Abstract
The research retrospectively analyzed cases of spinal chordoma and chondrosarcoma involving patients who received treatment at the two hospitals between 2001 and 2023. Among the 48 patients studied (39 chordoma and 9 chondrosarcoma cases), the average age was 53.9 ± 15.8 years, with a range of 17 to 86 years. Out of these patients, 43 underwent excision surgery and were categorized based on tumor margin into negative (R0) or microscopically positive (R1) margin (n = 14) and macroscopically positive (R2) margin (n = 29) groups. The mean overall survival (OS) for R0/R1 and R2 groups was 156.5 ± 19.3 and 79.2 ± 11.9 months, respectively (p value = 0.012). The mean progression-free survival (PFS) for R0/R1 and R2 was 112.9 ± 24.4 and 25.5 ± 5.5 months (p value < 0.001). The study showed that regardless of whether patients in the R0/R1 or R2 groups received radiation therapy (RT) or not, there was no significant improvement in OS or PFS. Specifically, the OS and PFS for the RT only group were 75.9 ± 16.6 and 73.3 ± 18.0 months. In conclusion, the recommended treatment approach for spinal chordoma and chondrosarcoma remains en bloc resection surgery with an appropriate margin. Patients who are unsuitable for or decline surgery may find a beneficial disease control rate with traditional external beam photon/proton therapy.
Similar content being viewed by others
Introduction
Spinal chordoma and chondrosarcoma are classified as low to intermediate grade primary malignant tumors of the vertebrae1,2. While chordoma constitutes a relatively small fraction (1.4%) of primary malignant tumors overall3, they notably represent over half of all primary sacral tumors4,5. Moreover, they comprise 15 to 20% of primary tumors in the mobile spine6,7, but are exceedingly rare (0.2%) in the skull base8. Chondrosarcoma constitutes a significant portion, approximately 27%, of all primary bone tumors9, with spinal chondrosarcoma estimated to account for about 26% of primary malignant tumors in the vertebrae10,11. These tumors predominantly arise in the thoracic and lumbar regions10. Both chordoma and chondrosarcoma present a spectrum of symptoms, including back pain, neurological deficits, muscle atrophy, urinary and bowel dysfunction, and potential reductions in life expectancy11,12,13. Crafting comprehensive treatment strategies is paramount in optimizing patients’ quality of life and improving their overall survival (OS) outlook.
Chordoma emerges from the remnants of the notochord and typically grows slowly, yet malignantly14,15,16. Spinal chondrosarcoma can emerge spontaneously or as a result of preexisting cartilage lesions such as osteochondroma or enchondroma13,17,18. Despite their resistance to radiotherapy and chemotherapy, total en bloc resection is necessary to enhance local control rates5,19,20,21,22,23. The preferred treatment for both chordoma and spinal chondrosarcoma is wide local excision, sometimes accompanied by adjuvant radiotherapy24,25. However, many patients experience complications post-surgery. The extent of surgical removal is directly linked to improved OS26. Neurological involvement and disability are common and significant side effects, varying in severity based on the tumor’s extent or location23.
For patients with positive margins or those with unfavorable local tumor characteristics, an aggressive radiotherapy (RT) approach incorporating techniques like intraoperative dural plaques brachytherapy and high-dose photon/proton therapy may achieve regional control17,27,28. When achieving negative margins is challenging, RT utilizing the heavy charged particle carbon ion, known for its sharper lateral penumbra, is seen as a promising treatment option29,30,31. Additionally, hypofractionated stereotactic body radiation therapy (SBRT) and stereotactic radiosurgery (SRS) are under consideration as potential therapies for unresectable vertebral chordoma and chondrosarcoma due to advancements in geometric accuracy32,33,34.
Since spinal chordoma and chondrosarcoma exhibit several overlapping characteristics, this study collected cases of both conditions and investigated how different treatment methods influenced patient outcomes, aiming to anticipate the development of standardized therapeutic guidelines.
Results
Patients
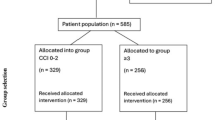
The patient characteristics are displayed in Table 1. The average age at diagnosis among the 48 patients was 53.9 ± 15.8 (ranging from 17 to 86) years, with 34 males and 14 females. Of these, 39 had vertebral chordoma and 9 had chondrosarcoma, all classified as 1B according to the Enneking classification. None of the patients exhibited lymph node or distant metastasis. Based on the AJCC 8th edition staging, there were 3, 21, 3, and 21 patients with T1, T2, T3, and T4 stages, respectively. Distribution across spinal regions was as follows: three in the cervical spine, five in the thoracic region, four in the lumbar spine, and thirty-six in the sacrum. The majority (n = 43) underwent tumor removal surgery, among which seven had negative margin (R0), seven had microscopically positive margin (R1), and 29 had macroscopically positive margin (R2). Among the R0 cases, two opted for adjuvant radiotherapy (RT), while five did not. Among the R1 cases, two chose RT, and five did not. Within the R2 cases, fourteen patients underwent RT, while 15 did not. Additionally, five patients received RT as a palliative measure instead of surgery. The follow-up period was computed starting on the date of treatment completion, including surgery and RT. At the last follow-up, 28 out of 48 individuals were alive, with an average follow-up duration of 58.1 ± 44.9 months.
The differences in results between R0/R1 and R2 margin
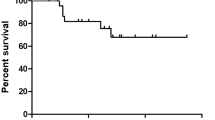
Patients who underwent excision surgery were divided into two groups based on histological findings: R0/R1 (n = 14) and R2 (n = 29) tumor margin. Figure 1 illustrates the OS and PFS curves. The mean OS for R0/R1 and R2 groups was 156.5 ± 19.3 and 79.2 ± 11.9 months, respectively (p value = 0.012). Both groups had estimated 5-year survival rates of 100.0% and 67.7 ± 9.8%, and estimated 10-year survival rates of 80.0 ± 17.9% and 15.1 ± 9.6%, respectively. The mean PFS for R0/R1 and R2 groups was 112.9 ± 24.4 and 25.5 ± 5.5 months, respectively (p value < 0.001).
(A) Overall survival (OS) of Patients with R0/R1 and R2 margin. For R0/R1 and R2 group, the mean OS was 156.5 ± 19.3 and 79.2 ± 11.9 months, respectively (p value = 0.012). The anticipated 5-year and 10-year survival rates for both groups were 100.0% and 67.7 ± 9.8%, and 15.1 ± 9.6% and 80.0 ± 17.9%, respectively. (B) Progression-Free Survival (PFS) of Patients with R0/R1 and R2 margin. For individuals with R0/R1 and R2 margin, the average PFS was 112.9 ± 24.4 and 25.5 ± 5.5 months, respectively (p value < 0.001).
The implications of adjuvant radiation on people with R0/R1 margin
Four out of the fourteen patients with negative or microscopically positive margin underwent RT, while ten did not. Figure 2 depicts the OS and PFS curves. Both groups had estimated 5-year survival rates of 100%, while the projected 10-year survival rates were 100% and 66.7 ± 27.2%, respectively. The average OS could not be calculated due to insufficient OS data. The mean PFS for R0/R1 patients with and without RT was 94.0 ± 21.9 and 111.0 ± 27.3 months, respectively (p value = 0.901).
(A) Overall survival (OS) between R0/R1-margined patients with or without radiation therapy (RT). Due to insufficient OS data, the average OS could not be calculated. However, the estimated 5-year survival rate was both 100%. Predicted 10-year survival rates were 66.7 ± 27.2% and 100%, respectively. (B) Progression-Free survival (PFS) between R0/R1-margined patients with or without radiation therapy. Among R0/R1 patients, the mean PFS was 94.0 ± 21.9 months for those undergoing RT and 111.0 ± 27.3 months for those who did not (p value = 0.901).
The effect of adjuvant radiation on patients with R2 margin
Fourteen out of the twenty-nine cases with R2 margin opted for RT, while 15 did not. The OS and PFS curves are depicted in Fig. 3. The estimated 5-year survival rates were 51.6 ± 17.8% and 78.0 ± 11.4%, and the estimated 10-year survival rates were 51.6 ± 17.8% and 9.8 ± 9.2%, respectively. For R2 patients with and without RT, the average OS was 105.5 ± 26.2 and 76.0 ± 9.2 months (p value = 0.734). The mean PFS for R2 patients with and without RT was 27.1 ± 6.4 and 22.9 ± 7.8 months (p value = 0.513), respectively.
(A) Overall survival (OS) between R2-margined patients with or without radiation therapy (RT). The mean OS for R2 patients, with and without RT, was 105.5 ± 26.2 and 76.0 ± 9.2 months, respectively (p value = 0.734). The predicted 5-year survival rates for each group were 51.6 ± 17.8% and 78.0 ± 11.4%, while the anticipated 10-year survival rates were 51.6 ± 17.8% and 9.8 ± 9.2%. (B) Progression-Free survival (PFS) between R2-margined patients with or without radiation therapy. Among R2 patients, the mean PFS was 27.1 ± 6.4 months for those who underwent RT and 22.9 ± 7.8 months for those who did not (p value = 0.513).
The results for patients with R0/R1 margin, with R2 margin, and with RT only
The outcomes following therapy were compared among patients with R0/R1 margin, individuals with R2 margin, and those who received only RT to assess whether RT could be beneficial for those unwilling or unable to undergo resection surgery. Figure 4 illustrates the OS and PFS curves. The estimated 5-year survival rates for each group were 100.0%, 67.7 ± 9.8%, and 60.0 ± 21.9%, while the estimated 10-year survival rates were 80.0 ± 17.9%, 15.1 ± 9.6%, and 30.0 ± 23.9%, respectively. The average OS for the three groups was 156.5 ± 19.3, 79.2 ± 11.9, and 75.9 ± 16.6 months (p value = 0.046). The PFS for the three groups was 112.9 ± 24.4, 25.5 ± 5.5, and 73.3 ± 18.0 months, respectively (p value < 0.001).
(A) Overall survival (OS) between patients with R0/R1 margin, with R2 margin, or with radiation therapy (RT) Only. The predicted 5-year survival rates for each group were 100.0%, 67.7 ± 9.8%, and 60.0 ± 21.9%, respectively, while the expected 10-year survival rates were 80.0 ± 17.9%, 15.1 ± 9.6%, and 30.0 ± 23.9%. The OS for the three groups was 156.5 ± 19.3, 79.2 ± 11.9, and 75.9 ± 16.6 months (p value = 0.046). (B) Progression-free survival (PFS) between patients with R0/R1 margin, with R2 margin, or with radiation therapy only. The PFS of the 3 groups was 112.9 ± 24.4, 25.5 ± 5.5, and 73.3 ± 18.0 months (p value < 0.001).
The treatment outcomes for patients receiving only RT were compared with those undergoing surgery (with R0/R1 or R2 margin) followed by RT, as shown in Fig. 5. The estimated 5-year survival rates were 60.0 ± 21.9% for the RT-only group and 61.3 ± 14.7% for the surgery group, while the predicted 10-year survival rates were 30.0 ± 23.9% and 61.3 ± 14.7%, respectively. The average OS was 75.9 ± 16.6 months for the RT-only group and 120.0 ± 21.9 months for the surgery group (p value = 0.707). The average PFS for the RT-only group and the surgery group was 73.3 ± 18.0 months and 43.0 ± 11.6 months, respectively (p value = 0.282).
(A) Comparison of Overall Survival (OS) between Patients Receiving Radiation Therapy (RT) only and Those Undergoing Surgery (with R0/R1 or R2 Margin) Followed by RT. The estimated 5-year survival rates for the RT-only group and the surgery-with-RT group were 60.0 ± 21.9% and 61.3 ± 14.7%, respectively, while the predicted 10-year survival rates were 30.0 ± 23.9% and 61.3 ± 14.7%. The average OS was 75.9 ± 16.6 months for the RT-only group and 120.0 ± 21.9 months for the surgery-with-RT group (p value = 0.707). (B) Comparison of Progression-Free Survival (PFS) between Patients Receiving RT only and those Undergoing Surgery with RT. The mean PFS was 73.3 ± 18.0 months for the RT-only group and 43.0 ± 11.6 months for the surgery-without-RT group (p value = 0.282).
The treatment outcomes were compared between patients receiving only RT and those undergoing surgery (with R0/R1 or R2 margin) without RT, as shown in Fig. 6. The estimated 5-year survival rates were 60.0 ± 21.9% for the RT-only group and 84.4 ± 8.6% for the surgery-only group, while the projected 10-year survival rates were 30.0 ± 23.9% and 23.0 ± 11.6%, respectively. The average OS was 75.9 ± 16.6 months for the RT-only group and 96.2 ± 12.7 months for the surgery-only group (p value = 0.713). The average PFS for the RT-only group and the surgery-only group was 73.3 ± 18.0 and 55.4 ± 14.3 months, respectively (p value = 0.379).
(A) Comparison of Overall Survival (OS) between Patients Receiving Radiation Therapy (RT) only and Those Undergoing Surgery (with R0/R1 or R2 margin) without RT. The estimated 5-year survival rates were 60.0 ± 21.9% for the RT-only group and 84.4 ± 8.6% for the surgery-only group, while the projected 10-year survival rates were 30.0 ± 23.9% and 23.0 ± 11.6%, respectively. The average OS was 75.9 ± 16.6 months for the RT-only group and 96.2 ± 12.7 months for the surgery-only group (p value = 0.713). (B) Comparison of Progression-Free Survival (PFS) between patients receiving RT only and those undergoing surgery without RT. The mean PFS was 73.3 ± 18.0 months for the RT-only group and 55.4 ± 14.3 months for the surgery-only group (p value = 0.379).
Treatment-related complications
Complications arising from surgery and adjuvant RT were documented according to Common Terminology Criteria for Adverse Events (CTCAE) version 5 and are outlined in Tables 2 and 3. Among the forty-three participants who underwent surgery in our study, twelve experienced grade 2 surgical complications. Of these, eight presented with neurological deficits, including dysuria or urinary incontinence, constipation or fecal incontinence, and decreased mobility. One case involved hematoma formation, two had wound infection, and one required tracheostomy care. Additionally, seven patients experienced grade 3 surgical complications, all of which were categorized as sepsis or deep wound infection. Among patients with R0/R1 and R2 margins, surgical complications ≥ CTCAE grade 2 occurred in 8 (57.1%) and 11 (37.9%) cases, respectively (p value = 0.235). Furthermore, 3 (21.4%) and 4 (13.8%) patients experienced surgical complications graded higher than CTCAE grade 3 (p value = 0.665), respectively. Twenty-three patients in our study opted for either palliative or adjuvant radiotherapy. Among them, twelve (52.2%) experienced acute CTCAE grade 2 RT-related complications, while one (4.3%) had acute CTCAE grade 3 complications. Additionally, one (5.3%), one (5.3%), and one (5.3%) patient experienced CTCAE grade 2, 3, and 4 late-onset RT toxicity, respectively.
Discussion
Primary vertebral neoplasms are uncommon in adulthood, with chordoma and chondrosarcoma comprising the majority. These tumors exhibit aggressive behavior and are prone to local recurrence25. The primary goal of treatment is excision surgery aiming for a negative or microscopically positive margin, which enhances local control and improves survival prospects for patients5,19,20,21,22,26. Nonetheless, surgical procedures often lead to complications due to delayed diagnosis and the tumors’ proximity to vital structures like arteries, viscera, and neural tissue24,35. En bloc resection of chordoma and spinal chondrosarcoma remains particularly challenging considering the potential postoperative side effects and functional impairments24. For instance, data from Maggiore Hospital in Bologna, Italy, indicated that only 20% of 52 patients treated over a 50-year period until 2002 achieved adequate margins post-surgery, with many experiencing local recurrence within 56–94 months36.
In our investigation, fourteen out of the 43 patients who opted for surgery achieved a negative or microscopically positive margin, demonstrating notably superior outcomes in terms of OS and PFS compared to those with a macroscopically positive margin The efficacy of therapy was found to be significantly influenced by complete surgical resection, corroborating findings from previous studies5,19,20,21,22,26,37,38. Previous clinical series have consistently highlighted that a negative surgical margin stands out as the most critical predictor of tumor recurrence rates and long-term survival1,4.
While total excision is considered the optimal treatment approach, it is essential to weigh the risks of perioperative complications, including damage to nearby structures, neurological impairments, wound complications such as dehiscence or infection, medical morbidity stemming from surgical trauma, and potential long-term device failure39,40. Li et al. carried out a systematic review of perioperative complications in 824 cases undergoing total en bloc resection, revealing various rates: 12.7% for neurological damage, 12.1% for hardware failure, 10.6% for dural tear and cerebrospinal fluid leakage, 7.6% for wound-related complications with nearly half necessitating revision, and 7.3% for vascular injury and bleeding41. Additionally, mortality rates due to surgical complications were reported at 1.2%41. In our dataset, among patients undergoing surgery, 55.8% (24/43) experienced complications graded as CTCAE 0 or 1, 27.9% (12/43) had grade 2 complications, and 16.3% (7/43) had grade 3 adverse events. Of those with complications grade 2 or higher, ten were related to wound issues, and eight were neurological. Besides, one patient succumbed to severe sepsis within a month after surgery, leading to the exclusion from our study.
Research strongly supports the effectiveness of conventional photon or proton dosages for chordoma treatment30,42. Studies have indicated that radical surgery combined with conventional radiation at doses below 60 Gy yields poor local control rates (ranging from 0 to 50%) for spinal and sacral chordoma43,44,45. Nonetheless, the maximum feasible dose of traditional photon radiation typically administered to spinal tumors is around 50 Gy, a level associated with minimal risk of paralysis, determined by the tolerance of the spinal cord46. Proton therapy presents a promising option for treating spinal malignancies due to its potential for reduced or delayed toxicity. In a study by Hug et al., employing combined photon-proton radiotherapy with an average dose of 73.9 Gy/CGE, 5-year local control rates of 53% and 100% were observed for chordoma and chondrosarcoma of the axial skeleton, respectively47. Another phase II clinical study at Massachusetts General Hospital administered combined photon-proton radiation with total doses of 70.2 Gy/CGE for microscopic positive resection and 77.4 Gy/CGE for gross disease, with long-term results revealing a 74% local control rate after eight years48,49. These findings underscore the importance of high-dose radiation therapy, whether using photon or proton, for effective chordoma and chondrosarcoma treatment.
In our investigation, four out of the 14 individuals having R0/R1 resection received adjuvant RT, with only one of them receiving photon/proton therapy at a dosage exceeding 60 Gy/CGE. The OS and PFS were not significantly influenced by the administration of RT in the R0/R1 group. Among the 29 patients with R2 margin in our study, fourteen opted for adjuvant RT, while fifteen did not. Concerning toxicity, nine of the fourteen patients received cumulative doses exceeding 60 Gy/CGE, while five received low-dose RT, with dosages ranging from 30 to 74 Gy/CGE. There was no discernible difference in therapeutic outcomes between R2 margin patients who underwent RT and those who did not.
For individuals with unresectable tumors, conventional photon beam radiation therapy administered at doses exceeding 60 Gy, although high and often associated with toxicity, may exhibit therapeutic efficacy44,50,51. A retrospective study by Chen et al. demonstrated that proton therapy, with a median total dose of 77.4 Gy, resulted in OS rates of 91.7% and 78.1%, as well as PFS rates of 90.4% and 79.8% at 3 and 5 years, respectively51.
In our study, only 5 out of the 48 people did not undergo tumor resection surgery due to considerations of adverse effects, unresectable tumor location or size, or both factors combined. Among these, three patients received high-dose photon therapy, one received low-dose photon therapy, and one patient’s record was misplaced. The findings revealed no notable difference in treatment outcomes between patients with R2 margin and those receiving RT only. Similarly, no significant difference was observed between patients who underwent surgery, regardless of margin type (R0/R1 or R2), followed by adjuvant RT, and those treated with RT alone. Additionally, there was no meaningful difference between patients who had surgery without adjuvant RT and those receiving only radiation therapy. Therefore, obtaining R0/R1 surgical margin remains the most important factor affecting treatment outcomes. Meanwhile, RT served as an alternative option for these particular individuals, potentially offering benefits to those who cannot undergo surgery, are unsuitable for it, or are likely to achieve an R2 margin if they do undergo resection surgery. Furthermore, the analysis confirmed that patients with R0/R1 margin had significantly longer PFS than others, consistent with findings from numerous previous studies5,19,20,21,22,26.
Radiation therapy has the potential to induce damage to cellular DNA, leading to both therapeutic effects on neoplasms and the occurrence of acute and late-onset toxicities52,53. Acute RT complications typically manifest within 3 months post-treatment and are sometimes unavoidable but typically self-limiting52,54. Following conventional photon RT (5500 cGy in 22 fractions) over sacrococcygeal area, one patient experienced cystitis with gross hematuria. The RT course was thus terminated. Conversely, late-onset complications usually emerge three months post-RT and are often deemed irreversible and progressive over time52. Fuller et al. documented a case series of 25 patients undergoing RT for gross residual spinal or cranial chordoma, with only one severe complication reported, involving skin and sacral necrosis occurring in a patient receiving EBRT at 70 Gy in 35 fractions19. In another study involving 24 patients with spinal chordoma receiving conventional EBRT after biopsy or partial resection, two cases experienced late skin necrosis following RT retreatment for recurrence44. In our study, three cases experienced late-onset RT complications exceeding grade 2. Among them, one patient developed lower limb soreness and weakness two years post-RT, while two patients suffered from severe soft tissue necrosis. None of these cases underwent resection surgery or reirradiation, receiving RT with photon beam at doses ranging from 6250 to 7450 cGy delivered in 25 to 37 fractions.
Presently, experts are actively pursuing the development of SBRT, which holds promise for curative treatment while minimizing adverse effects. Hypofractionated SBRT leverages advancements in radiation technology, such as micro-multileaf collimators, robotic systems, cone beam computed tomography scans, and real-time image guidance. This approach enables the delivery of ablative radiation doses to target tissues and spares neighboring normal tissues from excessive radiation exposure55. Jin et al. conducted a study where they utilized surgery and/or single-fraction SBRT (18–24 Gy) to treat 35 patients with de novo chordoma of the sacrum and mobile spine. The 3- and 5-year OS rates were reported as 90.0% and 84.3%, respectively, with corresponding local control rates of 86.2% and 80.5%33. Sherry et al. reported results from a study involving 15 patients with chordoma or chondrosarcoma affecting the sacrum or mobile spine, who were treated with a single fraction of SBRT (24 Gy); the OS rates at 2 and 5 years were 86% and 62%, while 2- and 5-year local control rates were 100% and 90%, respectively56.
The acute toxicity associated with spinal SBRT is minimal and exceedingly rare, with reported incidence rates of serious adverse effects (CTCAE grade 3 or higher) at 5% or lower57,58,59,60. However, a relatively common late-onset toxicity attributed to SBRT is vertebral compression fracture (VCF), with estimated risks ranging from 11–39%61,62,63. Evidence suggests that VCF risk increases when the dosage exceeds 20 Gy per fraction64. Radiation-induced myelopathy, a feared late effect, is exceptionally rare with the advent of modern SBRT techniques. Besides, separation surgery, where the tumor is excised to decompress the spinal cord, leaving a space for safe SBRT delivery, serves as a viable treatment option preceding SBRT65.
Currently, it is widely acknowledged that chemotherapy lacks efficacy against the majority of chordoma and chondrosarcoma cases, with no approved medications specifically indicated for their treatment66,67,68,69. However, Frezza et al. demonstrated that adjuvant therapy might confer a survival benefit for mesenchymal chondrosarcoma, a relatively rare and aggressive histologic subtype of chondrosarcoma70. In our study, only one out of the 48 patients diagnosed with chondrosarcoma received chemotherapy in 2002. Notably, immunotherapy was not administered to any case in this study. Numerous ongoing clinical trials hold the potential to yield promising therapeutic options for chordoma and chondrosarcoma in the future71.
Our study investigated the outcomes of individuals diagnosed with vertebral chordoma and chondrosarcoma who underwent either surgery or radiotherapy. However, several limitations of our investigation should be considered when interpreting the findings. One limitation is its retrospective nature, which can introduce challenges and biases such as selection bias and confounding factors. Additionally, the small sample size poses another constraint, as it can be challenging to recruit an adequate number of participants due to the rarity of these tumors. Furthermore, our research did not document data regarding post-treatment symptom improvement, which is crucial for assessing the overall quality of life of patients. Moreover, the study did not delve deeply into the specific surgical strategies employed in the treatment, making it difficult to accurately evaluate the success rates of different surgical methods. Another limitation is the relatively short follow-up period for many cases, which may result in an overestimation or underestimation of treatment effectiveness due to the lack of consideration for long-term effects. Consequently, the findings of the study should be interpreted with caution and cannot be extrapolated to larger populations without further investigation. Despite these limitations, the study offers valuable insights and contributes to the existing body of knowledge on the subject. To enhance the robustness and validity of future findings, researchers should aim to address these limitations by utilizing more diverse and up-to-date data sources.
Methods
Study design
Patients diagnosed with primary spinal chordoma or chondrosarcoma were identified through a search of the databases of two medical centers in Taiwan spanning from 2001 to 2023. Only individuals who underwent tumor resection surgery or radiotherapy at these institutions and whose histology was confirmed were included in the study. General patient information collected for the study included age at diagnosis, gender, tumor types and subtypes, primary lesion location, Enneking classification, American Joint Committee on Cancer (AJCC) 8th edition TNM staging, surgical margin (R classification), RT and chemotherapy regimens, post-intervention complications, duration of follow-up, status at last follow-up, OS, as well as PFS. The project received approval from the Institutional Review Boards of both medical centers (IRB No.202400714B0) and (IRB No.202403144RIND). All research were performed in accordance with the relevant guidelines and regulations, as well as the Declaration of Helsinki. Due to the retrospective nature of the study, both Chang Gung Medical Foundation Institutional Review Board and National Taiwan University Hospital Institutional Review Board waived the need of obtaining informed consent.
Patients with tumors located in the spine or pelvis were retrospectively reviewed from 2001 to 2023. The study excluded individuals with non-spinal pelvic tumors, untreated patients, those presenting with distant metastasis or recurrence of the original tumor at their initial visit, individuals with tumors originating in the bone marrow, and patients diagnosed with reticuloendothelial system or germ cell cancers. Additionally, those who were not followed up for more than three months after completing therapy, as well as patients with diagnoses other than chondrosarcoma or chordoma, such as osteosarcoma or Ewing’s sarcoma, were excluded. Pediatric patients, defined as those under 16 years old, were also excluded. Ultimately, the study included 39 cases of spinal chordoma and 9 cases of chondrosarcoma.
Comparison of the outcomes after various treatment
We compared OS and PFS among patients with different surgical margins (i.e., R0/R1, R2). Within each subgroup defined by surgical margin, we analyzed whether adjuvant radiotherapy (RT) contributed to OS and PFS. Additionally, we compared OS and PFS between patients who only received RT and those with R0/R1 or R2 margins. These analyses were conducted to assess the effectiveness of various treatment approaches. Recurrence, disease progression, or distant metastasis were identified by an increase in tumor size observed on magnetic resonance imaging (MRI) or computed tomography (CT), the onset of neurological deficits due to tumor enlargement, or worsening symptoms attributed to tumor growth. Treatment-related complications were documented following CTCAE version 5.0. Surgical complications among different surgical margin groups were also compared.
Statistical analysis
IBM SPSS Statistics (Version 25) was employed for the statistical analysis. Fisher’s exact test and one-way ANOVA were utilized for analyzing patient baseline characteristics, while treatment results were assessed using the Kaplan-Meier technique and the log-rank test. In addition, Chi-square test or Fisher’s exact test was utilized in order to compare the surgical complication rates among different groups. All continuous variables were deemed statistically significant at a p value of 0.05, which were showed as mean ± standard deviation.
Conclusion
Our study findings corroborated the hypothesis that excision surgery with a suitable margin stands as the gold standard treatment. Additionally, our research revealed that photon/proton radiation holds promise for achieving a favorable disease control rate in patients who are either unwilling or unable to undergo surgery.
Data availability
The accompanying author can provide the data supporting the results of the research upon reasonable request with the appropriate ethical and legal approvals. To gain access to the data, researchers with an interest can contact tsuangfy@gmail.com.
References
Sciubba, D. M., Chi, J. H., Rhines, L. D. & Gokaslan, Z. L. Chordoma of the spinal column. Neurosurg. Clin. N. Am. 19, 5–15 (2008).
McLoughlin, G. S., Sciubba, D. M. & Wolinsky, J. P. Chondroma/chondrosarcoma of the spine. Neurosurg. Clin. N. Am. 19, 57–63 (2008).
Healey, J. & Lane, J. Chordoma: a critical review of diagnosis and treatment. Qld. Gov. Min. J. 20, 417–426 (1989).
Fourney, D. R. & Gokaslan, Z. L. Current management of sacral chordoma. NeuroSurg. Focus 15, 1–5 (2003).
Cheng, E. Y., Özerdemoglu, R. A., Transfeldt, E. E. & Thompson, R. C. Jr Lumbosacral chordoma: prognostic factors and treatment. Spine 24, 1639 (1999).
Fuentes, J. & Benezech, J. Strategy of the surgical treatment of primary tumors of the spine. Neuro-chirurgie 35, 323–327 (1989).
Dahlin, D. C. Bone tumors. General Aspect and Data on 11087 cases. Charles C (1996).
Samii, A. et al. Chordomas of the skull base: surgical management and outcome. J. Neurosurg. 107, 319–324 (2007).
Giuffrida, A. Y. et al. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. JBJS 91, 1063–1072 (2009).
Kelley, S. P., Ashford, R. U., Rao, A. S. & Dickson, R. A. Primary bone tumours of the spine: a 42-year survey from the Leeds Regional Bone Tumour Registry. Eur. Spine J. 16, 405–409. https://doi.org/10.1007/s00586-006-0188-7 (2007).
Higinbotham, N. L., Phillips, R. F., Farr, H. W. & Hustu, H. O. Chordoma. Thirty-five-year study at Memorial Hospital. Cancer 20, 1841–1850 (1967).
Bernard, S. A., Brian, P. L. & Flemming, D. J. Primary osseous tumors of the spine. Semin. Musculoskelet. Radiol. 17, 203–220. https://doi.org/10.1055/s-0033-1343097 (2013).
Knoeller, S., Uhl, M., Gahr, N., Adler, C. & Herget, G. Differential diagnosis of primary malignant bone tumors in the spine and sacrum. The radiological and clinical spectrum: minireview. Neoplasma 55, 16–22 (2008).
Mindell, E. R. Chordoma. J. Bone Jt. Surg. Am. 63, 501–505 (1981).
Azzarelli, A. et al. Chordoma: natural history and treatment results in 33 cases. J. Surg. Oncol. 37, 185–191 (1988).
Sahakitrungruang, C. & Chantra, K. One-staged subtotal sacrectomy for primary sacral tumor. Ann. Surg. Oncol. 16, 2594–2594 (2009).
Sundaresan, N., Rosen, G. & Boriani, S. Primary malignant tumors of the spine. Orthop. Clin. N. Am. 40, 21–36 (2009).
Tessitore, E., Burkhardt, K. & Payer, M. Primary clear-cell chondrosarcoma of the cervical spine: case illustration. J. Neurosurg. Spine 4, 424–424 (2006).
Fuller, D. B. & Bloom, J. G. Radiotherapy for chordoma. Int. J. Radiat. Oncol. Biol. Phys. 15, 331–339 (1988).
Chandawarkar, R. Y. Sacrococcygeal chordoma: review of 50 consecutive patients. World J. Surg. 20, 717–719 (1996).
Bethke, K. P., Neifeld, J. P. & Lawrence, W. Jr Diagnosis and management of sacrococcygeal chordoma. J. Surg. Oncol. 48, 232–238 (1991).
Yang, H. et al. Analysis of risk factors for recurrence after the resection of sacral chordoma combined with embolization. Spine J. 9, 972–980 (2009).
Fourney, D. R. et al. En Bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J. Neurosurg. Spine 3, 111–122 (2005).
Samson, I. R., Springfield, D. S., Suit, H. D. & Mankin, H. J. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. JBJS 75, 1476–1484 (1993).
Walcott, B. P. et al. Chordoma: current concepts, management, and future directions. Lancet Oncol. 13, e69–e76. https://doi.org/10.1016/s1470-2045(11)70337-0 (2012).
Arshi, A. et al. Chondrosarcoma of the osseous spine: an analysis of epidemiology, patient outcomes, and prognostic factors using the SEER Registry from 1973 to 2012. Spine 42, 644–652. https://doi.org/10.1097/brs.0000000000001870 (2017).
Prevedello, D. M. S., Cordeiro, J. G., Koerbel, A., Ditzel, L. F. & Araújo, J. C. d. S. Management of primary spinal chondrosarcoma: report of two cases causing cord compression. Arq. Neuro-Psiquiatr. 62, 875–878 (2004).
Rao, G. et al. Surgical management of primary and metastatic sarcoma of the mobile spine. J. Neurosurg. Spine 9, 120–128 (2008).
Imai, R. et al. Carbon ion radiation therapy for unresectable sacral chordoma: an analysis of 188 cases. Int. J. Radiat. Oncol. Biol. Phys. 95, 322–327 (2016).
Schulz-Ertner, D. et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int. J. Radiat. Oncol. Biol. Phys. 68, 449–457 (2007).
Imai, R., Kamada, T. & Araki, N. Clinical efficacy of carbon ion radiotherapy for unresectable chondrosarcomas. Anticancer Res. 37, 6959–6964 (2017).
Berber, T., Numanoğlu, Ç., Uysal, E., Dinçer, S. & Yıldırım, B. A. Results of salvage treatment with CyberKnife® fractioned radiosurgery in recurrent large chordoma. Eur. Spine J. 32, 244–253. https://doi.org/10.1007/s00586-022-07399-1 (2023).
Jin, C. J. et al. Long-term outcomes of high-dose single-fraction radiosurgery for chordomas of the spine and sacrum. J. Neurosurg. Spine 32, 79–88 (2019).
Catanzano, A. A. et al. Revisiting the role of radiation therapy in chondrosarcoma: a National Cancer Database Study. Sarcoma 2019, 4878512. https://doi.org/10.1155/2019/4878512 (2019).
Huvos, A. G. Bone Tumors: Diagnosis, Treatment and Prognosis (1987).
Boriani, S. et al. Chordoma of the mobile spine: fifty years of experience. Spine 31, 493–503 (2006).
Yu, X. et al. Comparison of wide margin and inadequate margin for recurrence in sacral chordoma: a meta-analysis. Spine 45, 814–819. https://doi.org/10.1097/brs.0000000000003386 (2020).
Fuchs, B., Dickey, I. D., Yaszemski, M. J., Inwards, C. Y. & Sim, F. H. Operative management of sacral chordoma. JBJS 87, 2211–2216 (2005).
Boriani, S., Gasbarrini, A., Bandiera, S., Ghermandi, R. & Lador, R. Predictors for surgical complications of en bloc resections in the spine: review of 220 cases treated by the same team. Eur. Spine J. 25, 3932–3941. https://doi.org/10.1007/s00586-016-4463-y (2016).
Charest-Morin, R. et al. Perioperative adverse events following surgery for primary bone tumors of the spine and en bloc resection for metastases. J. Neurosurg. Spine 1–8. https://doi.org/10.3171/2019.6.Spine19587 (2019).
Li, Z. et al. A systematic review of perioperative complications in en Bloc resection for spinal tumors. Glob. Spine J. 13, 812–822. https://doi.org/10.1177/21925682221120644 (2022).
Kim, Y. J. et al. The volumetric change and dose-response relationship following hypofractionated proton therapy for chordomas. Acta Oncol. 53, 563–568 (2014).
Rich, T. A., Schiller, A., Suit, H. D. & Mankin, H. J. Clinical and pathologic review of 48 cases of chordoma. Cancer 56, 182–187 (1985).
Cummings, B. J., Hodson, D. I. & Bush, R. S. Chordoma: the results of megavoltage radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 9, 633–642 (1983).
Bjornsson, J., Wold, L. E., Ebersold, M. J. & Laws, E. R. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer 71, 735–740 (1993).
Schultheiss, T., Kun, L., Ang, K. & Stephens, L. Radiation response of the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 31, 1093–1112 (1995).
Hug, E. B., Fitzek, M. M., Liebsch, N. J. & Munzenrider, J. E. Locally challenging osteo-and chondrogenic tumors of the axial skeleton: results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 31, 467–476 (1995).
DeLaney, T. F. et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 74, 732–739 (2009).
DeLaney, T. F. et al. Long-term results of phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J. Surg. Oncol. 110, 115–122. https://doi.org/10.1002/jso.23617 (2014).
Catton, C. et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother. Oncol. 41, 67–72 (1996).
Chen, Y. L. et al. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine 38, E930–E936. https://doi.org/10.1097/BRS.0b013e318296e7d7 (2013).
De Ruysscher, D. et al. Radiotherapy toxicity. Nat. Rev. Dis. Primers 5, 13. https://doi.org/10.1038/s41572-019-0064-5 (2019).
Wang, K. & Tepper, J. E. Radiation therapy-associated toxicity: etiology, management, and prevention. CA Cancer J. Clin. 71, 437–454. https://doi.org/10.3322/caac.21689 (2021).
Brown, S., Kirkbride, P. & Marshall, E. Radiotherapy in the acute medical setting. Clin. Med. (Lond.) 15, 382–387. https://doi.org/10.7861/clinmedicine.15-4-382 (2015).
Redmond, K. J. et al. Radiotherapy for Mobile Spine and Sacral Chordoma: a critical review and practical guide from the Spine Tumor Academy. Cancers 15, 2359 (2023).
Sherry, A. D. et al. Management of chordoma and chondrosarcoma with definitive dose-escalated single-fraction spine stereotactic radiosurgery. J. Neurooncol. 164, 377–386. https://doi.org/10.1007/s11060-023-04432-1 (2023).
Al-Omair, A. et al. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro-oncology 15, 1413–1419 (2013).
Laufer, I. et al. Local disease control for spinal metastases following separation surgery and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J. Neurosurg. Spine 18, 207–214 (2013).
Bate, B. G., Khan, N. R., Kimball, B. Y., Gabrick, K. & Weaver, J. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J. Neurosurg. Spine 22, 409–415 (2015).
Folkert, M. R. et al. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int. J. Radiat. Oncol. Biol. Phys. 88, 1085–1091 (2014).
Cunha, M. V. R. et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int. J. Radiat. Oncol. Biol. Phys. 84, e343–e349. https://doi.org/10.1016/j.ijrobp.2012.04.034 (2012).
Boehling, N. S. et al. Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J. Neurosurg. Spine 16, 379–386 (2012).
Rose, P. S. et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J. Clin. Oncol. 27, 5075 (2009).
Lee, S. H. et al. Can the spinal instability neoplastic score prior to spinal radiosurgery predict compression fractures following stereotactic spinal radiosurgery for metastatic spinal tumor? A post hoc analysis of prospective phase II single-institution trials. J. Neurooncol. 126, 509–517. https://doi.org/10.1007/s11060-015-1990-z (2016).
Gerszten, P. C. Spine metastases: from radiotherapy, surgery, to radiosurgery. Neurosurgery 61, 16–25 (2014).
Diaz, R. J. & Cusimano, M. D. The biological basis for modern treatment of chordoma. J. Neurooncol. 104, 411–422 (2011).
Italiano, A. et al. Advanced chondrosarcomas: role of chemotherapy and survival. Ann. Oncol. 24, 2916–2922 (2013).
Stacchiotti, S. & Sommer, J. Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 16, e71–e83 (2015).
Stacchiotti, S. et al. Best practices for the management of local-regional recurrent chordoma: a position paper by the Chordoma Global Consensus Group. Ann. Oncol. 28, 1230–1242 (2017).
Frezza, A. M. et al. Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur. J. Cancer 51, 374–381 (2015).
Traylor, J. I., Pernik, M. N., Plitt, A. R., Lim, M. & Garzon-Muvdi, T. Immunotherapy for chordoma and chondrosarcoma: current evidence. Cancers 13, 2408 (2021).
Author information
Authors and Affiliations
Contributions
P.L.K. and Y.C.Y. conceived the study and drafted the main manuscript. P.L.K., Y.C.Y., and F.Y.T. conducted the analyses and developed the methods. K.C. and T.T.T. collected the study data. P.L.L. and F.Y.T. were involved in the study design and oversaw the analyses. All authors participated in interpreting the results and provided critical revisions to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kuo, PL., Yeh, YC., Chang, K. et al. Spinal chordoma and chondrosarcoma treatment experiences - a 20-year retrospective study from databases of two medical centers. Sci Rep 14, 23012 (2024). https://doi.org/10.1038/s41598-024-74317-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74317-4
Keywords
This article is cited by
-
Surgical Management of Large Sacral Spine Chordomas
Indian Journal of Surgical Oncology (2025)
-
Surgical outcomes in oncological sacrectomy: a detailed analysis of surgical site infections (SSI)
European Spine Journal (2025)
-
Risk calculator for long-term survival prediction of spinal chordoma versus chondrosarcoma: a nationwide analysis
Journal of Neuro-Oncology (2025)