Abstract
This study aimed to evaluate the association between the questionnaires SARC-F and SARC-CalF with risk of mortality in patients undergoing hemodialysis (HD). A cohort study, with patients on HD age ≥ 18 years, both sex, between June 2019 and April 2023. Body composition (anthropometry and bioelectrical impedance), muscle functional (handgrip strength and gait speed), screening of sarcopenia using the SARC-F and SARC-CalF, nutritional status and laboratory data were assessed. Follow-up for mortality up to 47 months. The sample consisted of 243 participants and the prevalence of risk of sarcopenia using SARC-F and SARC-CalF were 30% and 45%, respectively; 65 died for all reasons and three patients were censored due to transplantation. Multivariate analysis identified SARC-CalF as predictor of mortality in HD patients [hazard ratio 1.96; 95% CI (1.01–3.79); p = 0.04]. The survival analysis showed that there was a significant difference in the survival curves among the groups stratified by SARC-F and SARC-CalF for log-rank test. A higher specificity was found for SARC-CalF than SARC-F (80% vs. 77%) in receiver operating characteristic (ROC) curve. Both questionnaires were associated with anthropometric, parameters of body composition, physical measurements, and SARC-CalF was predictor of risk for mortality in HD patients.
Similar content being viewed by others
Introduction
Muscle wasting and cardiorespiratory fitness reduction have been described in chronic kidney disease (CKD) patients and especially in those with end-stage kidney disease (ESKD)1,2. The causes of muscle catabolism are diverse and associated with chronic inflammation, uremic toxins, hyperparathyroidism, resistance to anabolic hormones, metabolic acidosis, oxidative stress, mitochondrial dysfunction, nutrient losses into the dialysate and physical inactivity3,4.
Recently, the European Working Group on Sarcopenia in Older People (EWGSOP2) revised the definition for sarcopenia as the concomitant loss of muscle mass and low of muscle strength5. The primary sarcopenia occurs when the etiology is related to aging, and secondary sarcopenia occurs when it results from other conditions, as is the case in CKD/ESKD1.
Likewise, sarcopenia is associated with worse clinical outcomes such as physical disability, increased falls/fractures, hospitalization, poor quality of life and increase of mortality rate6. The prevalence of sarcopenia ranged from 4 to 42% according to the definition used, the population studied, the methods applied for the assessment of muscle mass and the stage of CKD1. Moreover, a higher prevalence is also expected in hemodialysis (HD) patients, ranging from 1.9–40%7.
The muscle strength can be easily assessed by surrogate measures such as a handgrip dynamometer, on the another hand, the assessment of muscle quantify and quality is particularly challenging in the clinical practice and can be more difficult to evaluate4. Bioelectrical impedance analysis (BIA), dual-energy X-ray absorptiometry (DXA), ultrasound, computed tomography and magnetic resonance imaging are mainly the methods used to assess muscle mass, however, this techniques are not yet available in clinical settings and often restricted to researches8. Therefore, the application of questionnaires-based tools can also be a valid option for sarcopenia screnning with a most adequate cost-benefit relation, valid, with cost-effective and repeatable.
In this context, SARC-F is the first tool validated with 5-itens (sluggishness, assistance in walking, rising from a chair, climbing stairs, falls) for the rapid screening for sarcopenia and recommended by EWGSOP 2019, to elicit self-reports from patients on signs that are characteristic of sarcopenia5,9. This questionnaire was validated in United States and the questions were based on exploratory investigations in data relating to geriatric issues by panel study of African American Health (AAH), the Baltimore Longitudinal Study of Aging (BLSA) and National Health and Nutrition Examination Survey (NHANES) cohorts10. Posteriorly, studies showed high scores SARC-F related with low physical performance by 3 tests (4 m walking speed, timed up and go test and short physical performance battery) and low muscle strength measured by grip strength in older community-living Chinese11,12.
The inclusion of calf circumference (CC) to SARC-F was suggested an indicator of muscle mass. The study conduced by Barbosa-Silva et al. (2016), with elderly (aged + 60 years) population-based survey from southern Brazilian city, showed that this addition CC measurement improved the sarcopenia screening ability, the diagnostic accuracy and sensitivity increased from 33 to 66%, and this questionnaire started to be called the SARC-CalF13.
Previous studies have shown that the SARC-F is associated with parameters of sarcopenia in CKD/ESKD, such muscle strength and physical functional14,15,16,17,18. The results of Marini et al. (2020), with 95 HD older patents, evidenced correlations between SARC-F and handgrip strength (HGS), SARC-F and gait speed (GS)17. In agreement, the study by Duarte et al. (2022), with 30 HD adults patients, showed moderate correlations between SARC-F and HGS, SARC-F and GS, SARC-F and five-time sit-to-stand test19.
However, the association between SARC-F and SARC-CalF with outcomes in CKD patients undergoing HD not been reported yet, and its clinical utility in these patients is largely unknown. Therefore, the aim of this study was to evaluate the performance of SARC-F and SARC-CalF scores to predict risk of mortality in HD patients.
Results
Patient characteristics
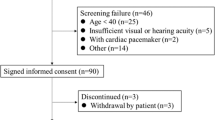
Initially, of 258 participants admitted during the data collection, nine were excluded and six participants had an unsatisfactory body assessment. The final sample consisted of 243 participants and were available for follow-up during 47 months, 65 (27%) died for all reasons and three were censored due to transplantation (see Supplementary Fig. S1 online).
Of the remaining 243 participants, average age was 55.66 ± 14.87 years, 151 (62%) were male, 132 (54%) were adults, median of dialysis vintage 4 (3-360) months, 113 (46%) had diabetes and 223 (92%) had hypertension (Table 1). The causes of CKD, diabetic nephropathy 38%, hypertension 36%, glomerulonephritis 4%, polycystic kidney disease 4%, other causes 6% and unknown 12%. Compared to the outcome, the survived participants had significantly higher percent standard of mid-arm circumference (MAC), triciptal skinfold (TSF), CC, fat tissue index (FTI), phase angle (PhA) and HGS.
The prevalence of risk of sarcopenia using SARC-F (≥ 4 points) and SARC-CalF (≥ 11 points) was 73 (30%) and 109 (45%), respectively (Table 2). Among the sample, 39 (16%) participants had confirmed sarcopenia, according to EWGSOP2. Probable sarcopenia by low HGS, low SMM or GS ≥ 0.8 m/s was 134 (55%), 59 (24%) and 66 (27%), respectively. Malnutrition assessed by 7-point subjective global assessment (7p-SGA) was present in 116 (48%) participants.
Correlations analyses
Among the parameters of body composition, SARC-F correlated significantly with PhA (r= -0.492, p < 0.001), HGS (r= -0.522, p < 0.001) and 7p-SGA (r= -0.512, p < 0.001) (see Supplementary Fig. S2 online). Furthermore, SARC-CalF correlated negatively with PhA (r= -0.505, p < 0.001) and 7p-SGA (r= -0.653, p < 0.001). Both questionnaires correlated positively with GS. In addition, CC correlated significantly with weight (r = 0.723, p < 0.001), body mass index (BMI) (r = 0.646, p < 0.001), MAC (r = 0.631, p < 0.001), mid-arm muscle circumference (MAMC) (r = 0.533, p < 0.001) and 7p-SGA (r = 0.508, p < 0.001).
Survival analysis and receiver operating characteristic (ROC) curve
The Cox regression analysis to identify the independent predictors of all-cause mortality, according to SARC-F (Table 3) and SARC-CalF (Table 4), after adjusted for the potencial confounders, the variable SARC-CalF was predictor of mortality in model adjusted for age (Hazard ratio 1.96; 95% CI 1.01–3.79; p = 0.04). The survival analysis showed that there was a significant difference in the survival curves among the groups stratified by SARC-F (log-rank test, p = 0.019) and SARC-CalF (log-rank test, p = 0.007) scores (see Supplementary Fig. S3 online). SARC-F provided higher sensitivity (50%), whereas SARC-CalF had the highest specificity (80%). The area under the operating curve (AUC) was 0.664 for SARC-F and 0.666 for SARC-CalF (Fig. 1). The detailed diagnostic performance is presented in Supplementary Table S1 online.
Discussion
The findings of the present study were both SARC-F and SARC-CalF are associated with anthropometric parameters of body composition, physical measurements, and SARC-CalF was predictor of risk for mortality in HD patients. A higher specificity was found for SARC-CalF than SARC-F (80% vs. 77%) in ROC curve. There are limited data regarding evaluating the association between these questionnaires and mortality in HD patients, especially, with longer follow-up time. Our results indicate that these tools can serve for risk stratification of poor outcomes in this population.
One study reported SARC-F score correlated with HGS and GS, in addition, the authors did not find the difference of CC between the groups according to SARC-F scores17. In our findings, the CC correlated with all anthropometric and could indicate a valuable and simple tool for the initial screening of sarcopenia among patients undergoing HD, since it was evaluated after dialysis session, to mitigate the influence of hydration status, and in combination with muscle function indicators.
In 179 HD patients, the authors investigated the reliability and validity of the SARC-F questionnaire as a screening tool for sarcopenia, and they found 33% of these participants with risk of sarcopenia, and low to moderate sensitivity and specificity of this tool, 42% and 70%, while for identify severe sarcopenia, the values were 66% and 72%18. These authors recommended careful interpretation of screening for sarcopenia using SARC-F alone and suggested a combination with objective assessments to clinical significance.
Furthermore, one study investigated possible associations of SARC-F and SARC-CalF with sarcopenia in 30 patients undergoing HD and both questionnaires associated with physical function, but not with muscle assessment19. The authors found a more considerable sensitivity for SARC-CalF than SARC-F in detecting probable sarcopenia by HGS (70% vs. 30%) and by HGS and/or five-time sit-to-stand test (STS-5) (63% vs. 44%). They suggested CC in the SARC-F questionnaire would represent a more robust agreement with sarcopenia traits.
A cohort study with 127 patients with CKD on HD showed the association of sarcopenia with a higher risk of mortality, and the authors assessed muscle mass by measuring the CC and accomplished with simple measurements, such as HGS and GS, despite the influence of hydration disorders20. The gold standard for muscle mass assessment, such dual-energy X-ray absorptiometry, was not available in clinical settings, therefore, the CC is a noninvasive, rapid, practical, and low-cost alternative tool to assist in the screening of sarcopenia in HD. The specific cutoff points for CC that we considered in our study were derived from the local population21.
A 3-year longitudinal study evaluated the impact of sarcopenia on hospitalization and mortality in 126 chronic HD patients. The authors found 14% prevalence of sarcopenia using European criteria and 26 (21%) participants died during the follow-up22. In addition, they related low HGS and slow GS associated with hospitalization, as well as with mortality. Similar our results, we found 16% prevalence of sarcopenia and 55% probable sarcopenia by HGS in the sample. These findings demonstrated the importance of physical performance assessment in CKD and early detection of sarcopenia to effective intervention.
The prospective cohort study conducted by Lin Yu-Li et al.16 evaluated the association between SARC-F and mortality during a 24-month follow-up with 271 HD patients, the AUC was 0.716, and the best cut-off was a score ≥ 1 that provided 85% sensitivity and 47% specificity to predict mortality. In addition, the authors found, in Kaplan-Meier analysis, a stepwise decline in survival with higher SARC-F scores and suggested that cut-off value may be different in CKD patients. According to the authors, the SARC-F were positively correlated with age and Charlson comorbidity index, and negatively correlated with HGS, MAMC and skeletal muscle index. However, these authors did not apply SARC-CalF.
According to our cohort, the AUC of the SARC-F and SARC-CalF was 0.664 and 0.666, with sensitivity 50% vs. 48% and specificity 77% vs. 80%, respectively. These results showed low accuracy of both tools and demonstrated limitation in screening ability for correctly identifying poor outcomes in HD patients, however, by association CC measurement, the performance improved and suggested the impact of this variable. The sensitivity of a test is usually higher in a population with a higher prevalence of the target disease18. The questionnaire SARC-F correlated moderately and negatively with HGS and 7-pSGA and positively with gait speed. These discriminative powers indicated an excellent diagnostic performance of SARC-F for identifying physical performance. The SARC-CalF correlated moderately and negatively with PhA and 7-pSGA, suggesting relationship with nutritional status in addition.
The phase angle has demonstrated a prognostic utility in multiple aspects of health and disease, especially in CKD, associated with changes in body composition, disability, hydration status and increased risk of death and incident heart failure23. The cell membrane integrity and better cell function have been referred with phase angle, while low phase angle values have been associated with impaired cell structure, aging biomarkers, higher proinflammatory status and parameters of oxidative stress, which is linked to cell damage and therefore has been implicated in the loss of muscle mass24. Previous studies in CKD had demonstrated that low phase angle was associated with clinical outcomes, quality of life, low muscle mass and strength, consequently, with the presence of sarcopenia, independent of age, sex, and comorbidity index25,26. In our cohort, we found correlation between the questionnaires and PhA.
In addition, the follow-up duration allowed we found 27% mortality rates in our sample, probably, because it coincided with the occurrence of the COVID-19 pandemic. Data of Brazilian Dialysis Survey showed the estimated overall crude annual mortality rate was 24% and 22%, and COVID-19 mortality rate was 4% and 5% in 2020 and 2021, respectively, of chronic dialysis patients, with higher rates compared previous years27.
There were some limitations and strengths in our study. First, we evaluated in a single center and our results may not generalized to overall HD population. Second, we assessed the muscle mass using bioimpedance before the dialysis session, which could lead to an overestimation of the muscle mass due to hydration status of HD patients, and we did not measure the muscle mass using a gold standard equipment. However, our results emphasize the possibility of the implementation of simple tools in clinical routine to screen sarcopenia and potencial to predict poor outcomes.
Conclusion
Our findings demonstrated that both SARC-F and SARC-CalF are associated with anthropometric, parameters of body composition, physical measurements, and SARC-CalF was predictor of risk for mortality in HD patients, and more studies are needed to confirm the best cut-off value for this population.
Methods
Participants and design
The present prospective cohort study was conducted in a nephrology center at Hospital Regional de Taguatinga, located in Brasília, Brazil, during the period between June 2019 and April 2023, using a convenience sampling method, following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines28.
This study was approved by Presentation Certificate for Ethical Appreciation (CAAE) number 04495618.1.0000.5553, and informed written consent was obtained from all participants, according to the general recommendations of the Declaration of Helsinki. All included participants were followed for mortality events for all causes up to 47 months, until death, transplantation, or end of the study, through electronic medical record or telephone contacts.
The eligible participants enrolled if they met the following criteria: age ≥ 18 years, both sex, with a glomerular filtration rate (eGFR) of less than 15 mL/minute/1.73 m², calculated using the Chronic Kidney Disease Epidemiology Collaboration equation29, undergoing HD 4-h dialysis three times per week for the previous three months. ESKD patients who, during the data collection, were hospitalized, with immunodeficiency syndrome and cancer, pregnant women, amputated limbs, or those unable to perform the physical performance test were excluded.
Baseline demographic and clinical data
Age, sex, dialysis vintage in months, causes of CKD and presence of comorbidities, including diabetes, hypertension, and history of COVID-19, were collected through interviews or electronic medical record, registered in a previously prepared questionnaire.
Anthropometry
Anthropometric measurements such as body weight, height, MAC, tricipital skinfold (TSF), CC and WC were obtained after HD sessions. The BMI was calculated as body weight (kg) and height squared (m²). In the no dominant side or contrary to the presence of the arteriovenous fistula, the measurement of MAC and TSF was measured at the midpoint between the acromion and olecranon using a flexible inextensible tape and a skinfold caliper (Lange, US Chemical, USA), respectively30. In a sitting position with the knee and ankle at a right angle, on the same side as the other anthropometric measurements, CC was measured at the point of greatest circumference using a flexible inextensible tape. The average value of the three TSF readings was accepted, and MAMC was calculated using the formula MAC (mm) – [3.14 x TSF (mm)]. The percentage adequacy values according to age and gender to MAC, TSF and MAMC were obtained by the ratio between the obtained value and the reference value of the measure (50th percentile). The results were classified according to Frisancho31.
Bioimpedance analysis
Body composition was evaluated using a bioimpedance spectroscopy (BIS), Body Composition Monitor (BCM), (Fresenius Medical Care, Bad Homburg, Germany), tetrapolar, multifrequency, was measured before HD sessions, on the same day that anthropometric measurements were collected.
The participants were instructed not to exercise eight hours before, not to consume alcohol in the previous 12 h, not to consume any type of food or drink for at least four hours before, not to use any kind of moisturizer lotion on the body and remove metal objects (cell phone, keys, belts), including those attached to the body such as earrings, rings, and watches. Participants remained in a supine position, the electrodes were positioned on the side against the arteriovenous fistula, in the dorsal region in the hand (one between the head of the ulna and the radius, and the other in the proximal phalanx of the third finger) and in the foot (an electrode between the medial and lateral malleoli and another in the third metatarsal region). All results were recorded in BCM software (Fluid Management Toll v.3, Fresenius Medical Care) for further analysis.
Total body water (TBW), extracellular water (ECW), intracellular water (ICW), and muscle and fat tissue were evaluated. Skeletal muscle mass (SMM) was derived from the following equation proposed by Janssen et al.32. Lean tissue index (LTI) and fat tissue index (FTI) calculated as lean tissue mass (kg) and fat tissue mass (kg) divided by height squared (m2), respectively. The value of the phase angle obtained, through the bioimpedance evaluation, was found at the frequency of 50 kHz.
Muscle function: handgrip strength and gait speed
The HGS was measured by the dominant side or contrary to the presence of the arteriovenous fistula using a dynamometer with a variation of 1-100 kgf and an accuracy of 0.5 kgf (Jamar, Patterson Medical, United Kingdom). At the time of measurement, participants remained seated in a chair suitable for height to ensure that their arms could rest comfortably. The hand-held dynamometer was adjusted to the second joint of the finger, with the arm adducted at the side and a 90-degree at the elbow. The participants were instructed to exercise their maximum strength, after the evaluator’s command, and three measurements were obtained with minimum intervals of one minute. The maximum value was used for the analysis33. For the GS test, the time spent by the individual to travel 4 m in his usual step for two consecutive times was recorded, which was considered the shortest time for the analysis5. Both measurements proceeded before the HD sessions.
Assessment of sarcopenia
The screening of sarcopenia was assessed using the SARC-F and SARC-CalF. The SARC-F questionnaire is comprised of five components: strength, walking ability, rising from a chair, stair climbing, and experiences with falls. Each component adds 0 to 2 points, and the total score ranges from 0 to 10 points; a score of 0 signifies the best condition and a score of 10 signifies the worst condition9.
SARC-CalF is composed of the SARC-F with an additional measurement of CC. Whenever the CC is > 34 cm for males and > 33 for females, it scores 0 points, when CC is ≤ 34 cm for males and ≤ 33 cm for females, it scores 10 points. Total SARC-F + CC scores can be 0 to 20 points. The scores between 0 and 10 are considered “no suggestive signs of sarcopenia at the time”; whereas scoring 11–20 points is considered “suggestive of sarcopenia”13. Patients with a total score in SARC-F ≥ 4 points and SARC-CalF ≥ 11 points were considered at sarcopenia risk. Diagnosis sarcopenia was defined as low HGS < 27 kgf for males and < 16 kgf for females and low muscle quantify by SMM < 20 kg for males and < 15 for females, according to the EWGSOP25. Probable sarcopenia was defined by low HGS or low SMM or GS ≥ 0.8 m/s.
7-Point subjective global assessment (7p-SGA)
This questionnaire is comprised of six domains: involuntary change in body weight in the last six months, food intake in the last two weeks, gastrointestinal symptoms persisting for more than two weeks, reduced functional capacity related to nutrition, state of diseases related to nutritional needs, Muscle mass loss and visible adipose tissue in at least three areas and the presence of edema related to malnutrition34. Each domain was scored according to the intensity of the alteration found, ranging from one to seven points. The most frequent score between the domains represented the final score. Individuals with a predominance of one or two points were classified as severely malnourished; those with three to five points were slightly or moderately malnourished; and those with more than six points were classified as well-nourished or at very slight risk for malnutrition.
Laboratory data
Fasting blood samples (~ 5 ml) were collected before dialysis and the results of laboratory data were obtained from the electronic medical records. The biochemical parameters considered were triglycerides (TG) (mg/dL); total cholesterol (mg/dL); high-density lipoprotein cholesterol (HDL-C) (mg/dL); low-density lipoprotein cholesterol (LDL-C) (mg/dL); very low-density lipoprotein cholesterol (VLDL-C) (mg/dL); albumin (g/dL); urea (mg/dL); creatinine (mg/dL) and C-reactive protein (CRP (mg/dL). Kt/V (K-dialyzer clearance of urea, t- dialysis time, V-volume of distribution of urea) was measured using the Daugirdas formula and evaluated the dialysis efficacy35.
Data analyses
The categorical data are presented as frequencies (relative and absolute) and the continuous data are presented as mean and standard deviation (SD) or median and interquartile range. The normality of the continuous variables was tested using the Kolmogorov-Smirnov test. To evaluate the association of SARC-F and SARC-CalF with muscle mass, strength and physical performance, a total of at least 172 patients should be enrolled, considering an effect size of 0.5, a power of 90%, and alpha level of 0.05.
The differences between groups were compared using T Student’s and Mann Whitney (for quantitative variables) or Pearson Chi-square or Fisher exact (for qualitative variables) tests. Pearson and Spearman correlation coefficients were used to verify the correlation between measurements of body composition, muscle function and scores of questionnaires.
Cox proportional hazard models were performed for overall mortality. The survival analyses were performed by the Kaplan-Meier graphic using the log-rank, Breslow and Tarone-Ware tests to compare the survival curves among the screening of sarcopenia based on SARC-F and SARC-CalF. The AUC for the ROC curve were used for predicting mortality using the questionnaires and to identify the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and Youden’s index. In all analyses, the probability of statistical significance was considered p-value < 0.05. All analyses were performed using IBM SPSS (Statistical Package for Social Science) software for Windows (version 26.0; IBM Corp., Armonk, NY, USA).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sabatino, A., Cuppari, L., Stenvinkel, P., Lindholm, B. & Avesani, C. M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 34(4), 1347–1372 (2020).
Eldehni, M. T. Frailty, multimorbidity and sarcopaenia in haemodialysis patients. Curr. Opin. Nephrol. Hypertens. 31(6), 560–565 (2022).
Watanabe, H., Enoki, Y. & Maruyama, T. Sarcopenia in chronic kidney disease: Factors, mechanisms, and therapeutic interventions. Biol. Pharm. Bull. 42(9), 1437–1445 (2019).
Troutman, A. D., Arroyo, E., Lim, K., Moorthi, R. N. & Avin, K. G. Skeletal muscle complications in chronic kidney disease. Curr. Osteoporos. Rep. 20(6), 410–421 (2022).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Aging. 48(1), 16–31 (2019).
Sánchez-Tocino, M. L. et al. Sarcopenia assessed by 4-step EWGSOP2 in elderly hemodialysis patients: Feasibility and limitations. PLoS ONE. 17(1), e0261459 (2022).
Chatzipetrou, V., Bégin, Marie-Josée, Hars, M. & Trombetti, A. Sarcopenia in chronic kidney disease: A scoping review of prevalence, risk factors, association with outcomes, and treatment. Calcif Tissue Int. 110, 1–31 (2022).
Prado, C. M. et al. Advances in muscle health and nutrition: A toolkit for healthcare professionals. Clin. Nutr. 41(10), 2244–2263 (2022).
Malmstrom, T. K. & Morley, J. E. SARC-F: A simple questionnaire to rapidly diagnose Sarcopenia. JAMDA. 14(8), 531–532 (2013).
Malmstrom, T. K. et al. SARC-F: A symptom score to predict persons with Sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle. 7, 28–36 (2016).
Cao, L. et al. A pilot study of the SARC-F scale on screening Sarcopenia and physical disability in the Chinese older people. J. Nut Health Aging. 18(3), 277–283 (2014).
Woo, J., Leung, J. & Morley, J. E. Validating the SARC-F: A suitable community screening tool for sarcopenia? JAMDA. 15(9), 630–634 (2014).
Barbosa-Silva, T. G., Menezes, A. M. B., Bielemann, R. M., Malmstrom, T. K. & Gonzalez, M. C. Enhancing SARC-F: Improving Sarcopenia screening in the clinical practice. J. Am. Med. Dir. Assoc. 17(12), 1136–1141 (2016).
Do, J. Y., Seo, Y., Kang, S. H. & J. H. & Validation of the SARC-F for assessing Sarcopenia in patients on peritoneal dialysis. J. Ren. Nutr. 32(3), 341–346 (2022).
Lin, Y. L. et al. A comparision of SARC-F, calf circunference, and their combination for Sarcopenia screening among patients undergoing peritoneal dialysis. Nutrients. 14(5), 923 (2022).
Lin, Y. L. et al. Association of SARC-F questionnaire and mortality in prevalent hemodialysis patients. Diagnostics. 10(11), 890 (2020).
Marini, A. C. B., Perez, D. R. S., Fleuri, J. A. & Pimentel, G. D. SARC-F is better correlated with muscle function indicators than muscle mass in older hemodialysis patients. J. Nut Health Aging. 24(9), 999–1002 (2020).
Imamura, K. et al. Limitations of SARC-F as a screening tool for Sarcopenia in patients on hemodialysis. Nephron. 146(1), 32–39 (2022).
Duarte, M. P. et al. SARC-F and SARC-CalF are associated with sarcopenia traits in hemodialysis patients. Nutr. Clin. Pract. 37(6), 1356–1365 (2022).
Ferreira, M. F., Böhlke, M., Pauletto, M. B., Frühauf, I. R. & Gonzalez, M. C. Sarcopenia diagnosis using diferente criteria as a predictor of early mortality in patients undergoing hemodialysis. Nutrition. 95, 111542 (2022).
Gonzalez, M. C., Mehrnezhad, A., Razaviarab, N., Barbosa-Silva, T. S. & Heymsfiel, S. B. Calf circumference: Cutoff values from the NHANES 1999–2006. Am. J. Clin. Nutr. 113(6), 1679–1687 (2021).
Lin, Y. L. et al. Impact of Sarcopenia and its diagnostic criteria on hospitalization and mortality in chronic hemodialysis patients: A 3-year longitudinal study. J. Formos. Med. Assoc. 119(7), 1219–1229 (2020).
García-García, B. D., Talluri, C., Lukaski, A., García-Almeida, J. M. & H. C. & Future lines of research on phase angle: Strengths and limitations. Rev. Endocr. Metab. Disord. 24(3), 563–583 (2023).
Norman, K., Herpich, C. & Müller-Werdan, U. Role of phase angle in older adults with focus on the geriatric syndromes Sarcopenia and frailty. Rev. Endocr. Metab. Disord. 24(3), 429–437 (2023).
Shin, J. et al. Phase angle as a marker for muscle health and quality of life in patients with chronic kidney disease. Clin. Nutr. 41(8), 1651–1659 (2022).
Kang, S. H., Do, Y. J. & Kim, J. C. Impedance-derived phase angle is associated with muscle mass, strength, quality of life, and clinical outcomes in maintenance hemodialysis patients. PLoS ONE. 17(1), e0261070 (2022).
Nerbass, F. B., Lima, H. N., Moura-Neto, J. A., Lugon, J. R. & Sesso, R. Brazilian dialysis survey 2022. Braz J. Nephrol. 46(2), 1–8 (2024).
Elm, E. V. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 61(4), 344–349 (2008).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604–612 (2009).
Durnin, J. V. G. A. & Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness measurements on 481 men and women aged 16–72 years. Br. J. Nutr. 32(1), 77–97 (1974).
Frisancho, A. R. New norms of upper limb at and muscle areas for assessment of nutritional status. Am. J. Clin. Nutr. 34(11), 2540–2545 (1981).
Janssen, I., Heymsfield, S. B., Baumgartner, R. N. & Ross, R. Estimation of skeletal muscle mass by biolectrical impedance analysis. J. Appl. Physiol. 89, 465–471 (2000).
Wilkinson, T. J. et al. A systematic review of handgrip strenght measurement in clinical and epidemiological studies of kidney disease: Toward a standardized approach. J. Ren. Nutr. 32(4), 371–381 (2022).
Lim, S. L., Lin, X. H. & Daniels, L. Seven-point subjective global assessment is more time sensitive than conventional subjective global assessment detecting nutrition changes. J. Parenter. Enter. Nutr. 40(7), 966–972 (2015).
Leypoldt, J. K. & Vonesh, E. F. Calculating standard kt/v during hemodialysis based on urea mass removed. Blood Purif. 47(1–3), 62–68 (2019).
Funding
This research was developed with financial support by Health Sciences Teaching and Research Foundation’s [grant numbers 06, 2020] and Research Support Foundation [grant numbers 03, 2023], in Brasília, Federal District, Brazil.
Author information
Authors and Affiliations
Contributions
S.B. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: S.B. and R.C.F. Development of methodology: S.B and R.C.F. Acquisition of data: S.B and R.C.F. Analysis and interpretation of data: S.B. and G.F.B.C. Writing, review, and/or revision of the manuscript: S.B and G.F.B.C. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Borges, S., Fortes, R.C., Ferreira Martins, T. et al. Performance of SARC-F and SARC-CalF scores to predict risk of mortality in hemodialysis patients: a cohort study. Sci Rep 14, 23262 (2024). https://doi.org/10.1038/s41598-024-74412-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74412-6
This article is cited by
-
SARC-F and six modified versions: prognostic role for prolonged hospital stay and 1-year mortality in older inpatients
BMC Geriatrics (2026)
-
Lean tissue mass is associated with adverse outcomes across different stages of chronic kidney disease: a systematic review and meta-analysis
Scientific Reports (2026)