Abstract
Aralia elata is closely related to Panax ginseng and contains high levels of saponins and other medicinal compounds. Successful A. elata micropropagation is commercially significant; however, the genomic stability of tissue culture-derived regenerants is unclear. In this study, callus-derived regenerated A. elata plants were obtained, and their cytogenomic constitutions were assessed. Using RepeatExplorer, pre-labeled oligonucleotide probes (PLOPs) were developed with newly mined tandem repeats from < 1× NGS whole-genome short reads, fluorescence in situ hybridization (FISH) was performed using six repeat probes, including three universal PLOPs, and genomic DNA content was estimated using flow cytometry. Regenerated A. elata plants (50) exhibited consistent ploidy, repeat distribution, and genome sizes compared with those exhibited by the mother plant. Six repeat probes were detected using FISH. Tandem repeat AeTR49 was identified as an excellent cytogenetic marker for homologous chromosomes, and AeTR161 and AeTR178 were localized in the centromeric and telomeric sections, respectively. Genomic DNA content (2C) was estimated at 2.46 ± 0.04 pg in the mother plant and 2.41 ± 0.05 pg in regenerated plants, with no significant variations in genome size or chromosome length. These results demonstrate that cytogenomics can be used to effectively evaluate chromosome-level genomic stability in regenerated A. elata plants.
Similar content being viewed by others
Introduction
Aralia elata (Miq.) Seem. is a deciduous perennial shrub in the Araliaceae (ginseng) family that is primarily found in Northeast Asian countries, such as Japan, Korea, and China1,2. A. elata has been used for centuries as a medicinal and edible plant because of its abundance of valuable active secondary metabolites, including triterpenes, diterpenes, flavonoids, coumarins, phenols, and protopectins3. Aralia saponins, also known as aralosides, are fundamental constituents of A. elata responsible for its medicinal properties4. Saponins isolated from the roots and bark of A. elata have been used in traditional folk medicine to treat neurodegenerative diseases, arthritis, and diabetes3,5,6. Furthermore, the shoots are widely regarded for their culinary appeal and nutritional benefits, and they are increasingly popular in micropropagation research for this species7,8,9,10.
Micropropagation is extensively used to meet the growing demand for fresh and processed A. elata products2,3. This technique is useful because it facilitates rapid multiplication of selected plants using in vitro culture techniques11,12. Tissue culture-derived micropropagation is more effective than natural and conventional propagation approaches such as seedlings, cuttings, layering, and grafting1,13. It has already been used to regenerate medicinal plants, orchids, cereals, vegetables, root crops, and trees14. Successful micropropagation of A. elata using the winter buds of 10-year-old trees9, leaflet petioles7, hairy roots8, cell suspensions1, leaves, shoots, stems, and root segments5, and mature embryos10 has been exploited for commercial purposes. However, the genomic stability of tissue culture-derived A. elata regenerants has not yet been evaluated.
Cytogenomic information such as chromosomal composition, karyotype, repeat distribution, and genome size is essential for investigating the genomic stability of micropropagated plants15,16,17. Fluorescence in situ hybridization (FISH) is a promising technique that can be used to physically observe the distribution of specific DNA segments and/or genome rearrangements in regenerated plants18,19,20. In addition, estimating genomic DNA content using flow cytometry is effective for screening ploidy, including mixoploidy and aneuploidy21,22.
In this study, chromosome composition, genomic repeat distribution, and genome size were analyzed in 50 tissue culture-derived regenerated A. elata plants. This is the first cytogenomic report on tissue culture-derived A. elata regenerants and provides helpful information for further cytogenomic research and breeding programs involving the ginseng family.
Methods
Plant materials
A. elata plants were provided by the Herbal Garden at Seoul National University, Korea, and were grown in a greenhouse at Sahmyook University to harvest fresh leaves. Leaf explants were washed with a detergent solution for 5 min and rinsed under running tap water for 10 min23. To ensure sterilization, the explants were treated with 70% (v/v) ethanol and 2% (v/v) sodium hypochlorite supplemented with two drops of Tween-20 for 1 and 15 min, respectively. Finally, the explants were rinsed meticulously with autoclaved distilled water five times5,24.
Tissue culture
Callus induction was performed on leaf explants cut into 2–10mm sections 23. The culture medium and incubation conditions were as previously described5,24,25. To initiate plant regeneration, callus sections were transferred to MS medium5 supplemented with 2.9 µM gibberellic acid (GA), 3% (w/v) sucrose, and 0.7% (w/v) plant agar to encourage shoot development. Once tiny shoots emerged, root induction was performed in another MS medium supplemented with 1.2 µM IBA, 2% (w/v) sucrose, and 0.2% (w/v) gelrite. These cultures were maintained at 25°C8,13 with a photoperiod of 16/8 h light/dark during shoot and root development5.
Plantlets were acclimatized in a local greenhouse for optimal adaptation to the natural environment. Plantlets (approximately 5–8 cm tall) were transplanted into 128-cell seedling trays filled with coco peat and moistened with water. The relative humidity of the greenhouse ranged from 50 to 70%, and the light intensity reached 130,000 lx. These environmental conditions were gradually introduced to the young regenerants until they were fully acclimated. Subsequently, the plants were placed in larger pots and watered every alternate day to ensure healthy growth5,13.
Repeat mining and probe preparation
The < 1× NGS whole-genome sequences of A. elata (NN-0919-000001) were obtained from the National Agricultural Biotechnology Information Center (NABIC) (https://nabic.rda.go.kr/). Subsequently, read quality trimming, sampling, and read clustering were performed using the Tandem Repeat Analyzer (TAREAN) workflow in the RepeatExplorer tool26. To identify genome-specific repeats, three tandem repeat sequences (AeTR49, AeTR63, and AeTR162) were selected to prepare probes.
A pre-labeled oligonucleotide probe (PLOP) labeled with Texas Red at the 5ʹ-end was designed exclusively for the 49-bp AeTR49 minisatellite to facilitate FISH analysis and procured from Bioneer Corporation, South Korea (https://www.bioneer.co.kr/). For the AeTR161 and AeTR178 minisatellites, reverse and forward primers were designed using Primer3Plus (http://www.primer3plus.com/cgi-bin/dev/primer3plus.cgi), and subsequent PCR amplification was conducted as previously described27. The PCR amplicons of both repeats were labeled using a nick-translation labeling method with digoxigenin-11-dUTP and biotin-16-dUTP (Roche, Germany) as FISH probes. DNA amplicons (approximately 2 µg) for AeTR161 and AeTR178 were separately labeled with biotin-16-dUTP and digoxigenin-11-dUTP, respectively (Table 1). The labeling mixtures were incubated at 15.6℃ for 3 h and this was then terminated by adding 0.5 M ethylenediaminetetraacetic acid (EDTA) and incubating for 10 min.
PLOPs targeting 5S rDNA, 45S rDNA, and Arabidopsis-type telomeric repeats have previously been synthesized and labeled with FAM, Cy3, and Texas Red, respectively, at the 5ʹ-end18.
Slide preparation
Chromosome spreads were prepared according to established protocols18,28,29. Root meristems were collected from 50 of the regenerated plants cultivated in the greenhouse. The roots were preliminarily treated with nitrous oxide at 0.5–0.6 MPa for 3 h. They were then fixed in an aceto-ethanol solution (1:3 v/v) overnight at room temperature (RT), transferred to a 70% (v/v) ethanol solution, and stored at 4°C. The meristematic root tips were carefully dissected and subjected to enzymatic digestion using 1% (w/v) pectolyase and 2% (w/v) cellulase for 45 min at 37°C. Following the enzymatic treatment, the hydrolyzed root tips were immersed in acetoethanol (1:3, v/v) and resuspended in a solution containing 9:1 (v/v) acetic acid and ethanol. The suspension (approximately 20 µL) was pipetted onto pre-warmed slides and air dried. To further process the chromosomes, glass slides were fixed in 2% (w/v) formaldehyde for 5 min and dehydrated in an ethanol series (70, 90, and 100%, v/v) for 3 min each before air drying.
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) using PLOPs based on tandem repeats was performed as previously described18,20,30. Briefly, a hybridization mixture (40 µL) was prepared and applied to the prepared slides, followed by denaturation at 80°C for 5 min and overnight hybridization at 37°C in a humid chamber. Subsequently, slides were washed sequentially in 2× SSC at RT for 10 min, 0.1× SSC at 42°C for 25 min, and again in 2× SSC at RT for 5 min, followed by dehydration in an ascending ethanol series (70, 90, and 100%, v/v) at RT for 3 min each, and then air-dried. Post air drying, chromosomes were counterstained with a 1:100 dilution of 4ʹ,6-diamidino-2-phenylindole (DAPI) (from a stock concentration of 100 µg mL− 1) in Vectashield (Vector H1000, Vector Labs, USA).
To detect nick-translated labeled minisatellite DNA, chromosomes were washed and reprobed according to a previously reported protocol27. For the hapten-labeled probes, an additional probe detection step was performed. After hybridization and stringent washes with 2× SSC, slides were treated with a blocking buffer (0.1 M Tris-HCl, 0.15 M NaCl, 1% (w/v) blocking reagent) for 5 min at RT. Subsequently, digoxigenin- and biotin-labeled transposable elements were detected using anti-digoxigenin FITC conjugate (Sigma) and streptavidin-Cy3 conjugate (Zymed, USA), respectively. Slides were then washed three times with TNT (0.1 M Tris-HCl, 0.15 M NaCl, 0.2% (v/v) Tween-20) at 37°C for 5 min each, dehydrated with an ethanol series, air-dried, and counterstained as described above.
Chromosomal images were captured using an Olympus BX53 fluorescence microscope (Olympus, Tokyo, Japan) equipped with a Leica DFC365 FS CCD camera (Leica Microsystems Inc., Germany) and analyzed using Cytovision ver. 7.2 (Leica Microsystems, Wetzlar, Germany). Further image contrast and level adjustments were performed using Adobe Photoshop CS6 (Adobe Inc., California, USA). Chromosome length was measured using ImageJ software (Wayne Rasband, Maryland, USA) and statistically analyzed using GraphPad Prism v7 (GraphPad Software, La Jolla, CA, USA). Homologous pairings and arrangements were determined based on chromosome length, satellite presence, and FISH signals, as described previously31,32.
Flow cytometry
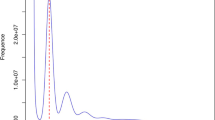
Dendropanax morbifera was used as the internal reference standard to estimate nuclear DNA content, with a known genome size of 2C = 4.09 pg DNA33. The genomic DNA content of the mother plant and tissue culture-derived regenerants was measured to assess variations in genome size. Young leaves of the reference standard and regenerated plants were simultaneously chopped in a glass Petri dish with LB01 buffer (500 µL)22,34. Nuclear suspensions were sequentially filtered through 50 and 30 μm nylon mesh into a 1.5 mL microtube on ice. Propidium iodide (PI; 50 µg mL− 1) was added to stain the nuclear DNA, and RNAse (10 µg mL− 1 ) was used to degrade RNA contamination34. A CytoFLEX BC04039 flow cytometer (Beckman Coulter Inc. USA) was used to measure the PI fluorescence of the stained nuclei (Fig. 3). More than 10,000 nuclei were counted for each measurement and analyzed using the built-in CytExpert software (Beckman Coulter, CA, USA). The sample 2C DNA content was estimated using standard computations21. The process was replicated three times on different days to obtain averages and standard deviations.
Results
Regeneration of the plants
Regenerated plants were successfully derived from callus tissue. Callus induction was observed after 4 weeks of inoculation, with the appearance of white-to-pale-yellow friable calli in the explants (Fig. 1A). Within 9 weeks, calli fragments were suspended in liquid MS medium to develop somatic embryos (Fig. 1B). After 8 weeks of culture, the calli generated globular-to-cotyledonary embryos (Fig. 1C). Two weeks later, complete plantlets with fully formed organs were observed (Fig. 1D) and transplanted into pots in a greenhouse. Fifty regenerated plants were obtained and used for cytogenomic analysis (Fig. 1E).
Novel repeats and probe development
Repetitive DNA elements in A. elata were analyzed to identify suitable FISH markers for karyotyping. RepeatExplorer analysis estimated that the repeats accounted for 52% of the A. elata genome. Three satellite repeats were identified (AeTR49, AeTR161, and AeTR178), showing typical satellite clustering graphs (Table 1 and Supplementary Fig. S1). The consensus sequences of AeTR49, AeTR161, and AeTR178 were 49, 161, and 178 bp long, representing 0.09, 0.33, and 0.05% of the A. elata genome, respectively.
Chromosome constitution
All 50 regenerated A. elata plants exhibited diploid 2n = 24 chromosome complements, demonstrating uniformity with no variation in chromosome number (Supplementary Fig. 1). Both the mother plant and regenerants exhibited consistent chromosomal constitutions and FISH karyotypes. The chromosome lengths of the mother plant ranged from 2.93 ± 0.50 to 4.79 ± 0.72 μm, and the short and long arms had average lengths of 1.77 ± 0.30 μm and 2.20 ± 0.39 μm, respectively (Table 2). The chromosome lengths of the regenerants ranged from 2.58 ± 0.39 to 4.45 ± 0.75 μm, and the short and long arms had average lengths of 1.58 ± 0.32 μm and 2.00 ± 0.41 μm, respectively (Table 3). No significant variation in chromosome length was observed. The mother plant and regenerants both had 11 pairs of metacentric chromosomes and one pair of submetacentric chromosomes. The total chromosome length (TCL) of the 50 regenerants varied slightly from that of the mother plant but not significantly (Supplementary Fig. 2).
Fluorescence in situ hybridization analysis
Multicolor FISH karyograms of both the A. elata mother plant and its regenerants were analyzed using six repeat probes: three newly developed AeTR49 (orange), AeTR161 (yellow), and AeTR178 (purple), and three additional universal probes for 5 S rDNA (green), 45 S rDNA (red), and telomeric repeats (blue) (Fig. 2). FISH probes exhibited clear signals within the chromosomes of the mother plant (Fig. 2A, B) and its regenerants (Fig. 2C, D). The FISH karyotypic idiogram in Fig. 2E illustrates the distribution of the repeat probes.
Distribution of repeat markers in Aralia elata mother plant and the regenerants. Respective fluorescence in situ hybridization (FISH) metaphase spread and karyogram of A. elata mother plant (A,B) and its regenerants (C,D) elucidate the distribution of the repeat marker in the chromosome complement of 2n = 24. The three repeat probes of 5S (green) and 45S (red) rDNAs, telomeric repeat (blue), and three genome-specific repeat sequences of AeTR49 (orange), AeTR161 (yellow), and AeTR178 (purple) were revealed as cytogenetic markers for chromosome pairing and identification. Chromosome pairing and identification were based on the distribution of the repeats, and they were arranged in decreasing length. (E) The ideogram illustrates the detailed distribution of the six repeats. The telomeric probe was illustrated equally regardless of the signal intensity. Scale bar = 10 μm.
All plants exhibited distinct 45S rDNA signals in the distal region of the short arm of chromosome 3 and 5S rDNA signals in the intercalary region of chromosome 1. Telomeric signals were present across all chromosome ends. AeTR49 was consistently detected along the intercalary regions of both the short and long arms of chromosomes, except in chromosomes 2 and 7, where the signals were restricted to the short arms. In contrast, AeTR161 was predominantly located at the centromeric regions, whereas AeTR178 was co-localized with Arabidopsis-type telomeric repeats at all chromosome ends (Fig. 2B,D; Tables 2 and 3).
AeTR49 exhibited chromosome-specific distribution and was identified as an excellent cytogenetic marker for each chromosome. In contrast, AeTR161 and AeTR178 were distributed throughout the genome as centromeric and telomeric repeats, respectively. FISH signal distribution using the six repeat probes confirmed the chromosome-level constitution of A. elata and its regenerated plants (Supplementary Fig. 3).
The FISH signal distribution patterns of the mother plant and all regenerants were consistent, and no chromosome-level variations were detected. The genomic stability of the regenerated plants was confirmed, at least for specific sequence-related chromosome constitutions.
Estimation of nuclear DNA content
A histogram of the relative 2C DNA content was obtained via simultaneous analysis of D. morbifera as an internal reference and individual A. elata plants. The G0/G1 peaks of the reference standard were located in channel 200 (Fig. 3). Simultaneous flow cytometry analyses of the mother and each selected regenerated plant revealed minor variations in DNA content, with 2C values of 2.46 ± 0.04 and 2.41 ± 0.05 pg, respectively. Regenerated plants had lower relative 2C nuclear DNA content than native plants. However, according to Tukey’s multiple comparison test, this variation was not statistically significant at the 95% (Table 4). In addition, the genome sizes of the regenerants were not correlated with the observed variation in their TCL (Supplementary Fig. S3).
Discussion
Micropropagation can enhance the regeneration frequency and survival rates of plants, and well-developed regenerated A. elata plants have been documented1,5,10,35. However, cytogenomic information such as ploidy, chromosomal constitution, specific sequence distribution on the chromosome, and genomic DNA content, which are useful for confirming the genomic stability of micropropagated plants, have not yet been reported for regenerated A. elata plants. This multistep regeneration process, which involves somatic embryo formation and maturation, can induce somaclonal variations, leading to genomic and epigenomic reprogramming1,14,36. Therefore, evaluation of the cytogenomic constitutions of regenerated plants is imperative because variations in ploidy, chromosome constitution, and genome size can significantly influence the phenotypic characteristics and overall quality of regenerated plants. Furthermore, cytogenomic information is crucial to genomic research and breeding programs for crop improvement37,38.
Flow cytometry and FISH have emerged as valuable tools for evaluating cytogenetic stability, as demonstrated in various studies on different plant species17,39. Specifically, PLOP-FISH enables comprehensive physical mapping, facilitating the monitoring of genomic changes and discrimination of homologous chromosomes18,20,28,32,33,40. FISH results for the 5S and 45S rDNA probes in this study were consistent with those of a previous report32. However, clear telomeric repeat signals were observed at the ends of the short and long arms of chromosomes 1 and 3, respectively, which differed from those previously reported (Fig. 2). Considering that the chromosome end sequence is essential for telomere formation across all chromosomes, the results of the FISH study appear to be more accurate. This discrepancy might be due to the metaphase chromosome quality, telomeric probe concentration, and choice of the labeled fluorochrome ATTO425, which has lower emission and excitation wavelengths than Texas Red, which was used in our experiment41.
FISH results using universal repeat probes of 5S and 45S rDNAs can provide useful information for identifying and pairing homologous chromosomes during karyotyping28,31 and telomeric repeats when investigating genomic differentiation in plant species18,40. In addition, three more tandem repeat probes were developed in this study, which appeared to be excellent chromosome-specific repeat markers that could be used to discriminate homologous chromosome pairs in the A. elata genome and to examine the chromosome-level genomic constitution of regenerated plants. A pre-labeled oligonucleotide probe was developed from the newly mined tandem repeat AeTR49A, but not from the tandem repeats of AeTR161 and AeTR178 because these two repeats consist of long repeat sequences; thus, PLOP development is impractical15. These newly developed repeat markers are expected to be useful in broader genomic research and breeding programs36,42.
The correlation between genome size (GS) and total chromosome length (TCL) has been studied in various plant species. In particular, this correlation has been highlighted in Triticum and rye species37, whereas similar observations have been made in Bromeliaceae38 and Fabaceae16. However, this study found no significant correlation between GS and TCL. The relationship between GS and TCL may be affected by different pretreatment agents, treatment durations, and chromosome stages43. Further research involving different plant taxa is required to clarify the correlation between GS and TCL.
Given the challenges associated with maintaining the integrity of genetic material during in vitro propagation, achieving genetic stability in tissue cultures represents a significant milestone in plant biotechnology11,44. The unpredictable nature of genetic variation among plant species presents a formidable obstacle to the reliable production of genetically stable regenerants. Numerous factors contribute to these variations, from the initial choice of culture method to the complexity of plant genetics, including culture period, explant type, and ploidy35,36,42. Therefore, developing a customized micropropagation protocol and regularly evaluating the genetic stability of regenerants is essential.
This study presents cytogenomic tools for evaluating the chromosome-associated genetic stability of regenerated A. elata plants, which will aid in the development of breeding strategies for crop improvement.
Data availability
The authors confirm that all data supporting the findings of this study are available in the article and supplementary material.
References
Karim, M. Z. et al. Efficient adventitious shoot regeneration from root explants of Aralia elata Seem. Int. J. Bot. 3, 390–393. https://doi.org/10.3923/ijb.2007.390.393 (2007).
Hong, J. & Gruda, N. S. The potential of introduction of Asian vegetables in Europe. Horticulturae. 6, 38. https://doi.org/10.3390/horticulturae6030038 (2020).
Zhang, Y. et al. Triterpene saponins with neuroprotective effects from a wild vegetable Aralia elata. J. Funct. Foods 45, 313–320. https://doi.org/10.1016/j.jff.2018.04.026 (2018).
Luo, Y., Dong, X., Yu, Y., Sun, G. & Sun, X. Total aralosides of Aralia elata (miq) seem (TASAES) ameliorate nonalcoholic steatohepatitis by modulating IRE1α-mediated JNK and NF-κB pathways in ApoE–/–mice. J. Ethnopharmacol. 163, 241–250 (2015).
Dai, J-L. et al. Rapid and repetitive plant regeneration of Aralia elata Seem. Via somatic embryogenesis. Plant. Cell. Tissue Organ. Cult. PCTOC. 104, 125–130. https://doi.org/10.1007/s11240-010-9801-x (2011).
Wei, H., Zhao, H., Chen, X. & He, X. Secondary metabolites, carbohydrate accumulation, and nutrient uptake in Aralia elata (Miq.) Seem seedlings exposed to shoot cutting and different LED spectra. Acta Physiol. Plant. 42, 1–15. https://doi.org/10.1007/s11738-020-03149-2 (2020).
Amemiya, K. & Mochizuki, T. Somatic embryo formation and plant regeneration in ‘Zaoh’line 2 of Japanese angelica tree (Aralia elata Seem). Plant. Biotechnol. 19, 383–387. https://doi.org/10.5511/plantbiotechnology.19.383 (2002).
Kang, H. J. et al. Production of transgenic Aralia elata regenerated from Agrobacterium rhizogenes-mediated transformed roots. Plant. Cell. Tissue Organ. Cult. 85, 187–196. https://doi.org/10.1007/s11240-005-9070-2 (2006).
Moon, H. K., Yi Jae Seon, Yi & Jae Seon. Somatic embryogenesis, plant regeneration, and field establishment from tissue culture of winter buds of 10-year-old Aralia elata. J. Korean Soc. Sci. 87, 57–61 (1998).
Zhao, X., Sun, F., Yin, J. & You, X. Induction of secondary somatic embryogenesis of Aralia elata (miq.) Seem. And anti-oxidative enzymes activities under high temperature stress. J. Northeast Univ. 41, 99–102 (2013).
George, E. F., Hall, M. A. & Klerk, G-J-D. Micropropagation: Uses and methods. Plant. Propag. Tissue Cult. 1 Backgr. 29–64. https://doi.org/10.1007/978-1-4020-5005-3_2 (2008).
Loberant, B. & Altman, A. Micropropagation of plants. Encycl Ind. Biotechnol. Bioprocess. Biosep Cell. Technol. Wiley N Y 3499–3515 (2010).
Nyum, L. N., Kyu, M. H., Je-Wan, L., Eui, C. Y. & So-Young, P. Somatic embryogenesis and plant regeneration from a 700-year-old Kalopanax septemlobus tree. Trees. 31, 1439–1451. https://doi.org/10.1007/s00468-017-1560-4 (2017).
Thorpe, T. A. History of plant cell culture. Plant. Tissue Cult. Tech. Exp. 1, 1–22. https://doi.org/10.1016/B978-0-12-415920-4.00001-3 (2013).
Kato, A., Lamb, J. C. & Birchler, J. A. Chromosome painting using repetitive DNA sequences as probes for somatic chromosome identification in maize. Proc. Natl. Acad. Sci. 101, 13554–13559. https://doi.org/10.1073/pnas.0403659101 (2004).
Mekki, L., Badr, A. & Fekry, M. Cytogenetic studies on nine genotypes of Phaseolus vulgaris L. cultivated in Egypt in relation to zinc efficiency. Pak J. Biol. Sci. PJBS. 10, 4230–4235. https://doi.org/10.3923/pjbs.2007.4230.4235 (2007).
Marasek-Ciolakowska, A. & Podwyszynska, M. Somaclonal variation in long-term micropropagated tulips (Tulipa gesneriana L.) determined by FISH analysis. Floric Ornam. Biotech. 2, 65–72 (2008).
Waminal, N. E. et al. Rapid and efficient FISH using pre-labeled oligomer probes. Sci. Rep. 8, 8224. https://doi.org/10.1038/s41598-018-26667-z (2018).
Peniton, E. A. Jr, Um, Y. & Kim, H. H. FISH karyotype comparison of Platycodon grandiflorus (Jacq.) A. DC.‘Jangbaek’and its colchicine-induced tetraploid ‘Etteumbaek. Plant. Breed. Biotechnol. 8, 389–395 (2020).
Campomayor, N. B. et al. Subgenome discrimination in Brassica and Raphanus allopolyploids using microsatellites. Cells. 10, 2358. https://doi.org/10.3390/cells10092358 (2021).
Doležel, J. & Bartoš, J. A. N. Plant DNA flow cytometry and estimation of nuclear genome size. Ann. Bot. 95, 99–110. https://doi.org/10.1093/aob/mci005 (2005).
Vrána, J., Cápal, P., Číhalíková, J., Kubaláková, M. & Doležel, J. Flow sorting plant chromosomes. Plant. Cytogenet. Methods Protoc. 119–134. https://doi.org/10.1007/978-1-4939-3622-9_10 (2016).
Yu, K-W., Gao, W-Y., Son, S-H. & Paek, K-Y. Improvement of ginsenoside production by jasmonic acid and some other elicitors in hairy root culture of ginseng (Panax ginseng CA Meyer). Vitro Cell. Dev. Biol-Plant. 36, 424–428. https://doi.org/10.1007/s11627-000-0077-4 (2000).
Kim, Y-S., Hahn, E-J., Yeung, E. C. & Paek, K-Y. Lateral root development and saponin accumulation as affected by IBA or NAA in adventitious root cultures of Panax ginseng CA Meyer. Vitro Cell. Dev. Biol-Plant. 39, 245–249. https://doi.org/10.1079/IVP2002397 (2003).
Choi, S. M. et al. Pilot-scale culture of adventitious roots of ginseng in a bioreactor system. Plant. Cell. Tissue Organ. Cult. 62, 187–193. https://doi.org/10.1023/A:1006412203197 (2000).
Novák, P., Neumann, P. & Macas, J. Global analysis of repetitive DNA from unassembled sequence reads using RepeatExplorer2. Nat. Protoc. 15, 3745–3776. https://doi.org/10.1038/s41596-020-0400-y (2020).
Zhou, H. C., Waminal, N. E. & Kim, H. H. In silico mining and FISH mapping of a chromosome-specific satellite DNA in Capsicum annuum L. Genes Genomics. 41, 1001–1006. https://doi.org/10.1007/s13258-019-00832-8 (2019).
Pellerin, R. J., Waminal, N. E. & Kim, H. H. FISH mapping of rDNA and telomeric repeats in 10 Senna species. Hortic. Environ. Biotechnol. 60, 253–260. https://doi.org/10.1007/s13580-018-0115-y (2019).
Peniton, E. J. A., Waminal, N. E., Yang, T-J. & Kim, H. H. Cell cycle synchronization in Panax ginseng roots for cytogenomics research. Hortic. Environ. Biotechnol. 63, 137–145. https://doi.org/10.1007/s13580-021-00383-6 (2022).
Nguyen, T. H. et al. Comparative triple-color FISH mapping in eleven Senna species using rDNA and telomeric repeat probes. Hortic. Environ. Biotechnol. 62, 927–935. https://doi.org/10.1007/s13580-021-00364-9 (2021).
Peniton, E. A., Waminal, N. E., Kim, T-H. & Kim, H. H. FISH karyotype comparison between wild and cultivated Perilla species using 5S and 45S rDNA probes. Plant. Breed. Biotechnol. 7, 237–244 (2019).
Zhou, H. C., Pellerin, R. J., Waminal, N. E., Yang, T-J. & Kim, H. H. Pre-labelled oligo probe-FISH karyotype analyses of four Araliaceae species using rDNA and telomeric repeat. Genes Genom. 41, 839–847 (2019).
Nguyen, T. H., Kang, B. Y. & Kim, H. H. Chromosomal dynamics in Senna: Comparative PLOP–FISH analysis of tandem repeats and flow cytometric nuclear genome size estimations. Front. Plant. Sci. 14, 1288220 (2023).
Galbraith, D. W., Lambert, G. M., Macas, J. & Dolezel, J. Analysis of nuclear DNA content and ploidy in higher plants. Curr. Protoc. Cytom. 2, 7.6.1-7.6.22. https://doi.org/10.1002/0471142956.cy0706s02 (1997).
Jogam, P. et al. Genetic stability analysis using DNA barcoding and molecular markers and foliar micro-morphological analysis of in vitro regenerated and in vivo grown plants of Artemisia vulgaris L. Ind. Crops Prod. 151, 112476. https://doi.org/10.1016/j.indcrop.2020.112476 (2020).
Rahman, M. & Rajora, O. Microsatellite DNA somaclonal variation in micropropagated trembling aspen (Populus tremuloides). Plant. Cell. Rep. 20, 531–536. https://doi.org/10.1007/s002990100365 (2001).
Lee, J-H. et al. Flow karyotypes and chromosomal DNA contents of genus Triticum species and rye (Secale cereale). Chromosome Res. 12, 93–102. https://doi.org/10.1023/B:CHRO.0000009327.45035.84 (2004).
Gitaí, J., Paule, J., Zizka, G., Schulte, K. & Benko-Iseppon, A. M. Chromosome numbers and DNA content in Bromeliaceae: Additional data and critical review. Bot. J. Linn. Soc. 176, 349–368. https://doi.org/10.1111/boj.12211 (2014).
Madon, M., Heslop-Harrison, J. S., Schwarzacher, T. & Hashim, A. T. Analysis of oil palm calli and regenerants using flow and image cytometry and 18S-25S ribosomal DNA fluorescence in situ hybridisation (FISH). J. Oil Palm. Res. 24, 1318–1329 (2012).
Ta, T. D., Waminal, N. E., Nguyen, T. H., Pellerin, R. J. & Kim, H. H. Comparative FISH analysis of Senna tora tandem repeats revealed insights into the chromosome dynamics in Senna. Genes Genom. 43, 237–249. https://doi.org/10.1007/s13258-021-01051-w (2021).
Solomatin, S. & Herschlag, D. Methods of site-specific labeling of RNA with fluorescent dyes. Methods Enzymol. 469, 47–68. https://doi.org/10.1016/S0076-6879(09)69003-0 (2009).
Kamińska, M., Tretyn, A. & Trejgell, A. Genetic stability assessment of Taraxacum pieninicum plantlets after long-term slow growth storage using ISSR and SCoT markers. Biol. (Bratisl). 75, 599–604. https://doi.org/10.2478/s11756-019-00377-x (2020).
Mártonfiová, L. A method of standardization of chromosome length measurement. Caryologia. 66, 304–312. https://doi.org/10.1080/00087114.2013.854565 (2013).
Bhatia, R., Singh, K. P., Sharma, T. R. & Jhang, T. Evaluation of the genetic fidelity of in vitro-propagated gerbera (Gerbera jamesonii Bolus) using DNA-based markers. Plant. Cell. Tissue Organ. Cult. PCTOC. 104, 131–135. https://doi.org/10.1007/s11240-010-9806-5 (2011).
Acknowledgements
This work was supported by the National Research Foundation of Korea (grant number NRF- 2021R1A2C1010269), Republic of Korea.
Author information
Authors and Affiliations
Contributions
E.A.P. performed the experiments, analyzed the data, and wrote the initial manuscript draft. N.E.W. designed and conceptualized the study, and analyzed the data. H.T.N. reviewed and revised the manuscript. K.H.H. and T.J.Y. supervised the study and revised the manuscript. All authors have approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Peniton, E.A., Nguyen, H.T., Waminal, N.E. et al. Cytogenomic evaluation of regenerated Aralia elata using PLOP-FISH and flow cytometry. Sci Rep 14, 30289 (2024). https://doi.org/10.1038/s41598-024-75004-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75004-0