Abstract
Weight-adjusted waist index (WWI) is a new marker of central obesity. This study explored the association of WWI with all-cause and cardiovascular disease (CVD) mortality in individuals with diabetes or prediabetes. 6551 participants with diabetes or prediabetes from the National Health and Nutrition Examination Survey (NHANES) records between 2005 and 2018 were included. The association of WWI with all-cause and CVD mortality was assessed using Kaplan-Meier survival analysis, Cox proportional hazards model (Cox regression), and restricted cubic spline (RCS). The predictive value of WWI for mortality was analyzed using time-dependent receiver operating characteristic curves (ROC). There were 1083 all-cause deaths and 360 CVD deaths. Multivariable-adjusted Cox regression analyses showed WWI was positively correlated with the risk of all-cause and CVD mortality in subjects with diabetes or prediabetes. Multivariate-adjusted RCS analyses showed a linear and positive correlation of WWI with all-cause mortality risk, and a nonlinear relationship with CVD mortality, with a threshold of 12.35. The area under the curve (AUC) for 3, 5, and 10-years survival for all-cause mortality was 0.795, 0.792, and 0.812, respectively, and for CVD mortality was 0.815, 0.833, and 0.831, respectively. WWI is a valuable predictor of all-cause mortality risk in patients with diabetes and prediabetes, and a valuable predictor of CVD mortality risk when patients with diabetes and prediabetes are considered as a whole.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a significant and growing public health issue worldwide. Studies estimate that over 500 million people aged 20–80 years are affected by diabetes globally, with this figure predicted to rise to 783.2 million1. DM can lead to vascular complications and is a significant cause of cardiovascular disease (CVD)2. CVD is a major cause of mortality globally, the number of people with CVD, exceeded 250 million worldwide in 2019, and the figure of deaths has risen by 6.5 million3. Notably, CVD continues to be a major cause of mortality in diabetic patients in the world4,5. People with DM tend to suffer from a higher risk of CVD and a worse outcome, manifesting higher rates of CVD and all-cause mortality. In 2018, all-cause mortality and CVD mortality rates for males with DM were 27.8 and 7.5 per 1,000 person-years, respectively, compared with 29.5 and 7.1 per 1,000 person-years for females6. A four-fold increase in CVD-related mortality has been reported in people with DM in comparison with people without DM. All-cause mortality is also increased in people with DM compared with people without DM7. Given the high morbidity and mortality associated with both DM and CVD, identifying modifiable risk factors affecting the prognosis of individuals with diabetes or prediabetes is critical to preventing DM complications and reducing mortality.
The correlation between obesity and the development of DM and CVD is well recognized. Abdominal obesity, in particular, is known to be an essential risk factor for DM and its complications, including diabetic kidney disease, osteoporosis, and CVD8. Several obesity indexes, including body mass index (BMI), waist circumference (WC), triglyceride-glucose index and visceral adiposity index, have been used in previous research to evaluate the relationship between obesity, DM, its complications, and CVD9. Each index has its strengths and limitations. Different obesity indexes in various populations yield inconsistent or contradictory results, known as the obesity paradox10. BMI and WC are classic clinical obesity assessments; however, BMI poorly distinguishes between fat distribution and muscle mass11. Similarly, WC is an inadequate independent predictor of obesity12,13.
The weight-adjusted-waist index (WWI) is a newly central obesity assessment marker introduced in 201814. WWI combines the strengths of WC and addresses the limitations of BMI, offering a more accurate assessment of fat and muscle mass. It focuses on abdominal obesity regardless of body-weight, providing a more robust measure of fat and muscle levels than other factors such as BMI, WC, and the visceral adiposity index VAI10,15,16,17. Emerging research indicates that increased WWI is an significant risk factor for metabolic disorders, including DM18, hypertension19 and CVD20. A study of adults from the United States (U.S.) indicated a positive correlation of WWI with CVD21, suggesting that WWI can be a reliable and comprehensive index of obesity20. While there is evidence of an association of WWI with diabetes and CVD, its role in predicting CVD outcomes, particularly those with in diabetes, has not been thoroughly investigated.
Given that WWI is an easy-to-measure surrogate for obesity, this retrospective cohort study aimed to explore the correlation of WWI with all-cause mortality and CVD mortality in participants from the National Health and Nutrition Examination Survey (NHANES) population screened for diabetes or prediabetes between 2005 and 2018.
Subjects and methods
Data sources
The data were extracted from the 2005 to 2018 NHANES records, a U.S. database that assesses representative health-related data on children and adults. The NHANES protocol was formally approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board, with informed consent obtained from all participants22. A detailed description can be obtained from https://www.cdc.gov/nchs/nhanes/.
Subjects
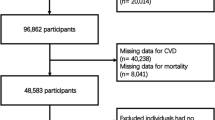
Data were collected from the NHANES from 2005 to 2018, including 6551 participants meeting specific criteria (Fig. 1, STROBE flowchart).
Inclusion criteria
(1) Participants with 20 ≤ age years; (2) Participants with diabetes or prediabetes.
Exclusion criteria
Participants with lacking data on WWI, all-cause mortality, or medical information were excluded.
Assessment of diabetes and prediabetes
According to the ADA criteria, participants were classified as having diabetes if they matched any of the following criteria: previous notification of diabetes, prior use of insulin or oral hypoglycemic drugs, fasting blood glucose (FBG) ≥ 126 mg/dL, or glycosylated hemoglobin (HbA1c) ≥ 6.5%. Participants were classified as having prediabetes if they matched any of the following criteria: previous notification of prediabetes, 100 mg/dL < FBG ≤ 126 mg/dL, and 6.5% > HbA1c ≥ 5.7%23.
Assessment of WWI and study participant grouping
WWI was calculated as: \(\:\text{W}\text{W}\text{I}=\frac{\text{W}\text{C}\left(\text{c}\text{m}\right)}{\sqrt{\text{w}\text{e}\text{i}\text{g}\text{h}\text{t}}\left(\text{k}\text{g}\right)}\)
Participants was categorized into the Q1, Q2, Q3, and Q4 groups according to the WWI quartiles.
Collection and assessment of variables
Data from the NHANES database, included gender, age, height, weight, BMI, poverty income ratio (PIR), race, education levels, with or without hypertension (hp), smoking status and drinking status. Smoking status was defined as “Not at all,” “Former,” or “Now.” Drinking status was categorized as “Not at all,” “Former,” “Mild,” “Moderate,” or “Severe” based on previous literature23.
Other covariates included aspartate-aminotransferase (AST), alanine-aminotransferase (ALT), gamma- glutamyltransferase (GGT), albumin (Alb), total protein (TP), triglycerides (TG), cholesterol (Chol), high-density lipoprotein cholesterol (HDL-C), serum creatinine (SCr), blood urea nitrogen (BUN), uric acid (UA), total bilirubin (TB), lactate dehydrogenase (LDH), HbA1c, and FBG.
Outcome measurements
The study’s outcome indicators were all-cause and CVD mortality. Both deaths from cardiovascular disease and cerebrovascular disease are defined as CVD mortality in the study. Survival data were collected from the NHANES public-use linked mortality file as of December 31 2019, linked to the National Center for Health Statistics (NCHS) and the National Death Index (NDI) using a probability matching algorithm.
Statistical methods
Statistical analysis was performed with R software (version 4.3.3). According to the NHANES guidelines, sample weights, clustering and stratification were applied. Factor variables are presented as n(%). Numeric variables are presented as mean ± standard deviation or median (quartiles). Differences between groups were compared by one-way ANOVA, Kruskal-Wallis test, and chi-square test according to the type of variable.
Kaplan-Meier curves were used to assess differences in survival rates among participants in each WWI quartile. The association of WWI with mortality was evaluated using a Cox regression model. Previous literature on survival in participants with diabetes guided the selection of covariates for adjustment, covariant variables were gender, race, age, drinking status, smoking status, education levels, with or without hypertension, BMI, and PIR in the models of multi-factor adjustment23.
After adjusting for confounders, the nonlinear correlation of WWI with all-cause or CVD mortality was assessed by selecting the restricted cubic spline (RCS) curves with the minimum Akaike information criterion value. Inflection points were identified through inflection point analysis. A two-step Cox regression model explained the nonlinearity of CVD mortality and WWI. Finally, subgroup and interaction analyses were conducted using the Cox regression model.
Results
Baseline characteristics of participants
Table 1 presents critical baseline demographic and clinical data stratified by WWI quartiles. The table shows that age, BMI, PIR, WC, HbA1c, AST, ALT, GGT, Alb, TP, TG, Chol, HDL-C, SCr, BUN, UA, TB, LDH, and gender demonstrated significant differences across the quartiles (p < 0.001). Moreover, the distribution of race, drinking status, smoking status, education levels, with or without hypertension, all-cause mortality, CVD mortality, diabetes and prediabetes also exhibited significant differences among the quartiles. Notably, gender distribution varied significantly, with a decreasing proportion of males and an increasing proportion of women as the quartile number increased. Race composition also differed significantly, with Non-Hispanic White participants comprising a higher percentage in higher quartiles. Furthermore, the prevalence of hypertension, all-cause mortality, and CVD mortality increased across quartiles.
Association of WWI with the risk of mortality in participants with diabetes or prediabetes
All-cause mortality in Q1 group was significantly lower compared to Q2, Q3 and Q4 groups (all p < 0.01), all-cause mortality in Q2 and Q3 groups significantly decreased compared to Q4 group (both p < 0.01) (Fig. 2A). Similarly, CVD mortality in Q1 group was significantly lower compared to Q2, Q3 and Q4 groups (all p < 0.01), CVD mortality in Q2 group significantly decreased compared to Q4 group (both p < 0.01) (Fig. 2B).
Association between WWI and mortality outcomes in participants with diabetes or prediabetes. (A) Kaplan-Meier curve for all-cause mortality, (B) Kaplan-Meier curve for CVD mortality, (C) The nonlinear relationship between the WWI and all-cause mortality, (D) The nonlinear relationship between the WWI and CVD mortality. Covariant variables were gender, race, age, drinking status, smoking status, education levels, with or without hypertension, BMI, and PIR in the model. WWI weight-adjusted-waist index, CVD cardiovascular disease, BMI body mass index, PIR poverty income ratio.
When WWI was considered as a continuous, Cox regression analyses revealed that WWI levels were positively correlated with the risk of all-cause mortality in Model 3 (HR = 1.23 (95%CI [1.12, 1.36], p < 0.001). When WWI was considered as acategorical, compared to the Q1 group, the adjusted HR for WWI and all-cause mortality risk in the Q4 group was 1.41(95%CI[1.15, 1.74], p = 0.001) in Model 3, with a significant trend (p for trend < 0.05) (Table 2).
When WWI was considered as a continuous, Cox regression analyses revealed that WWI levels were positively correlated with CVD mortality risk in Model 3 (HR = 1.19 (95%CI [1.01, 1.42], p = 0.043). When WWI was considered as a categorical, compared to the Q1 group, the adjusted HR for WWI and CVD mortality risk in the Q4 group was 1.30(95%CI [0.91, 1.87], p = 0.154) in Model 3 (Table 2).
The nonlinear correlation of WWI with all-cause and CVD mortality
The RCS curves indicated a positive linear correlation of WWI values with all-cause mortality (p for nonlinear relationship > 0.05) (Fig. 2C). Moreover a nonlinear correlation of WWI with CVD mortality was found (p for nonlinear relationship < 0.05) (Fig. 2D). Inflection point analysis showed the curve for WWI and CVD mortality inflected at WWI value of 12.35, indicating a positive correlation between CVD mortality risk and WWI ≥ 12.35 (HR = 1.88 [95%CI 1.29–2.74]). (Table 3).
Further analysis explored the nonlinear relationship of WWI with all-cause and CVD mortality in participants with prediabetes and diabetes. WWI showed no nonlinear relationship with all-cause and CVD mortality in participants with prediabetes (both p for nonlinear relationship = 0.794 and 0.632, respectively) (Fig. 3A and B). However, a nonlinear correlation of WWI with all-cause mortality in participants with diabetes was revealed (p for nonlinear relationship < 0.05), indicating a J-shaped dose–response curve (Fig. 3C). A nonlinear correlation of WWI with CVD mortality was also observed (p for nonlinear relationship < 0.05) (Fig. 3D). Inflection point analysis showed the curves for WWI and all-cause and CVD mortality inflected at WWI values of 11.2 and 12.3, respectively, with WWI being positively correlated with all-cause and CVD mortality risks above the threshold.
The nonlinear relationship between the WWI and all-cause and CVD mortality in participants with diabetes or participants with prediabetes. (A) The nonlinear relationship between the WWI and all-cause in participants with prediabetes, (B) The nonlinear relationship between the WWI and CVD in participants with prediabetes, (C) The nonlinear relationship between the WWI and all-cause in participants with diabetes. (D) The nonlinear relationship between the WWI and CVD in participants with diabetes. Covariant variables were gender, race, age, drinking status, smoking status, education levels, with or without hypertension, BMI, and PIR in the model. WWI weight-adjusted-waist index, CVD cardiovascular disease, BMI body mass index, PIR poverty income ratio.
Predictive power of WWI for all-cause and CVD mortality in participants with diabetes or prediabetes
Time-dependent-receiver-operating-characteristic (ROC) curves indicated that the area under the curve (AUC) for WWI combined with other covariates in predicting all-cause mortality was 0.795, 0.792, and 0.812 at 3, 5, and 10 years, respectively (Fig. 4A). For CVD mortality, the AUC for WWI combined with other covariates was 0.815, 0.833, and 0.831, at the same intervals (Fig. 4C). Moreover, time-dependent AUC curves demonstrated the better predictive power of WWI for both all-cause and cardiovascular mortality at different time intervals (Fig. 4B and D).
The predictive value of WWI for all-cause and cardiovascular mortality. (A) Time-dependent ROC curves of the WWI for predicting all-cause mortality; (B) Time-dependent AUC curves of the WWI for predicting all-cause mortality. (C) Time-dependent ROC curves of the WWI for predicting cardiovascular mortality; (D) Time-dependent AUC curves of the WWI for predicting cardiovascular mortality.
Subgroup and interaction analyses
To explore the effects of other factors on the correlation of WWI with all-cause or CVD mortality. Subgroups were classified by gender, age, BMI, race, education levels, drinking status, smoking status, and with or without hypertension. Cox regression analyses revealed that WWI values were positively correlated with the risk of all-cause mortality in patients with prediabetes (HR = 1.51, 95% CI [1.07, 2.12], p = 0.018) and those with diabetes (HR = 1.17 (95% CI [1.05, 1.30], p = 0.004). Further adjusted analyses revealed that WWI values were positively correlated with the risk of all-cause mortality in men (HR = 1.32, 95% CI [1.14, 1.54], p < 0.001) and those with diabetes (HR = 1.16 (95% CI [1.01, 1.33], p = 0.031).
These subgroup and interaction analyses suggested no significant interaction between WWI and gender, BMI, education levels, drinking status, smoking status, and with or without hypertension for all-cause and CVD mortality in participants. However, the interaction of WWI with age was significant, and a stronger correlation of WWI with all-cause and CVD mortality in participants with aged < 65 years was observed (Fig. 5A and B).
The relationship between WWI and all-cause and CVD mortality based on subgroups. (A) Subgroup and interaction analyses for all-cause mortality, (B) Subgroup and interaction analyses for CVD mortality. Covariant variables were gender, race, age, drinking status, smoking status, education levels, with or without hypertension, BMI, and PIR in the model, except when used as stratification variables. WWI weight-adjusted-waist index, CVD cardiovascular disease, BMI body mass index, PIR poverty income ratio.
Discussion
To the best of our knowledge, the study is the first to evaluate WWI in relation to all-cause mortality and CVD mortality in patients with diabetes or prediabetes. WWI demonstrated a significant linear relationship with all-cause mortality, and a nonlinear relationship with CVD mortality. Inflection point analyses indicated that the risk of CVD mortality increased significantly with WWI > 12.35. The results of the ROC analysis suggested that the baseline WWI values are a favorable predictor of all-cause and CVD mortality in all participants.
A previous study from Korea examined the association of WWI with CVD morbidity and mortality among the masses and incicated that WWI was positively linked to cardio-metabolic disease prevalence and mortality14. Another study in a Chinese general population also suggested a positive correlation of WWI with all-cause mortality was observed, and a positive association with all-cause mortality was observed at WWI values > 11.224. Similarly, cross-sectional research by Zheng et al. demonstrated a positive correlation of WWI with high abdominal aortic scores in unadjusted and multivariate models25. Moreover, WWI has been correlated with several CVD conditions, including subclinical atherosclerosis26, left ventricular hypertrophy27, heart failure28, and hypertension29. These findings indicate that WWI is a robust indicator of CVD risk, further supporting the results of our study. However, WWI also show different effects in different populations, a study exploring the relationship between WWI and the metabolic syndrome showed no correlation between WWI and the risk of all-cause and CVD mortality in patients with CKD, whereas it was positively associated with the risk of mortality in patients with normal kidney function.
Furthermore, the RCS results showed no nonlinear relationship between WWI and the risk of all-cause and CVD mortality in prediabetic patients, whereas a nonlinear relationship existed in diabetic patients. Subgroup and interaction analyses showed that the increased risk of all-cause mortality due to increased WWI was higher in prediabetic patients than in diabetic patients (HR = 1.51 and 1.17, respectively), although the difference was not significant. Similarly, the increased risk of CVD mortality due to increased WWI was higher in prediabetic patients than in diabetic patients (HR = 1.71 and 1.11, respectively), although no statistically significance was observed. This difference in risk may be due to insufficient awareness of serum glucose and health self-management in prediabetic patients, resulting in prediabetic patients with higher WWI value having a higher risk of death. In addition, some prediabetic patients’ serum glucose may recover to normal, whereas the present study did not record the subsequent progression of serum glucose of prediabetic patients and the sample size (deaths from CVD) may be insufficient, so the results may be biased. More detailed prospective studies may be needed for further confirmation in the future.The subgroup analyses also showed an interaction between WWI and age, suggested that participants aged < 65 years were at greater risk of all-cause and CVD mortality than those aged > 65 years with the same level of WWI elevation.
Several pathogenic and physiogenic mechanisms could explain the association of WWI with the risk of death in patients with diabetes or prediabetes. Obesity, particularly central obesity, is a recognized risk factor for CVD and mortality in adults30. High WWI value may predict adipose tissue dysfunction, especially increased visceral adiposity, which can lead to increased insulin resistance in diabetic patients31, increased release of inflammatory factors, such as TNF-α and IL-6, and decreased levels of lipocalin, all of which are risk factors for CVD32,33. Inflammatory factors can induce inflammation and low levels of lipocalin have a diminished inhibitory effect on inflammation and oxidative stress34, in addition adipose tissue accumulation produces excessive reactive oxygen species thereby depleting nitric oxide leading to endothelial cell dysfunction, which is directly related to the development of CVD35. Visceral obesity also induces cardiomyocyte hypertrophy and myocardial fibrosis in diabetic patients, and activates inflammatory pathways associated with macrophage infiltration and cytokine gene expression, leading to heart failure36,37. The main mechanism by which obesity, represented by high WWI values, leads to a poor prognosis likely involves lipid metabolism disorders38. In summary obesity with elevated WWI can induce dysregulated release of inflammatory cytokines and adipocytokines, promoting oxidative stress, inflammatory responses, endothelial dysfunction and microvascular remodelling20,39.
The paradoxical relationship between the obesity and survival in patients with chronic diseases has remained controversial41. It is a violation of common sense that a known positive correlation between obesity and increased mortality was observed, but research suggested that overweight and obese people are more likely to survive than lean people in people with chronic disease42. Growing evidence suggests that the obesity-survival paradox may result from the historical use of BMI and WC as indicators of obesity43. As previously mentioned, neither BMI nor WC adequately reflects visceral fat levels. Conversely, WWI combines the advantages of WC with the reduced relevance of BMI, making it a more accurate indicator of “true obesity” that reflects metabolic unhealthiness21. Therefore, this study is valuable for evaluating the correlation of obesity with mortality in participants with diabetes or prediabetes. Moreover WWI is an easy-to-measure clinical indicator that can be tested at the primary care level and in areas with limited medical resources, offering significant clinical utility.
Nevertheless, this study has limitations. Firstly, some clinical data such as glucose-lowering medication use and self-reported diabetes and prediabetes relied on participant recall rather than objective data, which may lead to information bias, and more research is needed to support our study. Secondly, the study was a single-center study, and it is unclear whether the results would be generalizable to other populations. Thirdly, due to the limitations of the NHANES data, all possible confounding factors could not be adjusted. Fourthly, while WWI was positively associated with the risk of CVD mortality in all patients when patients with diabetes and prediabetes were combined, this association was not observed in patients with prediabetes and diabetes, respectively, warranting further analysis in future prospective studies. Finally, we only used baseline WWI values and it is unknown whether variations in WWI values during the period of follow-up affect patient mortality.
Conclusion
WWI is a valuable predictor of the risk of all-cause mortality in patients with diabetes and prediabetes, respectively. Moreover, WWI is a valuable predictor of the risk of CVD mortality when patients with diabetes and prediabetes are considered together, with a nonlinear correlation observed for CVD mortality. The measurement of WWI may be useful in assessing mortality risk and predicting prognosis in participants with diabetes or prediabetes. It may be necessary in the future to explore the impact of altering WWI value on patients’ prognosis.
Data availability
All data can be downloaded from the official NHANES website, and the data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sun, H. et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract.183, 109119 (2022).
Ritchie, R. H. & Abel, E. D. Basic mechanisms of diabetic heart disease. Circul. Res.126(11), 1501–1525 (2020).
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol.76(25), 2982–3021 (2020).
Zheng, Y., Ley, S. H. & Hu, F. B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol.14(2), 88–98 (2018).
Virani, S. S. et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation. 141(9), e139–e596 (2020).
Pearson-Stuttard, J. et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: An epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol.9(3), 165–173 (2021).
Khumaedi, A. I. et al. The relationship of diabetes, periodontitis and cardiovascular disease. Diabetes Metabolic Syndrome: Clin. Res. Reviews. 13(2), 1675–1678 (2019).
Wan, H. et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc. Diabetol.19(1), 118 (2020).
Wang, Z. et al. The relationship between weight-adjusted-waist index and diabetic kidney disease in patients with type 2 diabetes mellitus. Front. Endocrinol. (Lausanne). 15, 1345411 (2024).
Liu, H., Ma, Y. & Shi, L. Higher weight-adjusted waist index is associated with increased likelihood of kidney stones. Front. Endocrinol. (Lausanne). 14, 1234440 (2023).
Kim, N. H. et al. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. 50(3), 780–786 (2020).
Antonopoulos, A. S. et al. From the BMI paradox to the obesity paradox: the obesity-mortality association in coronary heart disease. Obes. Rev. Off. J. Int. Assoc. Study Obes.17(10), 989–1000 (2016).
Clark, A. L., Fonarow, G. C. & Horwich, T. B. Waist circumference, body mass index, and survival in systolic heart failure: The obesity paradox revisited. J. Card. Fail.17(5), 374–380 (2011).
Park, Y. et al. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci. Rep.8(1), 16753 (2018).
Kim, N. H. et al. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. 50(3), 780–786 (2021).
Qin, Z. et al. The association between weight-adjusted-waist index and increased urinary albumin excretion in adults: A population-based study. Front. Nutr.9, 941926 (2022).
Yu, S. et al. Weight-adjusted-Waist Index predicts newly diagnosed diabetes in Chinese rural adults. J. Clin. Med.12(4) (2023).
Park, M. J. et al. A Novel Anthropometric parameter, weight-adjusted Waist Index represents Sarcopenic obesity in newly diagnosed type 2 diabetes Mellitus. J. Obes. Metabolic Syndrome. 32(2), 130–140 (2023).
Li, Q. et al. Transcriptome analysis reveals long intergenic non-coding RNAs contributed to intramuscular Fat Content differences between Yorkshire and Wei Pigs. Int. J. Mol. Sci.21(5), 1732 (2020).
Han, Y. et al. The weight-adjusted-waist index predicts all-cause and cardiovascular mortality in general US adults. Clin. (Sao Paulo Brazil). 78, 100248 (2023).
Fang, H. et al. Association between weight-adjusted-waist index and risk of cardiovascular diseases in United States adults: A cross-sectional study. BMC Cardiovasc. Disord.23(1), 435 (2023).
Johnson, C. L. et al. National health and nutrition examination survey: Sample design, 2011–2014. Vital and health statistics Series 2, Data evaluation and methods research. (162), 1–33 (2014).
Zhang, Q. et al. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol.22(1), 279 (2023).
Ding, C. et al. Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in China: A prospective cohort study. Nutr. Metab. Cardiovasc. Diseases NMCD. 32(5), 1210–1217 (2022).
Qin, Z. et al. The association between weight-adjusted-waist index and abdominal aortic calcification in adults aged ≥ 40 years: rRsults from NHANES 2013–2014. Sci. Rep.12(1), 20354 (2022).
Costo-Muriel, C. et al. Relationship between the novel and traditional anthropometric indices and subclinical atherosclerosis evaluated by carotid intima-media thickness (c-IMT). Front. Nutr.10, 1170450 (2023).
Cai, S. et al. The relationship between the weight-adjusted-waist index and left ventricular hypertrophy in Chinese hypertension adults. Hypertens. Res. Off. J. Japanese Soc. Hypertens.46(1), 253–260 (2023).
Zhang, D. et al. Association between weight-adjusted-waist index and heart failure: Results from National Health and Nutrition Examination Survey 1999–2018. Front. Cardiovasc. Med.9, 1069146 (2022).
Li, Q. et al. Association of weight-adjusted-waist index with incident hypertension: The rural Chinese cohort study. Nutr. Metab. Cardiovasc. Diseases NMCD. 30(10), 1732–1741 (2020).
Khan, S. S. et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol.3(4), 280–287 (2018).
Nam, K. W., Kwon, H. M. & Lee, Y. S. High triglyceride-glucose index is associated with early recurrent ischemic lesion in acute ischemic stroke. Sci. Rep.11(1), 15335 (2021).
Xiao, H. et al. Visceral adiposity index and chronic kidney disease in a non-diabetic Population: A cross-sectional study. Diabetes Metab. Syndrome Obesity Targets Therapy. 13:257–265 (2020).
Xie, F. et al. Association between the weight-adjusted-waist index and abdominal aortic calcification in United States adults: Results from the national health and nutrition examination survey 2013–2014. Front. Cardiovasc. Med.9, 948194 (2022).
Zha, D., Wu, X. & Gao, P. Adiponectin and its receptors in Diabetic kidney disease: Molecular mechanisms and clinical potential. Endocrinology. 158(7), 2022–2034 (2017).
Powell-Wiley, T. M. et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 143(21), e984–e1010 (2021).
Hu, G. et al. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 121(2), 237–244 (2010).
Levitan, E. B. et al. Adiposity and incidence of heart failure hospitalization and mortality: A population-based prospective study. Circ. Heart Fail.2(3), 202–208 (2009).
Long, J. et al. Lipid metabolism and carcinogenesis, cancer development. Am. J. cancer Res.8(5), 778–791 (2018).
Iozzo, P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 34(Suppl 2), S371–379 (2011).
Grundy, S. M. Metabolic syndrome update. Trends Cardiovasc. Med.26(4), 364–373 (2016).
Lu, C. Y. et al. Obesity indices and the risk of total and cardiovascular mortality among people with diabetes: A long-term follow-up study in Taiwan. Cardiovasc. Diabetol.22(1), 345 (2023).
Ades, P.A. & Savage, P.D. The obesity paradox: Perception vs knowledge. Mayo Clin. Proc.85(2), 112–114 (2010).
Orsi, E. et al. Body mass index versus surrogate measures of central adiposity as independent predictors of mortality in type 2 diabetes. Cardiovasc. Diabetol.21(1), 266 (2022).
Acknowledgements
The authors are grateful to the participants and staff of NHANES for their valuable contributions to this study. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
PP Z and YR W conceived and designed the study. PP Z, TQ D and Q Z collected clinical and biochemical data. PP Z, TQ D, Q Z and YR W contributed to the statistical analysis, results interpretation, drafting and revising the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The National Center for Health Statistics and Ethics Review approved the NHANES protocol and all participants provided written informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, P., Du, T., zhou, Q. et al. Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in individuals with diabetes or prediabetes: a cohort study from NHANES 2005–2018. Sci Rep 14, 24061 (2024). https://doi.org/10.1038/s41598-024-74339-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74339-y
Keywords
This article is cited by
-
Associations and predictive value of weight-adjusted waist index for cardiovascular outcomes in type 2 diabetes: evidence from the ACCORD study
Nutrition Journal (2025)
-
Weight-adjusted waist index and mortality in diabetic retinopathy: a NHANES 1999–2018 cohort study
Diabetology & Metabolic Syndrome (2025)
-
Triglyceride glucose-weight-adjusted waist index as a cardiovascular mortality predictor: incremental value beyond the establishment of TyG-related indices
Cardiovascular Diabetology (2025)
-
Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in hypertension: a prospective cohort study
BMC Public Health (2025)
-
Does a modified metabolic syndrome definition improve prediction of cardiovascular events and mortality? findings from a 17-year cohort study
Journal of Health, Population and Nutrition (2025)