prior to or concurrent with systemic symptoms and can aid in the diagnosis9,10.
Tortuosity and aneurysmal dilatations in retinal vessels are typical signs of the illness and are thought to be produced by glycosphingolipid formation in the tunica media of the tiny vessels and endothelial cells11. Optical coherence tomography angiography (OCTA) provides for non-invasive quantitative analysis of vascular alterations in the retina. The OCTA method can assess the density of retinal vascular plexuses and radial peripapillary capillaries12. These measures assist clinicians in evaluating retinal microvascular changes in a variety of illnesses13,14.
Several studies have found a link between Fabry disease and arterial stiffness. Arterial stiffness refers to the loss of arterial elasticity, resulting in decreased compliance of the arterial walls. Arterial stiffness in patients with Fabry disease has been analysed using a non-invasive method called pulse wave velocity15. The researchers found that patients with Fabry disease had significantly higher pulse wave velocity values, indicating increased arterial stiffness compared to healthy individuals.
The resistive index (RI) is a measure used in ophthalmology to assess vascular resistance in the central retinal artery. Color Doppler and gray scale sonography allow the visualization of the blood flow and morphology of the retrobulbar vessels. They can be useful for assessing the hemodynamic changes associated with various ocular diseases, such as glaucoma, diabetic retinopathy, and retinal vascular occlusion16. Moreover, so far, no data on RI of central retinal artery in patients affected by Fabry disease has been reported in literature.
This study aims to assess the effect of FD on the retinal microvasculature, arterial stiffness and the resistive index of the central retinal artery, in the early stage of the disease.
Materials and methods
Study design and setting
This observational, retrospective, and case-control study was conducted in the Department of Ophthalmology at “Azienda Ospedaliera dei Colli Monaldi” Hospital, Naples, Italy, and in the Department of Inherited and Rare Cardiovascular Diseases at the University “Luigi Vanvitelli”, between April 2022 and September 2023. The study was conducted in accordance with the tenets of the Declaration of Helsinki. The Ethical Committee of the Monaldi Hospital of Naples, Italy reviewed and approved the research protocol and study procedures. Before entering the study, all patients received detailed information about the study’s goals and procedures. They provided informed consent before any data collection began. Each participant confirmed their voluntary involvement and understanding of the study. The principles of confidentiality and privacy were strictly respected.
Participants
Twenty-nine patients diagnosed with FD and referred to the Azienda Ospedaliera dei Colli Monaldi Hospital were included, alongside 26 age-matched healthy controls. The inclusion criteria required an established diagnosis of FD with genetic testing, accompanied by indicative signs or symptoms, and participants aged over 18 years.
Written informed consent was obtained from the patients. Proof of informed consent is available upon request. Diagnosis can be achieved by the α-GAL A enzyme activity assay on several sample matrix types (dried blood spot DBS, peripheral white blood cells or plasma) and/or by molecular analysis. The samples were analyzed for α-GAL A enzyme activity using a multiplex MS/MS assay (PerkinElmer, Turku, Finland). Assay results were obtained after overnight incubation and enzyme activity was expressed as micromoles of substrate hydrolyzed per hour of incubation per liter of blood (µmol/L/h). For the samples with low activities confirmatory testing included molecular analysis. The GLA gene was sequenced using Next Generation Sequencing technology on genomic DNA isolated from peripheral leukocytes. Variants were classified according to published clinical reports and public databases including the International Fabry Disease Genotype-Phenotype Database and The Fabry Working Group Genotype Phenotype Database [International Fabry Disease Genotype-Phenotype Database. Available online: www.dbfgp.org]. Genetic counselling framework was offered and performed on the patients and his relatives.
Exclusion criteria encompassed the presence of comorbidities such as diabetes, atherosclerotic vasculopathy, glaucoma, or any macular or retinal disorders, as well as optical media opacity that could compromise the reliability of retinal functional assessments.
Data measurement
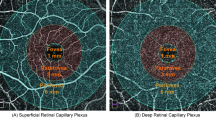
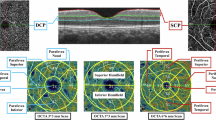
All images were acquired after pupil dilation using 1.0% tropicamide eyedrops. Assessment of SS-OCT and OCTA data was investigated to ascertain retinal and choroidal thickness (CT) along with vessel density (VD), employing the swept-source Topcon DRI OCT Triton (Topcon Corporation, Japan). Amidst all subjects, only the eye featuring the highest image quality scan was utilized for the study. The acquisition protocol included a macular line scan and a 6 × 6 mm macular cube scan for both OCT and OCTA. Each scan was centered on the fovea, and the IMAGENET 6.0 automated layer detection tool was applied to define retinal layers for analysis of the superficial capillary plexus (SCP), deep capillary plexus (DCP), and choriocapillaris (CC). 3D datasets were scrutinized to determine retinal thickness and VD within the five ETDRS sectors (central, nasal, temporal, inferior, and superior).
Only images with a quality score greater than 50 were considered acceptable and consequently comprised in the final analysis. VD was settled as the proportion of the total area covered by blood vessels. When using OCTA on the Topcon DRI OCT Triton, the unit of measurement is typically indicated as a dimensionless ratio or percentage. This metric assess the concentration of blood vessels within a specific sector of interest in the scanned tissue, representing the relative extent of the analyzed area occupied by blood vessels. This unit of measurement coordinates steadly with the established method for characterizing vessel density in OCTA imaging between different devices and platforms. The OCTA software produced vessel densities for each scan within the identical ETDRS regions outlined in our study protocol. The OCTA signal was identified and recorded for every ETDRS region, overlaid onto each OCTA en-face layer. Consequently, the average normalized VD of the full-thickness retina and the choriocapillaris in each ETDRS region was extrapolated from every subject, enabling further analysis.
The resistive index (RI) of the central retinal artery (CRA) is a sonographic parameter that reflects the vascular resistance and blood flow in the retina. It is calculated as the difference between the peak systolic velocity (PSV) and the end-diastolic velocity (EDV) divided by the PSV. (Boote 2003)
To measure the RI of the CRA, a high-frequency linear probe (7.5–10 MHz) is used to locate the ophthalmic artery (OA) along the medial border of the medial rectus muscle. The OA is then followed until it bifurcates into the CRA and the posterior ciliary artery. A 2–3 mm sample window is placed on the CRA and the spectral Doppler signal is recorded17.
The aortic PWV is a measure of arterial stiffness that reflects the elasticity and distensibility of the aorta. It is calculated as the ratio of the distance travelled by the pulse wave along the aorta to the time it takes to travel that distance18.
The Complior method is a non-invasive technique that uses two pressure transducers applied on the skin over the common carotid and femoral arteries. The transducers detect the pulse waves at both sites and send them to a computer that records the waveforms and calculates the transit time between them. The distance between the two transducers is measured using a tape measure and corrected by a factor of 0.8 to account for the tortuosity of the aorta19.
Variables and outcomes
The primary outcome was centered on evaluating the differences between the two groups across the parameters of CRA RI, SCP cVD, DCP cVD, CC cVD, and PWV. Secondary outcomes involved investigating the relationship of each of these variables between the FD patient and healthy control groups.
Statistical analysis
The descriptive analysis employed mean values with their respective standard deviations and numerical representations in percentages. Mann-Whitney test was performed to test for differences between the two groups in terms of age. Fisher’s test was used to test for differences between the two groups in terms of sex. To compare continuous variables between the Fabry and the control groups, the Student’s t-test was used. To investigate the relationship between the variables—CRA RI, SCP cVD, DCP cVD, CC cVD, and PWV—and the groups (Fabry/Controls), linear regression analysis was conducted. A visual representation of the relationships between the variables and groups was generated through boxplots. Subsequently, a linear regression analysis was conducted to investigate the relationship between the variables that we had analyzed. Confidence intervals for the coefficients were calculated at the 95% confidence level. A p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using R software version 4.2.2 (R Project for Statistical Computing, Vienna, Austria) with packages `stats`, `tidyr`, `ggplot2`, and `tableone`.
Results
A total of 55 eyes were included in this investigation, 29 from individuals diagnosed with Fabry disease and 26 from healthy control subjects. The mean age was 45.07 (SD = 8.15) years, with a gender distribution of 56.4% female and 43.6% male. Statistical analysis revealed no significant differences in age or gender between the two groups (p = 0.074 and p = 0.79, respectively) (Table 1).
Among the Fabry patients, 65.5% were undergoing Enzyme Replacement Therapy (ERT). Systemic involvement analysis showed 72.4% with heart involvement, 34.5% with central nervous system involvement, and no reported cases of kidney involvement. Regarding ocular presentations in Fabry disease, none of the patients exhibited cornea verticillate, 65.5% displayed conjunctival vessel tortuosity, 51.7% had retinal vessel tortuosity, and 20.7% presented with lenticular opacities (Table 1).
The mean CRA RI in the Fabry group was 0.78 (SD = 0.10), while in the control group, it was 0.56 (SD = 0.08). SCP cVDs were 16.27 (SD = 4.04) and 19.30 (SD = 1.45), DCP cVDs were 24.42 (SD = 4.73) and 27.87 (SD = 3.04), and CC cVDs were 26.18 (SD = 4.64) and 45.81 (SD = 5.51), in Fabry patients and healthy controls, respectively. All the means and standard deviations of the VDs for each retinal layer measured in the central, superior, inferior, nasal, and temporal regions are reported in Table 2.. PWV in the Fabry and control groups was 7.34 (SD = 0.91) and 6.85 (SD = 0.69), respectively.
CRA RI was significantly lower in the control group compared to the Fabry group (p < 0.001). The regression model indicated that the CRA RI in the Fabry group was estimated to have a mean increase of 0.22 units compared to the control group (95% CI [0.1; 0.27]) (p < 0.001). This finding is visualized in the boxplot in Fig. 1. A subgroup linear regression analysis was performed to compare CRA RI in Fabry patients with and without ocular vascular findings, specifically retinal and conjunctival vessel tortuosity. The analysis revealed no statistically significant difference in CRA RI between Fabry patients with and without retinal vessel tortuosity (β = -0.02, 95% CI [-0.1, 0.07], p = 0.7). The regression model demonstrated a very low R-squared value (R² = 0.005). Similarly, no significant difference was observed in CRA RI between Fabry patients with and without conjunctival vessel tortuosity (β = 0.01, 95% CI [-0.08, 0.09], p = 0.9). This model also showed an extremely low R-squared value (R² = 0.0008).
The boxplot figure depicts a clear and statistically significant difference in the resistive index of the central retinal artery between the control group and the Fabry group (p < 0.001). In the control group, the median resistive index appears notably lower, indicative of reduced vascular resistance in the central retinal artery.
Central VD was found to be significantly lower in the Fabry group compared to the control group in all the retinal layers (SCP (p < 0.001), DCP (p < 0.005), CC (p < 0.001)). Regarding SCP cVD, the analysis revealed that the SCP cVD in the Fabry group was estimated to have a mean decrease of 3.0 units compared to the control group (95% CI [-4.7; -1.3]) (p < 0.001) Fig. 2. Similarly, DCP cVD in the Fabry group was estimated to have a mean decrease of 3.4 units compared to the control group (95% CI [-5.6; -1.3]) (p = 0.003). Finally, CC cVD in the Fabry group was estimated to have a mean decrease of 20 units compared to the control group (95% CI [-22; -17]) (p < 0.001). These significant differences in cVDs across multiple retinal layers are graphically depicted in Fig. 3 through boxplots.
Upper-Right eye of a Fabry 44-year-old male reveals on Optical Coherence Tomography Angiography (OCTA) a reduced foveal vessel density in superficial capillary plexus (SCP) along with increased vessel morphological tortuosity. Bottom-On OCTA of the right eye of an healthy 43-year-old male shows normal indices of the superficial capillary plexus with physiological vascular texture.
The boxplot analysis reveals a compelling and statistically significant difference in retinal central vessel density between the control and Fabry groups in all assessed layers, including the superficial capillary plexus (p < 0.001), deep capillary plexus (p < 0.005), and choriocapillaris plexus (p < 0.001). The central vessel density was markedly lower in the Fabry group compared to the control group and the most pronounced difference was observed in the choriocapillaris plexus. VD: vessel density; SCP cVD: superficial capillary plexus central vessel density; DCP cVD: deep capillary plexus central vessel density; CC cVD: choriocapillaris plexus central vessel density.
Finally, PWV was significantly higher in the Fabry group than in the control group (p = 0.03). The analysis showed that PWV in the Fabry group was estimated to have a mean increase of 0.49 units compared to the control group (95% CI [0.06; 0.93]) (p = 0.028). This finding is visualized in the boxplot in Fig. 4.
The boxplot illustrates a notable and statistically significant difference in the pulse wave velocity of the aortic arterial stiffness between the Fabry group and the control group (p = 0.03). Pulse wave velocity, a key indicator of arterial stiffness, is consistently higher in the Fabry group compared to the control group, as evident from the distinct separation in the boxplot distributions.
Discussion
Patients with Fabry disease exhibit vascular injuries affecting heart, brain, kidneys, and retina20. The pathophysiology of this vasculopathy remains incompletely elucidated, with current hypotheses suggesting endothelial dysfunctions, alterations in cerebral perfusion, and a pro-thrombotic phenotype arising from alpha-galactosidase A deficiency21.
In patients with Fabry disease, diverse ocular manifestations have been observed, with occasional findings indicating vascular occlusive disorders arising from compromised choroidal and retinal vasculature6.
The objective of this study is to investigate vascular differences between Fabry patients and healthy controls, encompassing for the first time not only arterial stiffness but also exploring ocular aspects through the assessment of retinal angiopathy (specifically CSP-VD, DCP-VD, and CC-VD) and retinal arterial compromise (RI-CRA), using reliable non-invasive methods.
In our analysis, arterial stiffness resulted higher in the Fabry group than in the control group, aligning with findings from several prior studies22. This observation correlates with an increased incidence of cardiovascular events and challenges in peripheral microcirculation in this particular patient population23. Alterations in arterial stiffness and blood pressure metrics are increasingly investigated, suggesting a potential role not only in delaying the decline in kidney functions but also in contributing to retinal neurodegeneration24,25.
Comparison of data between the two cohorts, FD and controls, revealed a significant decrease in VD across the superficial, deep, and choriocapillaris plexuses. Our study identified the choriocapillaris as the most compromised, consistent with the knowledge that it is particularly vulnerable to damage in the presence of systemic diseases involving vascular complications26,27,28.
Alongside alterations in VD, the information obtained through ColorDoppler revealed a noteworthy increase in the resistive index of the central retinal artery in Fabry patients.
The resistive index is a measure of vascular resistance, and changes in this index can indicate alterations in blood flow characteristics within the central retinal artery. In diabetic retinopathy increased vascular resistance in the central retinal artery has been investigated and resulted from both macrovascular and microvascular changes associated with the pathology29.
This elevation is reflective of the compromised retinal circulation and impaired autoregulation in the context of diabetes and has been considered as a bioimaging biomarker for the severity of the disease16.
In our study, for the first time an increase in the resistive index of the central retinal artery has been registered in Fabry patients, probably due to the glycolipid accumulation leading to structural changes, potentially impacting blood flow dynamics and increasing vascular resistance.
Investigating and comparing RI changes in the central retinal artery can contribute to a deeper understanding of the vascular alterations specific to the analysed condition.
It’s essential to consider the potential implications of RI changes in guiding treatment strategies and predicting the risk of vision-related complications, such as the risk of thrombosis, which encompasses central retinal artery occlusion and retinal vein occlusion30,31,32. Notably, the individuals under examination did not exhibit symptomatic ocular complications; rather, the sole indicators of ocular vasculopathy were observed in the form of conjunctival vessel tortuosity (in 19 patients) and retinal vessel tortuosity (in 15 patients). In essence, these patients could be characterized from an ocular standpoint as being in a subclinical stage. The highlighted alteration in VD prompts consideration of the potential value in longitudinally monitoring these individuals. Eco ColorDoppler and OCTA emerge as early preferences, offering non-invasive and cost-effective means to delineate vascular changes in the absence of overt pathology. A key advantage of our study is the use of Color Doppler imaging, which provides additional information about blood flow quality and tissue perfusion in the eyes. This method can be particularly beneficial in cases with media opacities, where other imaging techniques might be limited. In addition, while OCTA is valuable for mapping the structure and density of retinal vessels, it does not provide insight into blood flow dynamics. In contrast, Color Doppler can measure blood velocity and arterial resistance, which are early indicators of vascular changes that may precede structural alterations detectable by OCTA.
This study is subject to certain limitations, notably the relatively small number of patients analyzed, a factor attributed to the rarity of the disease. Additionally, the short-term follow-up period raises a need for further investigations to evaluate longitudinal changes and potential correlations between ocular alterations and the emergence of signs and symptoms of pathology. Despite these limitations, the study gains strength from its innovative approach, employing for the first time a combination of eco ColorDoppler and OCTA in Fabry patients.
In conclusion, our study has revealed that FD patients exhibit not only the anticipated significant increase in arterial stiffness compared to healthy subjects but also an elevation in the resistive index of the central retinal artery and a reduction in retinal microvasculature density. These findings suggest the presence of subclinical ocular damage in these patients, underscoring the importance of ongoing monitoring. A focused screening for organ damage in FD patients is strongly recommended, emphasizing the necessity to identify parameters that can serve as pivotal benchmarks in assessing ocular vascular involvement. Such non-invasive bioimaging biomarkers might also serve to monitor the latest therapies available for Fabry patients, such as the enzyme replacement therapy, to assess treatment response and adjust therapy as needed. Certainly, further studies are needed to elucidate how variations in these parameters over time are associated with the pathology progression.
Data availability
Data is provided within the manuscript and the complete data source trough supplementary files if needed.
References
Desnick, R. J., Dean, K. J., Grabowski, G. A., Bishop, D. F. & Sweeley, C. C. Enzyme therapy XVII: metabolic and immunologic evaluation of alpha- galactosidase A replacement in fabry disease. Birth Defects Orig Artic Ser. 16, 393–413 (1980).
El-Abassi, R., Singhal, D. & England, J. D. Fabry’s disease. J. Neurol. Sci. 344, 5–19 (2014).
Germain, D. P. Fabry disease. Orphanet J. Rare Dis. 5, 30 (2010).
Poorthuis, B. J. et al. The frequency of lysosomal storage diseases in the Netherlands. Hum. Genet.105, 151–156 (1999).
Mehta, A. et al. Enzyme replacement therapy with agalsidase alfa in patients with Fabry’s disease: an analysis of registry data. Lancet. 374, 1986–1996 (2009).
Sodi, A., Ioannidis, A. & Pitz, S. Ophthalmological Manifestations of Fabry Disease. (2006).
Bitirgen, G., Turkmen, K., Malik, R. A., Ozkagnici, A. & Zengin, N. Corneal confocal microscopy detects corneal nerve damage and increased dendritic cells in fabry disease. Sci. Rep. 8, 12244 (2018).
Samiy, N. Ocular features of fabry disease: diagnosis of a treatable life-threatening disorder. Surv. Ophthalmol. 53, 416–423 (2008).
Pitz, S. et al. Ocular signs correlate well with disease severity and genotype in fabry disease. PLoS One. 10, e0120814 (2015).
Sodi, A. et al. Ocular manifestations of Fabry’s disease: data from the Fabry Outcome Survey. Br. J. Ophthalmol. 91, 210–214 (2007).
Libert, J. & Toussaint, D. Tortuosities of retinal and conjunctival vessels in lysosomal storage diseases. Birth Defects Orig Artic Ser. 18, 347–358 (1982).
Cennamo, G. et al. Optical coherence tomography angiography findings in Fabry Disease. J. Clin. Med. 8, (2019).
Cennamo, G. et al. The role of OCT- angiography in predicting anatomical and functional recovery after endoscopic endonasal pituitary surgery: a 1-year longitudinal study. PLoS One. 16, e0260029 (2021).
Chiosi, F. et al. Optical Coherence Tomography Angiography Analysis of Vessel Density Indices in early Post-COVID-19 patients. Front. Med. (Lausanne). 9, 927121 (2022).
Rubino, M. et al. Diagnosis and management of Cardiovascular involvement in Fabry Disease. Heart Fail. Clin. 18, 39–49 (2022).
Khatri, M. et al. Resistive index of central retinal artery is a bioimaging biomarker for severity of diabetic retinopathy. Int. J. Retina Vitreous. 5, 38 (2019).
Kalayci, M. & Tahtabasi, M. Assessment of Doppler flow parameters of the retrobulbar arteries and internal carotid artery in patients with glaucoma: the significance of ophthalmic artery peak ratio and the intima-media thickness of the internal carotid artery. Int. Ophthalmol. 40, 3337–3348 (2020).
Shahzad, R. et al. Quantification of aortic pulse wave velocity from a population based cohort: a fully automatic method. J. Cardiovasc. Magn. Reson. 21, 27 (2019).
Calabia, J. et al. Doppler ultrasound in the measurement of pulse wave velocity: agreement with the Complior method. Cardiovasc. Ultrasound. 9, 13 (2011).
Tuttolomondo, A. et al. Anderson-Fabry disease: a multiorgan disease. Curr. Pharm. Des. 19, 5974–5996 (2013).
Mishra, V. et al. Stroke and fabry disease: a review of literature. Cureus. 12, e12083 (2020).
Passaro, G. et al. Arterial involvement in fabry disease: state of the art and future diagnostic purposes. Eur. Rev. Med. Pharmacol. Sci. 25, 845–855 (2021).
Vlachopoulos, C., Aznaouridis, K. & Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 55, 1318–1327 (2010).
Huang, Y. et al. Effects of blood pressure and arterial stiffness on retinal Neurodegeneration: cross-sectional and longitudinal evidence from UK Biobank and Chinese cohorts. Hypertension. 80, 629–639 (2023).
Sedaghat, S. et al. Arterial stiffness and decline in kidney function. Clin. J. Am. Soc. Nephrol. 10, 2190–2197 (2015).
Chen, Y., Qi, Z., Hou, X. & Chen, L. Optical coherence tomography angiography for assessment of changes of the retina and choroid in different stages of diabetic retinopathy and their relationship with diabetic nephropathy. Endokrynol Pol. 74, 135–139 (2023).
Lejoyeux, R. et al. Choriocapillaris: fundamentals and advancements. Prog Retin Eye Res. 87, 100997 (2022).
Özdal, P. Ç. & Tugal-Tutkun, I. Choroidal involvement in systemic vasculitis: a systematic review. J. Ophthalmic Inflamm. Infect. 12, 14 (2022).
Pillai, G. S., Kutumbaka, A., Nazar, P. K., Chandiri, A. & Ravindran, G. C. Association between resistivity index of central retinal artery and severity of diabetic retinopathy. Indian J. Ophthalmol. 71, 3539–3543 (2023).
Oto, S., Kart, H., Kadayifçilar, S., Ozdemir, N. & Aydin, P. Retinal vein occlusion in a woman with heterozygous fabry’s disease. Eur. J. Ophthalmol. 8, 265–267 (1998).
Utsumi, K. et al. High incidence of thrombosis in Fabry’s disease. Intern. Med. 36, 327–329 (1997).
Utsumi, K. et al. Thrombosis in Japanese patients with fabry disease. J. Neurol. Sci. 283, 83–85 (2009).
Funding
None.
Author information
Authors and Affiliations
Contributions
F.C. and M.L.P. wrote the main manuscript; M.R. and L.D. analysed and commented results; M.C. did the genetic testing; M.R. and E.M. examined population with Fabry disease; F.N. collected data for PWV and RI of CRA; G.C. collected data from OCTA; A.R. and F.C. collected figures and data; G.L. and C.C. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Financial disclosures
The authors declare no financial conflicts of interest or competing interests related to this research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rinaldi, M., Chiosi, F., Passaro, M.L. et al. Resistive index of central retinal artery, aortic arterial stiffness and OCTA correlated parameters in the early stage of fabry disease. Sci Rep 14, 24047 (2024). https://doi.org/10.1038/s41598-024-74146-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74146-5
Keywords
This article is cited by
-
Optical coherence tomography angiography in non-alcoholic fatty liver disease: is it a disease affecting the microvascular system??
BMC Ophthalmology (2025)
-
Retinal microvasculature alteration in patients with acute pancreatitis: an observational OCTA study
European Journal of Medical Research (2025)
-
SS-OCTA assessment of fundus microvascular changes and their correlation with coronary lesion severity in severe coronary heart disease
Scientific Reports (2024)