Abstract
In recent years, the incidence of tibial plateau fractures (TPF) has been on the rise. Deep vein thrombosis (DVT) may lead to poor prognosis in patients. The systemic immune-inflammation index(SII) are novel biomarkers of inflammation, and this study aims to verify their predictive effect and construct the nomogram model. This study used binary logistic regression analysis to predict the predictive effect of SII on the occurrence of DVT in tibial plateau fracture patients. And use R studio to construct nomogram model. The results showed that Age (1.03 [1, 1.06], p = 0.032), SII (3.57 [1.68, 7.61], p = 0.04), and NC (7.22 [3.21, 16.26], p < 0.001) were independent predictive factors for DVT. The nomogram demonstrated good predictive performance with small errors in both the training and validation groups, and most clinical patients could benefit from them. The nomogram constructed based on SII can assist clinicians in early assessment of the probability of DVT occurrence.
Similar content being viewed by others
Introduction
Tibial plateau fracture (TPF) is a common intra-articular injury that often leads to gradually worsening chronic pain, limited knee joint mobility, post-traumatic arthritis, and other complications1. Many studies have been conducted to investigate the causes and treatment options for complications associated with tibial plateau fractures. Jin et al.2analyzed a sample of 300 patients who underwent internal plate fixation for tibial plateau fractures in a local hospital. They identified fracture classification, mechanism of injury, osteoporosis status, and time from injury to surgery as independent risk factors for the development of postoperative traumatic arthritis in the knee joint. Forna et al.3 performed a finite element analysis on three different methods of internal fixation for C1.1 (AO-41) tibial plateau fractures. They evaluated the stress distribution on the tibial plateau generated by each fixation method. However, there is limited research on blood markers related to tibial plateau fractures. Blood tests, such as red blood cell count and white blood cell count, are commonly performed upon admission and can provide important guidance for clinical treatment.
Deep vein thrombosis (DVT) is one of the common complications in orthopedic surgery. Severe fractures or high soft tissue injuries can lead to a stronger immune or inflammatory response after trauma4. The formation of deep vein thrombosis is closely related to white blood cells, platelets, and endothelial cells in the blood5. Once a local blood clot is dislodged from the lower extremities, it can cause pulmonary embolism and lead to severe consequences such as sudden death. Studies have shown that the incidence of DVT after trauma is approximately 9.1–11.1%6. In Zhang et al.‘s study7, 404 patients with lower extremity fractures were selected, and 143 patients (35%) were found to have lower extremity deep vein thrombosis during preoperative ultrasound examination. DVT often presents as asymptomatic in the early stages, and most researchers suggest that early diagnosis of DVT and early intervention and treatment can significantly reduce the occurrence of fatal outcomes8. Currently, the diagnosis of DVT in clinical practice mostly relies on vascular color Doppler ultrasound. However, errors in diagnosis can occur due to early limb swelling and insufficient clinical experience. Therefore, DVT requires more intuitive, simple, and early predictive indicators to meet the goals of early prevention and treatment in clinical practice9.
Hemostasis is a protective process in which platelets, white blood cells, and endothelial cells are the main cellular components. They localize the clotting reaction, ensuring the activation of clotting factors in the injured area. Therefore, when there are abnormalities in platelets, white blood cells and other major cellular components, uncontrolled clotting can occur, leading to the formation of pathological blood clots5. In the case of neutrophils, this may be related to the release of neutrophil extracellular traps10. Additionally, neutrophils themselves can influence partial cell apoptosis processes and promote blood clot formation. Currently, many researchers have focused on novel inflammatory factors to better predict the occurrence of deep vein thrombosis11. The systemic immune-inflammation index (SII), as a popular inflammatory factor in recent years, has been shown to predict preoperative deep venous thrombosis in elderly patients with intertrochanteric femoral fractures12. However, there is currently no research specifically indicating the relationship between SII and the occurrence of DVT in patients with tibial plateau fractures. Therefore, this study aims to collect blood routine and biochemical examination results upon patient admission and develop a predictive model for the occurrence of lower limb deep venous thrombosis in patients with tibial plateau fractures based on the SII at admission. This will enable a simple and effective assessment of the preoperative risk of DVT in patients with tibial plateau fractures.
Materials and methods
Patient section
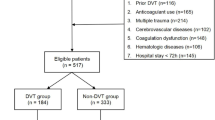
This is a single-center retrospective cohort study. The research question and assessment were predetermined. The study collected data from patients diagnosed with tibial plateau fractures at the First Affiliated Hospital of Guangxi Medical University from January 2017 to January 2023. Inclusion criteria: Patients diagnosed with tibial plateau fractures were diagnosed based on the following: (a) history of knee trauma; (b) clinical manifestations: pain in the affected knee, limited mobility, ecchymosis on the skin, tenderness and percussion pain at the tibial tuberosity of the affected limb, and positive longitudinal percussion pain in the lower limb; (c) imaging examinations: X-ray and CT examinations showed discontinuity of the tibial plateau bone cortex, with or without obvious displacement. Exclusion criteria: (1) Deep venous ultrasound results could not be obtained before surgery; (2) Multiple fractures or pathological fractures were diagnosed; (3) Anticoagulants or antiplatelet drugs were used due to previous diseases; (4) Combined immune system, hematology and other diseases. As this is a retrospective study, the ethics committee approved the study without requiring informed consent from the patients, in accordance with national laws and institutional agreements. In this study, patients’ personal identifying information will be anonymized.
Data collection and definition
This study collected baseline clinical data and laboratory test results including complete blood count, blood biochemistry, and coagulation function. The baseline clinical data included gender, age, affected side, history of hypertension, history of diabetes, history of hepatitis, history of alcohol consumption, and smoking history. History of hypertension was defined as a previous diagnosis of hypertension, history of diabetes was defined as a previous diagnosis of diabetes, and history of hepatitis was defined as a previous diagnosis of hepatitis.
In addition, the researchers obtained laboratory test results from patients at the time of their emergency department visit or initial hospital admission, which included the following information. White blood cell count (WBC), Red blood cell count (RBC), Hemoglobin (HGB), Mean corpuscular hemoglobin concentration (MCHC), Mean corpuscular volume (MCV), Neutrophils (NC), Lymphocyte (LYM), Monocyte (MONO), Eosinophils (EOS), Basophil (BASO), Albumin (ALB), Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Total bilirubin (TBIL), Creatinine (Cr), Blood urea nitrogen (BUN), Prothrombin time (PT), Activated partial thromboplastin time (APTT), Fibrinogen (FIB), D-Dimer and other relevant data. Among these, the inflammatory factor validated in this study is SII=(neutrophil count × platelet count)/lymphocyte count; MLR = Monocyte to lymphocyte ratio; NAR = Neutrophil to albumin ratio; ANRI = Aspartate aminotransferase to neutrophil ratio index.
Outcome
The outcome event of this study is whether DVT occurs before surgery in TPF patients. The swelling will subside 3–4 days after TPF occurs, and medication will be used to reduce the swelling. The indication for surgery is the subsidence of swelling and the appearance of skin wrinkles. Surgery is usually performed 5–7 days after TPF occurs. Before surgery, each patient underwent a comprehensive vascular color Doppler ultrasound examination. The presence of DVT can be determined by comparing positive results (indicating the presence of DVT) with negative results (indicating the absence of DVT).
The characteristics of vascular color Doppler ultrasound in diagnosing DVT are as follows: the inner diameter of the venous lumen at the embolization site is widened, and a relatively uniform substantive low echo or extremely low echo is seen inside, which is completely or incompletely filled in the lumen, the vascular wall is blurred and unclear, and the color blood flow signal cannot be measured at the embolization site13,14. The results will be reviewed and diagnosed by at least two color Doppler doctors.
It should be noted that in clinical work, we will carry out a series of standardized measures to prevent DVT in patients during the perioperative period and after surgery according to the requirements of the guidelines, such as early ankle pump exercise, oral rivaroxaban, pneumatic pump and other preventive measures. Our study is based on the premise of standardized prevention of DVT to predict the occurrence of preoperative DVT.
Statistical analysis
Firstly, this study grouped the data based on the presence or absence of DVT and compared various clinical and laboratory data using SPSS 21.0 (SPSS Inc., Chicago, IL). For continuous data, the Shapiro-Wilk test was used to assess normality. If the data followed a normal distribution, it was presented as mean ± standard deviation, and group comparisons were performed using independent one-way analysis of variance (ANOVA). If the data did not follow a normal distribution, it was presented as median (25th percentile, 75th percentile), and group comparisons were performed using the Kruskal-Wallis test. Categorical data were described using frequencies (percentages), and group comparisons were performed using the chi-square test or Fisher’s exact test. Graphpad Prism 9.5.0 was used to create box plots of the SII in the DVT and non-DVT groups, as well as to plot the ROC curve and calculate the area under the curve. A two-sided p-value less than 0.05 was considered statistically significant.
Secondly, we divide the collected independent variables into two groups according to their mean values. Variables below the mean are defined as group 1, and variables above the mean are defined as group 2. Using SPSS software to calculate the sample size and power, it was concluded that 300 or more patients need to be included to make the results more reliable15. We then performed univariate logistic regression analysis, and included the clinically significant factors found in the univariate analysis into multivariate logistic regression analysis to calculate independent predictive factors for DVT.
Finally, we used SPSS to randomly divide the data into a training set and a validation set in a 7:3 ratio. Based on the results of the multivariate logistic regression analysis, we used R Studio (version 4.2.2) to create a nomogram for the independent predictive factors. The predictive performance of the model was evaluated using the ROC curve and the area under the curve (AUC). The model’s average error was calculated using a calibration plot, and the clinical utility of the model was analyzed using a decision curve analysis (DCA) plot.
Result
Clinical factors and predictive relationship of DVT
A total of 370 patients were collected for this study, with 60 patients excluded due to the aforementioned factors. Ultimately, a retrospective study was conducted with 310 patients included, as shown in Fig. 1.
The data was grouped based on the presence or absence of DVT, with 224 patients in the DVT group and 86 patients in the No DVT group. The results showed that in the DVT group, there were 31 females (36.05%) and 55 males (63.95%). The number of patients with a history of hypertension, diabetes, hepatitis, smoking, and alcohol consumption were 9 (12.16%), 6 (8.11%), 2 (2.7%), 21 (28.38%), and 7 (9.46%) respectively. There were no statistically significant differences between the groups (p > 0.05), indicating comparability of the data. However, there were statistically significant differences between the groups in terms of red blood cell count, white blood cell count, absolute neutrophil count, lymphocyte count, monocyte count, eosinophil count, basophil count, prothrombin time, albumin, ALT, MLR, NAR, ANRI, and SII (p < 0.05) (Table 1). Subsequently, a box plot was created to visually display the distribution differences of SII between the two groups (Fig. 2A). Additionally, an ROC curve was plotted to assess the predictive ability of SII for DVT, calculating the optimal cutoff value, sensitivity, and specificity (Fig. 2B). The results showed that the optimal cutoff value for SII was 1328.172, with a sensitivity of 0.709 and specificity of 0.772.
The clinical data were dichotomized using the mean value and included in univariate binary logistic regression analysis. The results showed that Age (0.64 [0.38, 1.07]), WBC (7.25 [4.106, 12.81]), NC (10.32 [5.68, 18.77]), LYM (0.38 [0.22, 0.65]), MONO (1.96 [1.18, 3.25]), EOS (0.56 [0.32, 0.98]), BASO (0.50 [0.29, 0.85]), MLR (3.76 [2.24, 6.33]), NAR (6.95 [3.94, 12.27]), ANRI (0.32 [0.16, 0.64]), and SII (7.02 [4.06, 12.14]) were all risk factors for DVT (Table 2). The significant factors (p < 0.1) from the univariate analysis were included in the multivariate binary logistic regression analysis. The results showed that Age (1.03 [1, 1.06], p = 0.032), SII (3.57 [1.68, 7.61], p = 0.04), and NC (7.22 [3.21, 16.26], p < 0.001) were independent predictive factors (Table 3).
Construction and evaluation of nomogram
To further validate the predictive ability of various factors on tibial plateau fractures, a nomogram model was constructed using R Studio software based on the results of the multifactor analysis (Age, SII, NC) in American English style. The collected data was randomly sampled in a 7:3 ratio and divided into a training group and a validation group. The baseline data of the grouped training group and validation group were compared between groups, as shown in Table 4.The training group data was used to construct a nomogram, as shown in Fig. 3, and the ROC curve was plotted to validate the predictive performance of the nomogram graph in the training group (Fig. 4A), with C-index of 0.904, indicating good predictive ability. The calibration curve for the training group (Fig. 5A) showed an average error of 0.021. The decision curve analysis (DCA) for the training group (Fig. 6A) indicated good clinical benefits within the threshold range of 0.01–0.99. The validation group data was used for validation, and the ROC curve for the validation group (Fig. 4B) yielded a C-index of 0.775. The calibration curve (Fig. 5B) showed an average error of 0.033, and the DCA (Fig. 6B) indicated good clinical benefits within the threshold range of 0.06–0.99. It is evident that the nomogram graph exhibited good predictive performance with minimal error in both the training and validation groups, benefiting the majority of clinical patients.
Discussion
This study is based on the results of blood tests conducted on 310 patients with tibial plateau fractures who were admitted to our hospital. It was found that there were statistically significant differences in inflammatory markers such as NC, LYM, WBC, and SII among the 86 patients with perioperative DVT. Through univariate and multivariate analyses, age, SII, and NC were identified as independent predictive factors for the occurrence of DVT in patients with preoperative tibial plateau fractures. Tibial plateau fractures (TPF) are common and severe injuries following high-impact trauma, occurring not only in young patients but also in elderly patients with osteoporosis16. DVT is a common complication during the perioperative and postoperative period, especially in elderly patients, and it has a significant impact on postoperative recovery. The study found that local embolism in the lower limb vessels can lead to discomfort, swelling, and gait disturbances, and if it progresses, it can cause pulmonary embolism and ultimately result in death17. Literature has shown that the incidence of DVT in hip fracture patients without prophylaxis can reach 46-75%, highlighting the importance of early diagnosis and treatment of DVT18.
Current research suggests that the three main factors leading to DVT are static blood flow, venous wall damage, and hypercoagulable state19. TPFs often result in adjacent deep vein vessel damage, which can lead to vascular wall injury and the production of vascular inflammatory reactions20. Neutrophils, as one of the important factors involved in the inflammatory response, can be rapidly activated in the early stages of trauma, exacerbating endothelial tissue damage and accelerating thrombus formation21. Several days after the injury, inflammatory cells (neutrophils, lymphocytes, and monocytes) can infiltrate the edges of the thrombus, further triggering inflammatory reactions22, exacerbating thrombus formation and leading to a vicious cycle. In recent years, there has been an increasing amount of research on the predictive role of inflammatory factors in DVT events. SII, as a novel inflammatory marker reflecting the inflammatory status, has been widely used in various diseases23,24,25. In this study, by constructing a nomogram model for independent risk factors, the predictive role of SII was fully validated, enabling early prediction of DVT occurrence and early thrombus intervention. The study results also demonstrated that the model had good predictive efficacy, with a small average error and significant clinical benefits. It can assist clinicians in making relevant predictions and judgments upon patient admission, thereby implementing preventive measures.
This study has several strengths: (1) It is a retrospective study that involved the construction of a model using a training set and validation through a separate validation set, ensuring the reliability and ease of application of the model. (2) Routine blood tests and biochemical examinations are typically conducted upon patient admission. This study aimed to collect relevant data at the time of admission to achieve early predictive capabilities and benefit a larger number of patients. However, there are also some limitations to this study: (1) Postoperative and long-term follow-up rates of patients were not continued. (2) The data center and sample size need further expansion. (3)We did not collect CT and CTA data for the patients we included, so we were unable to assess their vascular damage, vascular plaques, etc., which may have a certain impact on our results.
Conclusion
Age, SII, and NC are independent predictive factors for DVT in patients with tibial plateau fractures upon admission. The nomogram constructed based on SII can assist clinical doctors in early assessment of the probability of DVT occurrence, enabling early prevention and treatment of DVT, benefiting a larger number of patients.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- TPF:
-
Tibial plateau fracture
- DVT:
-
Deep vein thrombosis
- BMI:
-
Body mass index
- SII:
-
Systemic immune-inflammation index
- MLR:
-
Monocyte to lymphocyte ratio
- NAR:
-
Neutrophil to albumin ratio
- ANRI:
-
Aspartate aminotransferase to neutrophils ratio index
- WBC:
-
White blood cell count
- RBC:
-
Red blood cell count
- HGB:
-
Hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- NC:
-
Neutrophils
- LYM:
-
Lymphocyte
- MONO:
-
Monocyte
- PLT:
-
Platelet
- EOS:
-
Eosinophils
- BASO:
-
Basophil
- PCT:
-
Platelet hematocrit
- ALB:
-
Albumin
- GGT:
-
Glutamyl transpeptidase
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- TBIL:
-
Total bilirubin
- PT:
-
Prothrombin time
- APTT:
-
Activated partial thrombin time
- FIB:
-
Fibrinogen
- Cr:
-
Creatinine
- BUN:
-
Blood urea nitrogen
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- AUC:
-
The area under the curve
- DCA:
-
Decision curve analysis
- ROC:
-
Receiver operating characteristic
References
Mattiassich, G. et al. Radiographic and clinical results after surgically treated tibial plateau fractures at three and twenty two years postsurgery. Int. Orthop. 38, 587–594. https://doi.org/10.1007/s00264-013-2174-0 (2014).
Jin, X., Li, D., Yang, L., Han, F. & Jia, P. Analysis of risk factors and surgical strategy of knee traumatic arthritis after internal plate fixation in the treatment of tibial plateau fracture. Comput. Math. Methods Med. 2022, 9146227. https://doi.org/10.1155/2022/9146227 (2022).
Forna, N. et al. Treatment of C1.1 (AO-41) tibial plateau fracture: A finite element analysis of single medial, lateral and dual plating. Exp. Ther. Med. 23, 198. https://doi.org/10.3892/etm.2022.11121 (2022).
Sun, T., Wang, X., Liu, Z., Chen, X. & Zhang, J. Plasma concentrations of pro- and anti-inflammatory cytokines and outcome prediction in elderly hip fracture patients. Injury. 42, 707–713. https://doi.org/10.1016/j.injury.2011.01.010 (2011).
Colling, M. E., Tourdot, B. E. & Kanthi, Y. Inflammation, infection and venous thromboembolism. Circ. Res. 128, 2017–2036. https://doi.org/10.1161/circresaha.121.318225 (2021).
Shin, W. C. et al. Preoperative prevalence of and risk factors for venous thromboembolism in patients with a hip fracture: An Indirect Multidetector CT Venography Study. J. Bone Joint Surg. Am. 98, 2089–2095. https://doi.org/10.2106/jbjs.15.01329 (2016).
Zhang, B. F. et al. Perioperative deep vein thrombosis in patients with lower extremity fractures: An observational study. Clin. Appl. Thromb. Hemost. 26, 1076029620930272. https://doi.org/10.1177/1076029620930272 (2020).
Wendelboe, A. M. & Raskob, G. E. Global burden of thrombosis: epidemiologic aspects. Circ. Res. 118, 1340–1347. https://doi.org/10.1161/circresaha.115.306841 (2016).
Wang, J. et al. Current challenges in the prevention and management of post-thrombotic syndrome-towards improved prevention. Int. J. Hematol. 118, 547–567. https://doi.org/10.1007/s12185-023-03651-6 (2023).
Mussbacher, M. et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front. Immunol. 10, 85. https://doi.org/10.3389/fimmu.2019.00085 (2019).
Gromadziński, L. et al. Transcriptomic profiling of femoral veins in deep vein thrombosis in a Porcine Model. Cells. 10, https://doi.org/10.3390/cells10071576 (2021).
Zeng, G. et al. A nomogram model based on the combination of the systemic immune-inflammation index, body mass index, and neutrophil/lymphocyte ratio to predict the risk of preoperative deep venous thrombosis in elderly patients with intertrochanteric femoral fracture: a retrospective cohort study. J. Orthop. Surg. Res. 18, 561. https://doi.org/10.1186/s13018-023-03966-4 (2023).
Bernardi, E. & Camporese, G. Diagnosis of deep-vein thrombosis. Thromb. Res. 163, 201–206. https://doi.org/10.1016/j.thromres.2017.10.006 (2018).
Di Nisio, M., van Es, N. & Büller, H. R. Deep vein thrombosis and pulmonary embolism. Lancet. 388(10063), 3060–3073. https://doi.org/10.1016/S0140-6736(16)30514-1 (2016).
Schmidt, S. A. J., Lo, S. & Hollestein, L. M. Research Techniques made simple: Sample size estimation and power calculation. J. Invest. Dermatol. 138(8), 1678–1682. https://doi.org/10.1016/j.jid.2018.06.165 (2018).
Kerschbaum, M. et al. The Tibial Plateau Map: Fracture line morphology of intra-articular proximal tibial fractures. Biomed. Res. Int. 2021, 9920189. https://doi.org/10.1155/2021/9920189 (2021).
Kim, K. A., Choi, S. Y. & Kim, R. Endovascular treatment for lower extremity deep vein thrombosis: An overview. Korean J. Radiol. 22, 931–943. https://doi.org/10.3348/kjr.2020.0675 (2021).
Agnelli, G. et al. A randomised, double-blind, placebo-controlled trial of Dermatan sulphate for prevention of deep vein thrombosis in hip fracture. Thromb. Haemost. 67, 203–208 (1992).
Borgel, D., Bianchini, E., Lasne, D., Pascreau, T. & Saller, F. Inflammation in deep vein thrombosis: A therapeutic target? Hematology 24, 742–750. https://doi.org/10.1080/16078454.2019.1687144 (2019).
Bortolotti, P., Faure, E. & Kipnis, E. Inflammasomes in tissue damages and immune disorders after trauma. Front. Immunol. 9, 1900. https://doi.org/10.3389/fimmu.2018.01900 (2018).
Granelli-Piperno, A., Vassalli, J. D. & Reich, E. Secretion of plasminogen activator by human polymorphonuclear leukocytes. Modulation by glucocorticoids and other effectors. J. Exp. Med. 146, 1693–1706. https://doi.org/10.1084/jem.146.6.1693 (1977).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689. https://doi.org/10.1038/nri2156 (2007).
Wang, R. H. et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front. Immunol. 14, 1115031. https://doi.org/10.3389/fimmu.2023.1115031 (2023).
Huang, H. et al. Prognostic value of preoperative systemic Immune-inflammation index in patients with cervical Cancer. Sci. Rep. 9, 3284. https://doi.org/10.1038/s41598-019-39150-0 (2019).
Wang, P. X. et al. A nomogram combining plasma fibrinogen and systemic immune–inflammation index predicts survival in patients with resectable gastric cancer. Sci. Rep. 11, 10301. https://doi.org/10.1038/s41598-021-89648-9 (2021).
Acknowledgements
Not applicable.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Z.H. and W.S. designed this study. H.L. and W.C.L. are responsible for writing articles, conducting statistical analysis, reviewing articles, and creating images. G.Y.D. and Y.H.L. and R.B.L. are responsible for collecting data and conducting statistical analysis.All authors reviewed the manuscript.All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the First Affiliated Hospital of Guangxi Medical University Ethics Review Committee (2023-E583-01).
As this article is a retrospective study, the clinical data obtained in the study are all from case data and test reports. Therefore, with the approval of the First Affiliated Hospital of Guangxi Medical University Ethics Review Committee, the requirement for informed consent was waived.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ling, H., Li, W., Deng, G. et al. Development of a nomogram for deep vein thrombosis in patients with tibial plateau fractures based on systemic Immune-inflammation index. Sci Rep 14, 23888 (2024). https://doi.org/10.1038/s41598-024-74644-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74644-6