Abstract
The long-term use of aspirin for preventing cardiovascular disease has been recommended for decades. However, there is currently uncertainty regarding the long-term effects of aspirin use on the risk of all-cause, cardiovascular, and cancer mortality in cancer patients. The aim of this work was to analyze the connection between the prophylactic use of low-dose aspirin and the risk of all-cause death, cardiovascular death, and carcinoma death in carcinoma patients in the United States. A cohort study was conducted using National Health and Nutrition Examination Survey (NHANES) data (2011–2012, 2013–2014, 2015–2016, and 2017–2018) and associated mortality data. The 95% confidence intervals (CIs) and hazard ratios (HRs) between non-aspirin use and prophylactic low-dose aspirin use and the risk of death were measured via Cox proportional hazard regression models. A total of 1819 participants were included in the present research, of whom 945 were nonaspirin users and 874 were prophylactic aspirin users. Compared with non-aspirin users, prophylactic low-dose aspirin users had a decreased risk of all-cause death (HR = 0.647, 95% CI = 0.489–0.857). There was no statistically significant difference in the risk of cardiovascular death (HR = 0.623, 95% CI = 0.362–1.074) or cancer death (HR = 0.709, 95% CI = 0.410–1.226). Prophylactic use of low-dose aspirin may lower all-cause mortality in individuals with cancer but does not have a substantial effect on cardiovascular risk or cancer-specific mortality in this patient population.
Similar content being viewed by others
Introduction
Although the pathogenesis of cancer is well known, the global burden of cancer has not decreased, and cancer continues to be a primary contributor to the global mortality rates associated with cancer1. It is estimated that 9 out of 10 deaths per year are cancer deaths2. Furthermore, the American Cancer Society estimates that in 2024, there will be approximately 2,001,140 new cancer cases and 611,720 fatalities due to cancer in the United States. This number equates to approximately 5,480 diagnoses and 1,680 deaths on a daily basis3, indicating that cancer affects a substantial segment of the population. It is imperative to reduce the global burden of cancer.
Aspirin, also known as acetylsalicylic acid, which is known for its antipyretic and analgesic properties, is a widely used nonprescription drug suitable for the bulk of the global population4. With further research on the biological effects of aspirin5, the relationship between aspirin and survival has also begun to gain widespread attention. A study conducted on a cohort of 10,854 individuals in the United States who were over 40 years old concluded that the preventive use of low-dose (75–100 mg/day) aspirin did not have a substantial effect on mortality from any cause6. A growing body of evidence shows that it is also linked to cancer7. Researchers believe that aspirin has the potential to impede cancer progression by directly targeting and disrupting cyclooxygenase (COX), inhibiting crucial enzymes associated with cancer cell proliferation, and intervening in cancer-related inflammation as well as platelet-induced cancer-promoting activity8. A nationwide study in Sweden revealed that aspirin use during follow-up in colon cancer patients was associated with an increased risk of death (hazard ratio [HR] = 1.09, 95% confidence interval [CI]: 1.04–1.15)9, whereas a Danish study of endometrial cancer reported no association between low-dose aspirin use and mortality (HR = 1.10, 95% CI: 0.90–1.33)10. Thus,
The relationship between aspirin use and death in patients with cancer remains uncertain. According to the Patient Mortality Detection, Epidemiology, and End Results (SEER) program, cardiovascular disease mortality within one year of cancer diagnosis is extremely high11. Moreover, cardiovascular disease accounts for 11% of cancer patient deaths. Therefore, whether aspirin, a cardiovascular prevention agent12, can also improve cardiovascular events in cancer patients warrants further study.
Therefore, in this survey, we utilized the National Health and Nutrition Examination Survey (NHANES) and related mortality data bank to examine the relationships between the prophylactic use of low-dose aspirin and the risk of all-cause, cardiovascular, and cancer-related death among United States sufferers with carcinoma.
Materials and methods
Study population
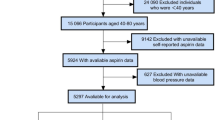
A total of 39,156 participants from 4 periods of the NHANES (2011–2012, 2013–2014, 2015–2016, and 2017–2018) were used and linked with subsequent mortality data (NHANES Mortality Public Use File, 2011–2018). We excluded 36,984 participants who did not have cancer or who were uncertain about whether they had cancer, 2 participants who had unqualified or undisclosed mortality data, 318 participants who had unclear aspirin use, and 33 participants whose diabetes or hypercholesterolemia data were unclear. Ultimately, 1819 participants were included in this research (Fig. 1). The research protocol was approved by the Institutional Review Board of the National Center for Health Statistics (NCHS), and informed agreement was signed by all participants. All studies were conducted in strict accordance with the Declaration of Helsinki.
Assessing exposure
The independent variables for this study were derived from questionnaires that reported aspirin use for prophylaxis and recommended low-dose aspirin use. A trained interviewer, through a computer-aided personal interview (CAPI) system, asked about aspirin use-related issues to determine aspirin use. “Doctors and other health care providers sometimes recommend taking low-dose aspirin daily to prevent heart attacks, strokes, or cancer. Were you told to do so?” and “Are you currently taking low-dose aspirin as recommended?”
Cancer status
In the NHANES, the Medical Conditions section provides information on self-reported health conditions. The diagnosis of cancer was made by a trained interviewer at home via the CAPI system. These methods are based on the following two questions: (1) “Have you ever been told by a doctor or other health professional that you have cancer or any malignancy?” (2) “What kind of cancer is this? When was it diagnosed?”
Death status
The National Center for Health Statistics released the NHANES Public Use Associated Mortality Archive for 1999–2018, providing follow-up data on fatality status, cause of death, and fatality from the date of the participant’s investigation through December 31, 2019. We categorized the reasons for death on the basis of the International Statistical Classification of Diseases, 10th Revision (ICD-10) code. The major events of our research were all-cause mortality, cardiovascular illness (codes I00-I09, I11, I13, and I20-I51), cancer (codes C00-C97), and mortality for other reasons.
Other covariates
The relevant covariates were extracted from the four data blocks of the NHANES database (demographic data, examination data, laboratory data and questionnaire data), which included the following two categories of covariates. The first type of continuous variable included age, BMI, total cholesterol, and glycosylated hemoglobin. Category 2 Categorical variables: sex, race, education, marital status, alcohol consumption status (no alcohol consumption, low to moderate alcohol consumption: < 1 drink/day for females, < 2 drinks/day for males, high alcohol intake or alcohol abuse: ≥1 drink/day for women, ≥ 2 drinks/day for men or history of alcohol abuse, not recorded), hypertension status (no, yes: 3 systolic or diastolic blood pressures ≥ 140/90 mmHg, or treatment with antihypertensive drugs), hypercholesterolemia status (no, yes: TC ≥ 6.2 mmol/L or use of hyperlipidemic drugs), diabetes status (no, yes: HBA1c ≥ 6.5% or uninterrupted usage of insulin or hypoglycemic pharmaceuticals), physical activity status (inactive: no moderate exercise and vigorous exercise per week, not too active: vigorous activity ≤ 75 min per week or moderate exercise ≤ 150 min per week, active: vigorous activity > 75 min per week or moderate exercise > 150 min per week, not recorded), smoking status (smoking from the questionnaire data - cigarette use asking “Do you currently smoke” was divided into never smoking, occasional smoking, daily smoking, not recorded).
Statistical analysis
The data used in this research are accessible at https://www.cdc.gov/nchs/nhanes/.
The participants were divided into two groups according to their aspirin use status: no aspirin use and prophylactic low-dose aspirin use. For normally distributed data, the median (interquartile distance) was used, whereas for normally distributed data, the mean ± standard deviation was considered, and differences between two groups were compared via t tests. Count data are expressed as frequencies (weighted percentages), and the χ2 test was used to compare differences between two sets. The results were compared between the two sets via hazard ratios (HRs) (95% CIs). Cox proportional hazard regression models were used to calculate the percentages of all-cause death, cardiovascular disease death (CVD), and carcinoma death. Two patterns were created: Model 1 was revised for gender, age, race and BMI; Model 2 was revised for gender, race, education, marital status, alcohol use, hypertension status, hypercholesterolemia status, diabetes status, physical activity, smoking status and the continuous variables of age, BMI, HBA1c, and total cholesterol. Additionally, we performed subgroup analyses for age, sex, hypertension status, hypercholesterolemia status, diabetes status, physical activity level, and smoking status via Model 2. Given aspirin’s susceptibility to cardiovascular disease, participants with preexisting heart failure, coronary artery disease, angina, myocardial infarction, or cerebrovascular accidents were excluded from the sensitivity analysis. If P < 0.05 for both sides, the difference was considered statistically significant, and all the statistical analyses were performed with Stata Statistical Software version 17 (StataCorp., T.X., USA). We can obtain the software at https://www.stata.com/.
Results
Baseline data of the participants
A combined sample of 1,819 participants participated in the research, of whom 945 were not treated with aspirin, and 874 were treated with low-dose aspirin prophylaxis. The mean age of the overall research population was 65.75 years, and the sample included 874 men and 945 women. Compared with the non-aspirin group, the prophylactic aspirin group had a greater mean age (62.39 ± 11.55 vs. 69.75 ± 9.22 years, P < 0.0001) and elevated HBA1c levels (5.74 ± 0.69 vs. 6.04 ± 0.93 mmol/L) but also had lower total cholesterol levels (5.22 ± 1.09 vs. 4.81 ± 1.03 mmol/L, P < 0.0001). Males, non-Hispanic whites, those with a senior high school degree, those who were less active, those who were not smokers and who occasionally had HBP, hypercholesterolemia, diabetes, and heart disease were more likely to use low-dose aspirin (Table 1).
Association between prophylactic low-dose aspirin use and mortality
We used the number of person-months of follow-up from the beginning of the interview to the date of death or the end of the follow-up period. The number of person-years of follow-up was 7856 (median follow-up, 4.5 years) in the group with no aspirin use and 7848 (median follow-up, 5.08 years) in the group with prophylactic low-dose aspirin use. After adjusting for potential confounders of sex, age, race, and BMI, the risk of all-cause death was lower in cancer patients treated with prophylactic low-dose aspirin than in patients not treated with aspirin (HR = 0.647, 95% CI: 0.489–0.857), but the risks of cardiovascular death (HR = 0.623, 95% CI: 0.362–1.074) and cancer death (HR = 0.709, 95% CI: 0.410–1.226) were not substantially different between the two groups. After adjusting for all factors, prophylactic administration of low-dose aspirin was associated with a decreased risk of all-cause mortality in cancer patients (HR = 0.604, 95% CI: 0.455–0.800), and neither the risk of death from cardiovascular disease (HR = 0.577, 95% CI: 0.323–1.020) nor the risk of cancer death (HR = 0.712, 95% CI: 0.447–1.133) had a significant effect. In addition, we found that the number of cancer deaths per 1000 person-years was slightly lower in the low-dose aspirin group (6.881 vs. 7.383) than in the non-aspirin group, but the difference was not statistically significant (Table 2).
Subgroup analysis
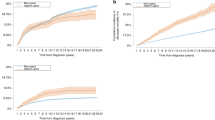
In addition, subgroup percentages of all-cause death, cardiovascular disease, and carcinoma death among cancer patients were analyzed for sex, age, alcohol consumption, smoking status, physical activity, and the presence of hypertension, hypercholesterolemia, and diabetes. Our study revealed that prophylactic use of low-dose aspirin was associated with a decreased risk of all-cause death in patients in all cancer subgroups. There was no significant difference in the incidence of cardiovascular death or cancer death between the subgroups (Fig. 2).
Sensitivity analysis
Considering the sensitivity of cardiovascular disease to aspirin13, we excluded 444 participants and 3 unrecorded participants with preexisting cardiac failure, ischemic heart disease, stenocardia, heart attack or stroke; ultimately, 1372 participants were included in the sensitivity analysis. Consistent with these results, compared with non-aspirin use, prophylactic low-dose aspirin was a protective factor against all-cause death but had no statistically significant effect on the risk of cardiovascular death or cancer death. Cancer mortality per 1000 person-years (5.566 vs. 7.993) was slightly lower in the prophylactic aspirin status group than in the non-aspirin status group, but the difference was not statistically significant (Table 3).
Discuss
In this cohort study based on a cancer population in the United States, we observed that the prophylactic use of low-dose aspirin was correlated with a decreased risk of all-cause mortality in cancer patients but was not significantly associated with cardiovascular death or carcinoma death in cancer patients. Sensitivity analyses that excluded participants with a record of cardiac failure, ischemic heart disease, stenocardia, heart attack or apoplexy did not change the results. This finding also shows the reliability and stability of the results of this study on the basis of the NAHNES 2011–2018 cancer population in the United States.
In 1981, a comprehensive prospective study was carried out to explore the potential link between the use of aspirin and the likelihood of fatal prostate cancer (PC), as well as postdiagnosis survival following PC, among a cohort of 22,071 healthy male physicians who were randomly allocated to receive either aspirin or placebo. As of 2009, a total of 502 instances of fatal PCs had been identified. The utilization of aspirin on a regular basis, whether in the present or past, is linked to a reduced likelihood of developing fatal PC compared with individuals who never used aspirin. Subsequent survival analysis revealed that the use of aspirin following diagnosis was linked to a decreased risk of fatal PC (HR = 0.68, 95% CI: 0.52–0.90) and gross mortality (HR = 0.72, 95% CI: 0.61–0.9)14. A systematic review of aspirin usage in populations eligible for CVD primary prevention revealed that both primary and secondary prevention trials demonstrated a reduction in colon cancer mortality over 20 years with aspirin (RR = 0.67; 95% CI: 0.52, 0.86). Furthermore, the initiation of aspirin therapy resulted in a decrease in the incidence of colon cancer, with an RR of 0.60 (95% CI: 0.47, 0.76) after a period of 10–19 years15. A meta-analysis of 118 studies on aspirin and 18 cancer types revealed that aspirin was associated with a decrease in all-cause mortality in carcinoma patients (HR = 0.80, 95% CI: 0.74–0.76)16. This study is consistent with these results, and our subgroup analysis also revealed that prophylactic low-dose aspirin use was involved in all-cause mortality in patients with carcinoma effects. Those with carcinoma who were ≥ 70 years of age; women; those with occasional alcohol use; those with no history of smoking or inactivity; and those with no history of hypertension, hypercholesterolemia, or diabetes were more likely to benefit from preventive low-dose aspirin use. A study of breast cancer revealed that aspirin can regulate M1/M2 macrophage subtypes by inhibiting crosstalk between 4T1 and RAW 264.7 cells and regulating angiogenesis and the production of inflammatory mediators to achieve adjuvant therapeutic effects17. Therefore, we believe that aspirin may increase cancer survival and thus affect all-cause death in the cancer population.
Previous studies have shown that aspirin can reduce the risk of cardiovascular death18,19. However, no significant impact of aspirin use on the risk of cardiovascular death was detected in healthy older adults ≥ 70 years old or adults ≥ 40 years old in the United States6,20. The study also revealed no statistically significant association between aspirin and cardiovascular death in the carcinoma population, including subsequent subgroup analyses that did not change this finding. This may be due to the decreased efficacy of low-dose aspirin in inhibiting platelet function in the cancer population21.
It is currently believed that aspirin can inhibit the upregulation of COX-2 in carcinoma cells for anticancer purposes22. However, studies at the cellular level23,24 have shown that aspirin impedes cell propagation and initiates cell cycle progression and apoptosis in multiple carcinoma cell lines, regardless of whether cancer cells express COX-2. There is also evidence of other potential targets for aspirin in the treatment of cancer25. In conclusion, the specific mechanism of the biological effects of aspirin in cancer is still not well understood. However, this study revealed that prophylactic low-dose aspirin use had no significant effect on cancer mortality and was not statistically significant in any subgroup. This is consistent with the results obtained by Elwood et al.26. In addition, our study revealed that prophylactic aspirin use may affect cancer mortality per 1000 person-years; even though this reduction was not statistically significant in this trial, it can be further observed in an expanded study population.
This research also has several limitations. First, the research was observational, and no causal relationship could be established between prophylactic low-dose aspirin use and mortality in carcinoma patients. Second, the identification of cancer patients and the use of aspirin were obtained through questionnaires, which may have resulted in certain memory bias. Finally, in our study, we did not explore aspirin-related deaths from side effects.
Overall, prophylactic use of low-dose aspirin diminishes the risk of all-cause death in carcinoma sufferers, specifically those who are aged more than 70 years, are female, have a history of occasional alcohol consumption, have no history of smoking, are inactive, and have no history of hypertension, hypercholesterolemia, or diabetes.
Data availability
The data used in this research are obtainable at https://www.cdc.gov/nchs/nhanes.
References
Cao, W., Chen, H. D., Yu, Y. W., Li, N. & Chen, W. Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. (Engl.) 134 (7), 783–791. https://doi.org/10.1097/cm9.0000000000001474 (2021).
Fitzmaurice, C. et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 5 (12), 1749–1768. https://doi.org/10.1001/jamaoncol.2019.2996 (2019).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74 (1), 12–49. https://doi.org/10.3322/caac.21820 (2024).
Hall, D. C. N. & Benndorf, R. A. Aspirin sensitivity of PIK3CA-mutated colorectal cancer: Potential mechanisms revisited. Cell. Mol. Life Sci. 79 (7), 393. https://doi.org/10.1007/s00018-022-04430-y (2022).
Ornelas, A. et al. Beyond COX-1: The effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 36 (2), 289–303. https://doi.org/10.1007/s10555-017-9675-z (2017).
Chen, Y. et al. Low- or high-dose preventive aspirin use and risk of death from all-cause, cardiovascular disease, and cancer: A nationally representative cohort study. Front. Pharmacol. 14, 1099810. https://doi.org/10.3389/fphar.2023.1099810 (2023).
Hybiak, J. et al. Aspirin and its pleiotropic application. Eur. J. Pharmacol. 866, 172762. https://doi.org/10.1016/j.ejphar.2019.172762 (2020).
Elwood, P. et al. Aspirin and cancer: Biological mechanisms and clinical outcomes. Open. Biol. 12 (9), 220124. https://doi.org/10.1098/rsob.220124 (2022).
Shahrivar, M. et al. Low-dose aspirin use and colorectal cancer survival in 32,195 patients-A national cohort study. Cancer Med. 12 (1), 315–324. https://doi.org/10.1002/cam4.4859 (2023).
Sperling, C. D. et al. Low-dose aspirin use and endometrial cancer mortality-a Danish nationwide cohort study. Int. J. Epidemiol. 49 (1), 330–337. https://doi.org/10.1093/ije/dyz253 (2020).
Sturgeon, K. M. et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 40 (48), 3889–3897. https://doi.org/10.1093/eurheartj/ehz766 (2019).
Yang, B. & Shi, J. Developing new cancer nanomedicines by repurposing old drugs. Angew Chem. Int. Ed. Engl. 59 (49), 21829–21838. https://doi.org/10.1002/anie.202004317 (2020).
Patrono, C. & Baigent, C. Role of aspirin in primary prevention of cardiovascular disease. Nat. Rev. Cardiol. 16 (11), 675–686. https://doi.org/10.1038/s41569-019-0225-y (2019).
Downer, M. K. et al. Regular aspirin use and the risk of lethal prostate cancer in the physicians’ health study. Eur. Urol. 72 (5), 821–827. https://doi.org/10.1016/j.eururo.2017.01.044 (2017).
Chubak, J. et al. Aspirin for the prevention of cancer incidence and mortality: Systematic evidence reviews for the U.S. preventive services task force. Ann. Intern. Med. 164 (12), 814–825. https://doi.org/10.7326/m15-2117 (2016).
Elwood, P. C. et al. Aspirin and cancer survival: A systematic review and meta-analyses of 118 observational studies of aspirin and 18 cancers. Ecancermedicalscience 15, 1258. https://doi.org/10.3332/ecancer.2021.1258 (2021).
Liu, J., Zheng, F., Yang, M., Wu, X. & Liu, A. Effect of aspirin use on survival benefits of breast cancer patients: A meta-analysis. Med. (Baltim) 100 (33), e26870. https://doi.org/10.1097/md.0000000000026870 (2021).
Bowman, L. et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl. J. Med. 379 (16), 1529–1539. https://doi.org/10.1056/NEJMoa1804988 (2018).
Guirguis-Blake, J. M., Evans, C. V., Perdue, L. A., Bean, S. I. & Senger, C. A. Aspirin use to prevent cardiovascular disease and colorectal cancer: Updated evidence report and systematic review for the US preventive services task force. Jama 327 (16), 1585–1597. https://doi.org/10.1001/jama.2022.3337 (2022).
McNeil, J. J. et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl. J. Med. 379 (16), 1519–1528. https://doi.org/10.1056/NEJMoa1803955 (2018).
Brotons, C., Benamouzig, R., Filipiak, K. J., Limmroth, V. & Borghi, C. A systematic review of aspirin in primary prevention: Is it time for a new approach? Am. J. Cardiovasc. Drugs 15 (2), 113–133. https://doi.org/10.1007/s40256-014-0100-5 (2015).
Khan, M. N. & Lee, Y. S. Cyclooxygenase inhibitors: Scope of their use and development in cancer chemotherapy. Med. Res. Rev. 31 (2), 161–201. https://doi.org/10.1002/med.20182 (2011).
He, Y. et al. Combined effects of atorvastatin and aspirin on growth and apoptosis in human prostate cancer cells. Oncol. Rep. 37 (2), 953–960. https://doi.org/10.3892/or.2017.5353 (2017).
Huang, Y. et al. Antitumor and antiangiogenic effects of aspirin-PC in ovarian cancer. Mol. Cancer Ther. 15 (12), 2894–2904. https://doi.org/10.1158/1535-7163.Mct-16-0074 (2016).
Dai, X. et al. Aspirin inhibits cancer metastasis and angiogenesis via targeting heparanase. Clin. Cancer Res. 23 (20), 6267–6278. https://doi.org/10.1158/1078-0432.Ccr-17-0242 (2017).
Elwood, P. C. et al. Aspirin in the treatment of cancer: Reductions in metastatic spread and in mortality: A systematic review and meta-analyses of published studies. PLoS One 11 (4), e0152402. https://doi.org/10.1371/journal.pone.0152402 (2016).
Funding
Supported by the National Natural Science Foundation of China: Regional Science Foundation of China, No.81,960,425.
Author information
Authors and Affiliations
Contributions
H.H. and J.-p.X. conceived and designed the study. W.-j.C. and C.S. collected and evaluated the data. H.H., W.-j.C., Z.-y.X. and L.-f.L. analyzed and interpreted the data, and H.H. wrote the manuscript. H.H. and J.-p.X. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics and Consent Statements
The research protocol was espoused by the Institutional Review Board of the National Center for Health Statistics (NCHS), and informed consent was signed by all participants.
Consent for publication
All authors agree to publish this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, H., Chen, Wj., Xiong, Zy. et al. Association of prophylactic low-dose aspirin use with all-cause and cause-specific mortality in cancer patients. Sci Rep 14, 25918 (2024). https://doi.org/10.1038/s41598-024-75612-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75612-w