Abstract
This study explored the factors influencing serum 25(OH)D3 levels and the effects of Vitamin D deficiency (VDD) and VD supplementation on 25(OH)D3 levels and neuropsychobehavioral development in premature infants, to provide a theoretical basis for improving their prognosis. Physical examination, neuropsychobehavioral development and serum 25(OH)D3 levels were assessed regularly in 158 preterm infants supplemented with VD formulation. 25(OH)D3 levels were analyzed at 3, 6, 9, 12, and 18 months after birth. The Gesell neuropsychological development test was conducted at 6, 9, 12, and 18 months after birth to obtain the developmental quotient (DQ). Based on the serum 25(OH)D3 levels at 42 days of age, the infants were divided into VDD and non-VDD groups. Preterm infants in the VDD group were supplemented with more VD until their 25(OH)D3 levels were normal, and were divided into sustained VDD (SVDD) and corrected VDD (CVDD) groups according to serum 25(OH)D3 levels at 3 months of age. Appropriate statistical methods were chosen to compare differences in 25(OH)D3 and DQ between or among different groups, screen for the factors influencing 25(OH)D3 levels in preterm infants at 42 days of age, and analyze the relationship between 25(OH)D3 and DQ. The 25(OH)D3 levels of preterm infants at 42 days of age were positively correlated with VD supplementation during pregnancy, and before 42 days after birth (P < 0.05). The 25(OH)D3 levels in preterm infants at 42 days and 3 months of age were positively correlated with the DQ levels at 6, 9, 12, and 18 months of age (P < 0.05). The DQ level in the VDD group, especially SVDD group, was lower than that in CVDD and non-VDD groups at the same time point (P < 0.05). This research thus demonstrates that VD supplementations during pregnancy and after birth is a major factor affecting 25(OH)D3 levels in premature infants. Early VDD and SVDD can affect their neuropsychobehavioral development, and effective VD supplementation can gradually correct VDD and mitigate this influence.
Similar content being viewed by others
Introduction
Preterm birth rates are increasing in most countries, including China, and the number of premature babies in China ranks second worldwide1. With the rapid development of perinatal and neonatal emergency medicine, the ability to treat preterm infants has greatly improved. This progress has led to the survival of an increasing number of critically ill preterm infants, such as very low birth weight/extremely low birth weight (VLBW/ELBW) infants. The prognosis of preterm infants after discharge has received increasing attentions, especially in terms of physical growth, bone metabolism and neuropsychobehavioral development, which are the main problems faced by preterm infants during their growth and development2.
Vitamin D (VD) is an indispensable fat-soluble vitamin in the human body that plays critical roles in multiple cellular processes, including maintaining skeletal and extraskeletal health3. 25(OH)D3 is recognized as the best indicator of VD nutritional status because of its stability and long half-life in the body4. Preterm infants are at high risk of vitamin D deficiency (VDD, defined as a serum 25(OH)D3 level < 50 nmol/L or 20 ng/mL) due to congenital deficiencies and limited nutrient supply after birth. Preterm infants are also prone to osteopenia of prematurity (OOP) because bone mineralization and calcium and phosphorus deposition in the fetus mainly occurs in the last 3 months of pregnancy. In addition, many postnatal factors including insufficient intake of VD, calcium and phosphorus, long-term parenteral nutrition, and the application of drugs that affect bone metabolism, can aggravate bone mineralization deficiency and result in OOP, which can lead to growth and development delay in childhood5,6. It has also been shown that VD, which is thought to function as a neurosteroid, may be an important modulator of brain development, and VDD during pregnancy has been associated with worse neurodevelopmental outcomes in infants7,8. Therefore, VD formulations may prove to extremely important for preterm infants.
However, only a few relevant studies have been conducted in China. Regarding the postnatal VD levels in preterm infants, whether VDD status can be prevented and corrected after VD supplementation, whether sustained VDD (SVDD) will lead to delayed neuropsychobehavioral development of preterm infants, and whether timely correction of VDD can prevent and improve OOP and neuropsychobehavioral development of preterm infants, these issues can be considered urgent for follow-up of preterm infants in pediatric healthcare clinic. Therefore, the purpose of this study was to explore the factors influencing serum 25(OH)D3 levels in premature infants, as well as the effects of VDD and VD supplementation on 25(OH)D3 levels and neuropsychobehavioral development in premature infants, to provide a theoretical basis for improving the prognosis of premature infants.
Materials and methods
Subjects
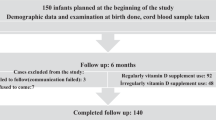
This prospective study was approved by the Ethics Committee of Shanxi Medical University (2019LL183). We confirm that all research was performed in accordance with relevant guidelines and regulations. The patient’s guardian signed a written informed consent form before participating in the study and consented to the publication of the study results. Data were collected from 158 premature infants (gestational age between 28 and 36 weeks) who underwent regular physical examination, developmental assessment and serum 25(OH)D3 level detection at the Department of Pediatric Health Care, Shanxi Children’s Hospital Affiliated to Shanxi Medical University between January 2020 and October 2021. General data on the preterm infants were recorded, including sex, gestational age, birth weight, birth length, head circumference, feeding status, serum 25(OH)D3 level and Gesell neuropsychological development test results.
Inclusion criteria and exclusion criteria
The inclusion criteria were as follows: (1) no serious complications after discharge, such as brain abnormalities or central nervous system disorders, major congenital/chromosomal anomalies, and bone, gastrointestinal, or kidney disease, and so on; (2) no serious pregnancy-related complications during the mother’s pregnancy; (3) no diseases or use of drugs that affect calcium and VD metabolism; and (4) good compliance and timely follow-up. The exclusion criteria were as follows: (1) serious complications after discharge (2), pregnancy-related complications during the mother’s pregnancy (3), having bone fractures or injuries, and disorders of calcium or VD metabolism, and (4) poor compliance.
Interventions and detections
The Vitamin D Deficiency and Vitamin D Deficiency Rickets Prevention and Treatment Recommendations released by the National Rickets Prevention and Treatment Research Collaboration Group in 20159 proposed that the main indicator for the early diagnosis of VDD and rickets is the serum 25(OH)D3 level. The optimal nutritional status of VD is a serum 25(OH)D3 level of 50–250 nmol/L, whereas serum 25(OH)D3 levels < 50 nmol/L or > 250 nmol/L are considered to be VDD or excess VD. Preterm infants were divided into VDD group (25(OH)D3 < 50 nmol/L) and non-VDD group (NVDD, 25(OH)D3 ≥ 50 nmol/L) according to the serum 25(OH)D3 level at 42 days of age after the first follow-up. According to the guidelines9,10, participants were instructed to add 800 IU/d VD (vitamin D3 drops) until 3 months after birth, and then regularly visited to the hospital for follow-up. Venous blood (2 ml) was collected at 3, 6, 9, 12, and 18 months after birth and serum 25(OH)D3 levels were detected using kits supplied by Roche Diagnostics GmbH according to the manufacturer’s protocol to judge whether VDD in preterm infants had been prevented and corrected after VD supplementation. The supplemental dosage of VD was adjusted according to the 25(OH)D3 level until it reached to normal levels and was continued until 2 years of age. At the end of all follow-ups, preterm infants with VDD were further divided into two groups according to serum 25(OH)D3 levels at 3 months of age: the SVDD and corrected VDD group (CVDD).
According to the the guidelines9,10, the intervention measures of VD were performed as follows: for all infants aged 42 days to 3 months: 800 IU/day VD; for the infants above 3 months of age, VD was supplemented according to the latest serum 25(OH)D3 level in combination with outdoor sun exposure time, such as 400–600 IU/day VD if 25(OH)D3 ≥ 50 nmol/L, 600–800 IU/day VD if 25(OH)D3 was between 40 and 50 nmol/L, and 800 IU/d VD if 25(OH)D3 < 40 nmol/L.
The Gesell neuropsychological development test was performed by professionals at 6, 9, 12 and 18 months after birth, and the developmental quotient (DQ) was obtained in five functional areas: gross motor, fine motor, adaptive behavior (cognition), language, and personal-social behavior. Interpretation of the results: DQ score ≥ 85 points, indicating normal neurobehavioral development; DQ score ≥ 75 and < 85 points, indicating suspicious delayed neurobehavioral development; DQ score < 75 points, abnormal, indicating delayed development. If the DQ score ≥ 75 and < 85 points, it is a “suspicious DQ case”, and if the DQ score < 75 points, it is an “abnormal DQ case”.
Statistical analysis
In this study, the statistical software GraphPad Prism5.0 was used for statistical analysis of the data. Measurement data consistent with normal distribution or approximately normal distribution were represented by mean ± standard deviation (x ± s). When the variance was homogeneous, t-test was used for comparisons between two groups, one-way analysis of variance (one-way ANOVA) was used for comparisons among multiple groups. Otherwise, Welch’s corrected t-test or the Kruskal–Wallis rank sum test was used. Enumeration data were expressed as the number of people and composition ratio (%). Pearson’s linear correlation analysis and multivariate linear regression analyses were used to screen for factors influencing serum 25(OH)D3 levels in preterm infants at 42 days of age. Pearson’s linear correlation analyses was used to analyze the relationship between 25(OH)D3 and DQ. The comparison of 25(OH)D3 or DQ at different time points was performed within and between groups to determine whether early or sustained VDD had an impact on the serum 25(OH)D3 level and neuropsychobehavioral development of preterm infants, and whether timely correction of VDD could prevent and improve their neuropsychological development. P < 0.05 indicated that the difference was statistically significant.
Results
General characteristics of the participants
A total of 158 preterm infants meeting the inclusion criteria were included in this study; however, 52 had incomplete data due to failure of follow up. Therefore, a total of 106 preterm infants (46 boys and 60 girls) were finally included in the study.
The supplementation of VD increased 25(OH)D3levels and decreased VDD cases at different time points after birth in all preterm infants
As shown in Fig. 1; Table 1, the average 25(OH)D3 level in preterm infants at 42 days after birth was 49.85 nmol/L, and the proportion of VDD cases was 54.7%. After adding a sufficient amount of VD (800 IU/d) according to the guidelines9,10, the average 25(OH)D3 level at 3 months of age increased to 68.08 nmol/L, and the proportion of VDD cases decreased to 19.8%. After 3 months of age, VD supplementation was adjusted to 400–800 IU/d according to the latest level of 25(OH)D3 at the follow-up. The average 25(OH)D3 levels in all premature infants at 6, 9, 12, and 18 months were > 60 nmol/L, and the proportion of VDD decreased to 1.9% at 9 months and 0% at 18 months. There was a rebound in the proportion of VDD cases in preterm infants owing to a reduction in supplemented VD in some infants after 3 months of age.
Comparisons of serum 25(OH)D3 levels in all preterm infants at different time points. ( ** indicates the comparison of 25(OH)D3 levels between other time points and 42 days, P < 0.01; # indicates the comparison of 25(OH)D3 levels between 3 m and 6 m, P < 0.05, * indicates the comparison of 25(OH)D3 levels between 6 m and 12 m, P < 0.01 ).
Preterm infants with VDD at 42 days of age needed more time and higher level of VD to correct their VDD
As shown in Fig. 2; Table 1, serum 25(OH)D3 levels in the VDD group at 42 days, and 3, 6, 9, and 12 months of age were lower than those in the NVDD group at the same time point (P < 0.05), and there was no statistically significant difference in 25(OH)D3 levels between the two groups at 18 months of age (P > 0.05), indicating that preterm infants with VDD at 42 days of age needed more time and higher level of VD to correct their VDD. Notably, there was a small increase or rebound in the proportion of VDD in both the VDD and NVDD groups owing to the reduction in supplemented VD after 3 months of age. In the NVDD group, 4.2% of preterm infants developed VDD at 6 months of age because VD supplementation decreased to 400 IU between 3 and 6 months of age, but it was completely corrected at 9 months of age after increasing VD supplementation to 600–800 IU between 6 and 9 months of age.
Preterm infants with SVDD needed more time and higher level of VD to correct their VDD
As shown in Fig. 3, among the SVDD, CVDD, and NVDD group, the serum 25(OH)D3 level in the SVDD group was the lowest at 3, 6, 9, and 12 months of age and significantly lower than that in the NVDD group at these same time points (P < 0.05). In contrast, the 25(OH)D3 level in the CVDD group was only lower than that in the NVDD group at 3 and 6 months of age. This indicates that preterm infants with SVDD needed more time and higher level of VD to correct their VDD.
25(OH)D3 levels in SVDD, CVDD, and NVDD groups at different time points. (A) The comparisons of serum 25(OH)D3 levels among SVDD, CVDD, and NVDD groups at the same time points. (B) Trend plot of 25(OH)D3 levels in SVDD, CVDD, and NVDD groups. (* indicates P < 0.01, # indicates P < 0.05). SVDD, sustained VDD; CVDD, corrected VDD; NVDD, non-VDD.
In addition, from the trend plot of 25(OH)D3 levels in the SVDD, CVDD, and NVDD groups over time, the serum 25(OH)D3 level in the CVDD and NVDD groups decreased or increased slowly because of the reduced dose of VD supplementation (400–600 IU) after 3 months, whereas the 25(OH)D3 levels in the SVDD group continued to increase after higher doses of VD supplementation (800 IU), indicating that maintaining a consistently high level of 25(OH)D3 requires supplementation with a higher dose of VD.
VD supplementation during pregnancy and after birth is important for preterm birth
As shown in Table 2, Pearson’s correlation analysis of possible factors influencing 25(OH)D3 level in premature infants at 42 days old revealed that there was a positive correlation between the 25(OH)D3 level at 42 days after birth and VD supplementation before 42 days after birth (200, 400, 500, 600, 800 or 900 IU), VD supplementation during pregnancy (200 or 400 IU) and birth weight (P < 0.05). There were no correlations between 25(OH)D3 level and gestational age and birth month (P > 0.05). However, when multivariate linear regression analysis was used to screen for factors influencing 25(OH)D3 levels (Table 3), it was found that there was a positive correlation between 25(OH)D3 levels at 42 days of age and VD supplementation during pregnancy and birth month (P < 0.05).
DQ levels of preterm infants in the VDD and SVDD groups were lower than the CVDD and NVDD groups at different time points
As shown in Figs. 4 and 5; Table 4, the DQ level of premature infants in the VDD group at the four time points was lower than that in the NVDD group (P < 0.05), and the DQ level of the SVDD group was lower than that in the CVDD and NVDD group (P < 0.05). DQ levels of all preterm infants and each group showed an overall upward trend over time, whereas the proportions of abnormal and suspicious DQ cases in all preterm infants and in each group showed an overall downward trend. The proportions of abnormal and suspicious DQ cases in the VDD group at the four time points were significantly higher than those in the NVDD group. The same situation was observed in the SVDD, CVDD, and NVDD group. The proportions of abnormal and suspicious DQ cases in the SVDD group at the four time points were significantly higher than those in the CVDD and NVDD group.
DQ levels of SVDD, CVDD, and NVDD groups at different time points. (A) The comparisons of DQ levels among SVDD, CVDD, and NVDD groups at the same time points. (B) Trend plot of DQ levels of SVDD, CVDD, and NVDD groups increasing with age at different months. (* indicates P < 0.01) DQ, developmental quotient; SVDD, sustained VDD; CVDD, corrected VDD; NVDD, non-VDD.
25(OH)D3 levels were positively correlated with DQ levels in the early postnatal period in preterm infants
As shown in Table 5, the 25(OH)D3 levels in preterm infants at 42 days and 3 months after birth were positively correlated with the DQ level at 6, 9, 12, and 18 months of age (P < 0.05).
Discussion
Previous studies have found that VD not only can maintain bone health, but also modulate brain development3,7,8. Bone health and neuropsychobehavioral development after discharge are important problems in the growth and development of preterm infants, who are at a high risk for VDD. Serum 25-(OH)D3 level can reflect the nutritional status of VD in the body, and is the main diagnostic indicator of VDD4. Monitoring the level of serum 25(OH)D3 can guide the supplementation of VD preparations in preterm infants. Therefore, we explored the factors influencing serum 25(OH)D3 levels in premature infants, and analyzed the effects of VDD and VD supplementation on serum 25(OH)D3 and neuropsychobehavioral development by monitoring serum 25(OH)D3 levels and DQ.
The demand for VD during pregnancy increases four to five times11, and the VD level during pregnancy will directly affect the VD level of the newborns12. There was a significant and strong relationship between the serum VD level of mothers and preterm newborns13. As shown in this study, both Pearson’s linear correlation analysis and multivariate linear regression analyses showed that the 25(OH)D3 levels at 42 days after birth were significantly positively correlated with VD supplementation during pregnancy. However, there were some inconsistencies between the Pearson’s linear correlation analysis and the multivariate linear regression analysis, such as VD supplementation before 42 days of age and birth weight. For VD supplementation before 42 days of age, its importance could not be ignored because of its direct effect of increasing 25(OH)D3 levels14, although the P value (0.0639) was slightly more than 0.05 in the multivariate linear regression analysis, which may be due to the small sample size. Generally, the older the gestational age, the greater the birth weight, and the longer VD can be supplemented during pregnancy, which increases 25(OH)D3 level. However, there was no correlation between 25(OH)D3 level at 42 days after birth and gestational age in this study, suggesting that VD supplementation during pregnancy and after birth is more important than gestational age. Although serum 25(OH)D3 levels at 42 days after birth were positively correlated with birth weight in the Pearson’s linear correlation analysis, low birth weight should be considered a consequence of low postnatal VD levels. Gestational VD deficiency can result in a variety of adverse health outcomes for both mothers and offspring, including fetal intrauterine growth restriction, higher risk of preterm birth and low birth weight, poor bone development and deformities, cognitive and psychological impairments15,16,17. For example, a meta-analysis involving 10 studies found that the average serum 25(OH)D3 level of mothers of preterm infants was less than 20 ng/ml (50 nmol/L) and the risk of preterm birth significantly increased with low variability, suggesting that VDD during pregnancy significantly increases the risk of preterm birth17. Adequate VD supplementation during pregnancy could decrease the rates of preterm birth and low birth weight and benefit the fetus18. It has also been found that more weeks of gestation at birth are significantly associated with better maturity of the nervous system8. Therefore, more attention should be paid to VD supplementation during pregnancy and after birth to minimize the adverse effects on the fetus and children caused by VDD.
It has been shown that among 128 preterm infants aged 26–32 weeks, the small-for-gestational-age infants and suitable-for-gestational-age infants had lower 25(OH)D3 levels at 2 weeks, 6 weeks and 6 months after birth19, and the supplementation of 1000 IU/d VD to premature infants after birth could make the 25(OH)D3 level reach the same level as that of full-term infants at 3 months20. In this study, preterm infants were managed after discharge according to the guidelines9,10, and the serum 25(OH)D3 levels and neuropsychobehavioral development levels were monitored with regular follow-up. This study found that the average 25(OH)D3 level at 42 days after birth in the enrolled preterm infants was lower than the normal level (50 nmol/L), and the proportion of VDD was up to 54.7%. In addition to insufficient VD supplementation during pregnancy, the causes of VDD may be more related to inadequate supplementation after birth due to a long hospital stay after birth, feeding intolerance, lack of sunlight exposure and other reasons. After adding a sufficient amount of VD according to the guidance9,10, the average 25(OH)D3 levels in preterm infants after 3 months of age increased to normal levels, and the proportion of VDD cases gradually decreased to 1.9% at 9 months and 0% at 18 months, indicating that VDD had been completely corrected. The 25(OH)D3 level in the VDD group, especially the SVDD group, was lower than that in the NVDD group before 12 months of age; however, the difference in 25(OH)D3 levels between the two groups gradually narrowed with increasing age until 18 months of age, when there was no significant difference. The proportion of VDD cases in the VDD group also gradually decreased to 0% at 18 months of age, indicating that VDD could be gradually corrected with sufficient VD supplementation. The small increase or rebound in the proportion of VDD cases in the VDD and NVDD groups indicates that individualized VD supplementation, which can be flexibly adjusted according to the serum 25(OH)D3 levels, is important and can effectively prevent and correct the occurrence of VDD. In addition, the trend plot of 25(OH)D3 levels of the SVDD, CVDD, and NVDD groups over time suggests that supplementation with a higher dose of VD is necessary to maintain a consistently high level of 25(OH)D3.
Currently, there is no uniform regimen for VD supplementation in preterm infants with different gestational ages or birth weights. The World Health Organization recommends that VD supplementation of VLBW (BW < 1,500 g) infants should be 400–1000 IU/d during the first 6 months of life21. The American Academy of Pediatrics (AAP) recommends a daily nutritional intake of VD in the range of 200–400 IU for VLBW. A higher intake in the range of 400–1000 IU is recommended for LBW (BW < 2,500 g) infants, which is similar to the recommended intake for full-term newborns22,23. A higher daily dose of VD in the range of 800–1,000 IU (irrespective of BW) was recommended by the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) Committee on Nutrition24. The guidelines9,10 in China proposed that high-risk populations (including premature infants, LBW infants, and twins) should be supplemented with 800–1000 IU/d VD after birth, and 400–800 IU/d after 3 months and until 2 years old. The newest version (2022) on nutrient intake and nutritional management for preterm infants provided by the ESPGHAN Committee on Nutrition in 2023 recommends a daily VD intake of 400–700 IU/kg/d for stable preterm infants, with a maximum recommended routine intake of 1000 IU/d; however, preterm infants with VDD can be temporarily supplemented with higher doses25. Another study showed that individualized VD supplementation in early infancy appeared to be more effective than standardized supplementation in the treatment of VDD and neurobehavioral development26. Therefore, to ensure the biological function of VD, further studies are needed to determine the requirement and supplementary dose of VD in preterm infants, especially VLBW infants, and ensure that preterm infants receive adequate VD supplementation without adverse effects. Individualized VD supplementation based on relevant guidelines and monitoring of serum level of 25(OH)D3 may be more advisable.
In this study, the 25(OH)D3 level in preterm infants at 42 days and 3 months after birth was positively correlated with the DQ level at 6, 9, 12 and 18 months, indicating that VDD before 3 months of age can affect the neuropsychological and behavioral development in preterm infants. The DQ levels of preterm infants in the VDD group was lower than that in the NVDD group at these four time points, and the DQ level in the SVDD group was also lower than that in the CVDD and NVDD groups at the same time point, indicating that early and sustained VDD would affect the neuropsychobehavioral development of preterm infants, while timely correction of VDD is very important for the normal neuropsychobehavioral development. However, although timely correction of VDD can improve the DQ level of preterm infants, it remains lower than that of non-vitamin D deficient preterm infants at 18 months of age. Supplementation of VD (400 IU/d) effectively reduced the prevalence of VDD and led to an increase in 25(OH)D3 level at 36 weeks postmenstrual age (PMA) in premature infants delivered in the NICU, while improving physical growth and neurodevelopment27. Therefore, preterm infants, especially those who need to be hospitalized for a long time after birth, should be supplemented with sufficient VD in the range of 400–1,000 IU/d until 3 months of age, after which the dose of VD can be adjusted to 400–800 IU/d according to the 25(OH)D3 level at follow-up.
In addition to neurodevelopmental problems in the early postnatal period of preterm infants, long-term mental health issues, including anxiety, depressive disorders, autism, and somatization, may affect social relationships and quality of life, and should be taken seriously28. Some animal experiments have confirmed that VD deficiency has an important effect on cognitive dysfunction29,30. A series of population epidemiological surveys have shown that maternal 25(OH)D3 levels during pregnancy positively correlate with the mental and psychomotor development in infants after birth31. Autism spectrum disorder (ASD) is a common neurobehavioral developmental disorder in children. Cannell et al.32 found that reduced VD levels during the fetal period or early childhood are important environmental risk factors for ASD. In 2016, Wang et al.33 conducted a meta-analysis of serum 25(OH)D3 levels in children with ASD. Eleven studies, including 870 children with ASD and 782 healthy controls, showed that 25(OH)D3 levels were significantly lower in the ASD group than in the control group, suggesting VD deficiency in children with ASD. Many studies34,35 have shown that VD supplementation is helpful in improving ASD symptoms in children. Soni et al.36 found that the risk of cognitive dysfunction in elderly individuals with VD deficiency was four times higher than that in those with VD sufficiency. Therefore, it was speculated that persistent VDD may lead to abnormal neuropsychobehavioral development in preterm infants and may even affect neuropsychobehavioral development in children and adults.
In this study, serum 25(OH)D3 and DQ levels in 106 preterm infants after VD supplementation were observed continuously for up to 18 months and even now some participants are still being followed up, which is a relatively long-lasting observational study. It was found that the DQ levels of premature infants can be decreased by early and sustained VDD and cannot catch up with those without VDD even after their VDD correction, which is a novel finding. However, a study with larger sample size and longer duration is required to obtain a comprehensive understanding of the protective effects of VD on neuropsychobehavioral development in preterm infants, which is also what we’re doing right now. We hope to continue the current research to draw useful conclusions and provide references for future clinical practice.
In summary, VDD is common in premature infants owing to congenital deficiency, postnatal feeding intolerance, and insufficient VD intake. Based on the results of this study, and considering the many beneficial effects of VD on newborn and children’s development and health37, to ensure a good prognosis for premature infants, doctors in obstetrics departments, neonatology departments, and pediatrics health care departments, and maternal and child health workers should pay more attention to the prevention of VDD in late pregnancy and early birth of newborns. The importance of timely and accurate supplementation of VD formulation and monitoring of serum 25(OH)D3 levels in the growth and development of preterm infants should be promoted and popularized through pregnant women’s schools, parents’ classes, newborn visits, and the doctors’ attention at all levels.
Conclusions
Early and sustained VDD in premature infants can lead to the decrease of DQ levels, and individualized and effective VD supplementation can gradually correct VDD, whereas their DQ levels even after VDD correction cannot catch up with those without VDD which is a new finding, suggesting that we should pay more attention to the prevention of VDD. VD supplementations during pregnancy and after birth is a major factor affecting serum 25(OH)D3 levels in premature infants, and there is even a more significant positive correlation between 25(OH)D3 levels at 42 days of age and VD supplementation during pregnancy, suggesting that the prevention of VDD should begin from pregnancy. So, in order to prevent the occurrence of VDD, and mitigate its influence on neuropsychobehavioral development, it is important to raise awareness among health care providers and pregnant women about the importance of VD supplementation during pregnancy and to implement it in practice.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Walani, S. R. Global burden of preterm birth. Int. J. Gynaecol. Obstet. 150, 31–33 (2020).
Glass, H. C. et al. Outcomes for extremely premature infants. Anesth. Analg. 120, 1337–1351 (2015).
Khazai, N., Judd, S. E. & Tangpricha, V. Calcium and vitamin D: skeletal and extraskeletal health. Curr. Rheumatol. Rep. 10, 110–117 (2008).
Cashman, K. D., Vitamin, D. & Deficiency Defining, Prevalence, causes, and strategies of addressing. Calcif Tissue Int. 106, 14–29 (2020).
Weiler, H. A., Yuen, C. K. & Seshia, M. M. Growth and bone mineralization of young adults weighing less than 1500 g at birth. Early Hum. Dev. 67, 101–112 (2002).
Rehman, M. U. & Narchi, H. Metabolic bone disease in the preterm infant: current state and future directions. World J. Methodol. 5, 115–121 (2015).
Harms, L. R., Burne, T. H., Eyles, D. W. & McGrath, J. J. Vitamin D and the brain. Best Pract. Res. Clin. Endocrinol. Metab. 25, 657–669 (2011).
Voltas, N. et al. Effect of vitamin D status during pregnancy on infant neurodevelopment: the ECLIPSES Study. Nutrients 12, 3196 (2020).
National Scientific Research Collaborative Group for the Prevention and Treatment of Rickets. Recommendations for prevention and treatment of vitamin D deficiency and vitamin D deficiency rickets. Chin. J. Child. Health Care 23(7), 781–782 (2015).
Editorial Board, Chinese Journal of Pediatrics; Subspecialty Group of Child Health Care, Society of Pediatrics, Chinese Medical Association; Subspecialty Group of Neonatology, Society of Pediatrics, Chinese Medical Association. Recommendations for feeding preterm or low birth weight infants after hospital discharge. Zhonghua Er Ke Za Zhi. 54, 6–12 (2016).
Mulligan, M. L., Felton, S. K., Riek, A. E. & Bernal-Mizrachi, C. Implications of vitamin D deficiency in pregnancy and lactation. Am. J. Obstet. Gynecol. 202, 429e421–429e429 (2010).
Song, S. J. et al. Vitamin D status in Chinese pregnant women and their newborns in Beijing and their relationships to birth size. Public. Health Nutr. 16, 687–692 (2013).
Motlagh, A. J., Davoodvandi, A. & Saeieh, S. E. Association between vitamin D level in mother’s serum and the level of vitamin D in the serum of pre-term infants. BMC Pediatr. 23, 97 (2023).
Ge, H. et al. Effects of early vitamin D supplementation on the prevention of bronchopulmonary dysplasia in preterm infants. Pediatr. Pulmonol. 57, 1015–1021 (2022).
Zhang, H. et al. Relationship between maternal vitamin D Levels and adverse outcomes. Nutrients. 14 https://doi.org/10.3390/nu14204230 (2022).
Pet, M. A. & Brouwer-Brolsma, E. M. The impact of maternal vitamin D status on offspring brain development and function: a systematic review. Adv. Nutr. 7, 665–678 (2016).
Qin, L. L., Lu, F. G., Yang, S. H., Xu, H. L. & Luo, B. A. Does maternal vitamin D Deficiency increase the risk of preterm birth: a meta-analysis of observational studies. Nutrients 8, 301 (2016).
Liu, C. C. & Huang, J. P. Potential benefits of vitamin D supplementation on pregnancy. J. Formos. Med. Assoc. 122, 557–563 (2023).
Giapros, V. I. et al. Vitamin D and parathormone levels of late-preterm formula fed infants during the first year of life. Eur. J. Clin. Nutr. 66, 224–230 (2012).
Delvin, E. E. et al. Oral vitamin A, E and D supplementation of pre-term newborns either breast-fed or formula-fed: a 3-month longitudinal study. J. Pediatr. Gastroenterol. Nutr. 40, 43–47 (2005).
Organization, W. H. EN elena: titles: micronutrient supplementation in low-birth-weight and very-low-birth-weight infants (2014).
Wagner, C. L. & Greer, F. R. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 122, 1142–1152 (2008).
Abrams, S. A. Calcium and vitamin d requirements of enterally fed preterm infants. Pediatrics. 131, e1676–1683 (2013).
Agostoni, C. et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 50, 85–91 (2010).
Embleton, N. D. et al. Enteral Nutrition in Preterm infants (2022): a position paper from the ESPGHAN Committee on Nutrition and Invited experts. J. Pediatr. Gastroenterol. Nutr. 76, 248–268 (2023).
Hou, Q. Y., Lin, M. Y. & Yuan, T. M. Vitamin D level in umbilical cord blood of late preterm infants and the effect of vitamin D(3) supplementation on the behavioral development of infants and young children: a prospective randomized controlled study. Zhongguo Dang Dai Er Ke Za Zhi 24, 1189–1194 (2022).
Tang, W. Q. et al. Vitamin D supplementation improved physical growth and neurologic development of Preterm infants receiving Nesting Care in the neonatal Intensive Care Unit. BMC Pediatr. 23, 248 (2023).
Shaw, R. J. et al. Neurodevelopmental, mental health, and parenting issues in preterm infants. Child. (Basel) 10, 1565 (2023).
Mizwicki, M. T. et al. Genomic and nongenomic signaling induced by 1α,25(OH)2-vitamin D3 promotes the recovery of amyloid-β phagocytosis by Alzheimer’s disease macrophages. J. Alzheimers Dis. 29, 51–62 (2012).
Taghizadeh, M., Djazayery, A., Salami, M., Eshraghian, M. R. & Zavareh, S. A. Vitamin-D-free regimen intensifies the spatial learning deficit in Alzheimer’s disease. Int. J. Neurosci. 121, 16–24 (2011).
Morales, E. et al. Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics. 130, e913–920 (2012).
Cannell, J. J. Autism and vitamin D. Med. Hypotheses. 70, 750–759 (2008).
Wang, T. et al. Serum concentration of 25-hydroxyvitamin D in autism spectrum disorder: a systematic review and meta-analysis. Eur. Child. Adolesc. Psychiatry. 25, 341–350 (2016).
Azzam, H. M. E. et al. Autism and vitamin D: an intervention study. Middle East Curr. Psychiatr. 22, 9–14 (2015).
Feng, J. et al. Clinical improvement following vitamin D3 supplementation in Autism Spectrum Disorder. Nutr. Neurosci. 20, 284–290 (2017).
Soni, M. et al. Vitamin D and cognitive function. Scand. J. Clin. Lab. Invest. Suppl. 243, 79–82 (2012).
Mansur, J. L., Oliveri, B., Giacoia, E., Fusaro, D. & Costanzo, P. R. Vitamin D: before, during and after pregnancy: effect on neonates and children. Nutrients 14, 1900 (2022).
Acknowledgements
We thank the Department of Pediatric Health Care, Shanxi Children’s Hospital Affiliated to Shanxi Medical University for its help during the research.
Patient consent to participate and consent for publication
The patient’s guardian signed a written informed consent form before participating in the study and consented to the publication of the study results, because there was not any personal information or privacy involved.
Funding
This study was supported by the Provincial Natural Youth Fund of Shanxi Provincial Department of Science and Technology (NO.201901D211344).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology by JJ Wei and H Guo; Collection of clinical data by H Guo; Analysis of data by JN Xie, XY Yu and Y Tian; Writing-Original Draft Preparation by H Guo, JN Xie, MQ Guan and Y Tian; Writing-Review and Editing by JJ Wei; Supervision by JJ Wei.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of Shanxi Medical University (Approval Number: 2019LL183).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, H., Xie, J., Yu, X. et al. Effects of vitamin D supplementation on serum 25(OH)D3 levels and neurobehavioral development in premature infants after birth. Sci Rep 14, 23972 (2024). https://doi.org/10.1038/s41598-024-75191-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75191-w