Abstract
The controlled synthesis of recyclable hematite in the leaching solution of secondary zinc oxide powder is an urgent problem in the chemical industry and metallurgy fields. In this paper, the effects of the temperature, agitation speed, and seed addition on the contents of iron, sulfur, potassium, sodium, and zinc in the iron removal residues, as well as the iron concentration in the supernatant after iron removal were systematically studied. In addition to the temperature control already reported, we found that the agitation speed can also control the transformation between jarosite and hematite phases. The content of jarosite in the residues can be effectively controlled by adjusting the agitation speed, thereby significantly improving the quality of hematite product, as indicated by SEM-EDS and XRD results. Under temperature of 185 °C, an agitation speed of 500 rpm, and a seed addition of 15 g/L, the iron, sulfur and zinc contents in the filter residues and the iron concentration in the supernatant were 59%, 3.22%, 0.92%, and 4.182 g/L, respectively. Kinetic studies show that the rapid oxidation kinetics is not conducive to the formation of high-quality hematite products. These results can directly guide the process of transformation of harmful jarosite into recyclable hematite from the leaching solution of secondary zinc oxide powder, which is of great practical significance for the controlled and waste-free removal of iron from aqueous solutions in the chemical industry and metallurgy fields.
Similar content being viewed by others
Introduction
During the production of iron and crude steel, a significant volume of metallurgical hazardous wastes with different compositions, such as slag, dust, and sludge, are generated from the basic oxygen furnace (BOF), blast furnace (BF), and electric-arc furnace (EAF)1,2,3,4,5. On average, the production of one tonne of crude steel produces around 23 kg of iron and steelmaking dusts and sludges via the blast furnace-basic oxygen furnace (BF-BOF) route and 13 kg for the EAF route6. In 2020, the total crude steel production worldwide exceeded 1878 million tonnes—a figure that is continually increasing7. This means that nearly 67.6 million tonnes of iron and steelmaking dusts and sludges need to be properly handled and treated in 2020. Due to the lack of a suitable recycling process, large portions of iron and steelmaking dusts and sludges are placed into landfills or long-term storage, which is a significant recycling challenge at integrated steelmaking plants8. These dusts and sludges usually contain significant amounts of iron and zinc with considerable quantities of other valuable metals such as potassium, sodium, manganese, magnesium, indium, germanium, gallium, and lead9,10. The construction of new storage or landfills is very costly and occupies large amounts of ground11. Ultimately, the present government regulations and environmental philosophies prefer reuse and recovery to disposal, while also avoiding the release of heavy metals into the earth4.
The direct reuse of dust and sludge back to steel production is hindered due to the presence of zinc, which readily evaporates because of the high temperature in the furnace. It subsequently condenses on the furnace walls at lower temperatures, causing considerable disturbances during the steelmaking process12,13. To recycle dust and sludge towards achieving zero-waste processes, several pyrometallurgical processes, hydrometallurgical processes and combined pyro-hydrometallurgical processes have been developed3,14,15,16. Although the pyrometallurgical process can obtain a high zinc recovery, it cannot simultaneously recover other valuable metals such as indium, germanium, or gallium, and still has some economic drawbacks due to construction expenses, high working costs, and high energy requirements3,17. The hydrometallurgical process can significantly improve the leaching rate of valuable metals such as zinc, copper, and cadmium, and the residue rate is also low4. Meanwhile, the precious metals in dust and sludge can be enriched in the leaching residue, which is conducive to the subsequent recovery of valuable metals. The zinc content of the iron and steelmaking dusts and sludges varies between 0.1% and 40%4. To maximize the recovery of dusts and sludges, secondary zinc oxide powder (SZOP) can be obtained by pyrometallurgically pretreating these hazardous wastes with different zinc contents. Then, a hydrometallurgical process can be used to recover zinc and other valuable metals18.
The hydrometallurgical leaching of the SZOP is usually carried out using sulfuric acid19. Valuable metals, such as zinc, lead, iron, potassium, sodium, and indium, enter the solution at the same time during leaching process. Iron must be removed from the leaching solution because it can significantly disturb the electrowinning of zinc through electrochemical short-circuiting20,21. Consequently, the separation of zinc and iron is a key step in the hydrometallurgical zinc process. To produce high-quality metal electrical products and a high current efficiency, three main processes have been applied to remove excess iron from leaching circuits: the jarosite (MFe3(SO4)2(OH)6, M = K+, Na+, H3O+ or NH4+) process, the goethite (α-FeOOH) process and the hematite (α-Fe2O3) process that are named according to the main iron compound precipitated during the process22,23,24,25. Nevertheless, the jarosite and goethite products are characterized as hazardous and toxic waste materials because they contain heavy metal elements (Zn, Cd, As, etc.); thus, they need to be stored in controlled and specialized lined tailings sites26,27,28. The iron contents (dry basis) of jarosite and goethite residues is only 10–40%25, which makes them difficult to reuse economically. Together with increasingly stringent global environmental regulations, the hematite process, which can be used as a pigment or as a raw material in the steel making industry or cement-manufacturing industry, is one of the most promising and environmentally friendly alternatives to the jarosite and goethite processes for the separation of zinc and iron. It is also less hazardous and more economical.
Iron removal by the hematite process, as practiced in the zinc industry, has been extensively studied29,30,31. Unlike the hydrometallurgical zinc processes based on the ore (sphalerite, marmatite, sulfide concentrate), the leaching solution of SZOP contains a high concentration of zinc, potassium, and sodium ions, which makes it difficult to apply the previous research conclusions obtained for the hematite process for this solution32. Previous studies have shown that ferric ions can be easily hydrolyzed to form jarosite rather than hematite in the presence of potassium and sodium ions23. Therefore, it is of great importance to systematically study the controllable technology of conversion of harmful jarosite from high concentration K+ and Na+ leaching solution to recoverable hematite in the hydrometallurgical SZOP process.
To this end, we studied the hematite process of the leaching solution from the SZOP under different conditions, focusing on the effects of the temperature, agitation speed and seed addition on the contents of iron, sulfur, potassium, sodium, and zinc in the iron removal residues, as well as the iron concentration in the supernatant after iron removal. Different methods of converting jarosite into hematite are shown in Table 1. In the previous research reports, the different types of jarosite were used as raw materials by hydrometallurgy, pyrometallurgy, or pyro-hydrometallurgical processes to decompose jarosite to obtain hematite. These methods are taking the idea of pollution first and then treatment. Our research work is to implement the precipitation of iron as recoverable hematite instead of jarosite in solutions with high potassium and sodium ion concentrations. The idea of this work is to convert pollution sources into pollution-free products. In addition to the temperature control already reported30, we show for the first time that the agitation speed can also control the transformation between the jarosite and hematite phases. Subsequently, the control mechanism was studied by kinetic. Finally, the zinc and iron in the leaching solution from the SZOP with high potassium and sodium ion concentrations were separated by the hematite process in an environmentally-friendly manner. This study provides vital experimental data for hydrometallurgical SZOP process.
Materials and methods
Apparatus and materials
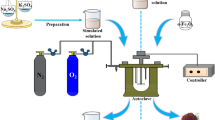
A Büchiglas-eloclave (49.32232 polyclave Type 2.0 It.), a stirrer with a rotation speed of 0–1500 rpm and the working temperature in the range of 0–300 °C, was used as the reaction vessel for the precipitation tests. The autoclave was equipped with a heating circulation bath, a sampling system, a proportional-integral-differential temperature controller, data logging software on a personal computer, which meet the requirements of the hematite process.
Analytical-grade of Fe2O3 was obtained from commercial sources and used without further purification as the seed of the hematite process. The sample of an industrial feed solution with pH 4.68, obtained after the indium precipitation process by the leaching solution of the SZOP, was provided by a clean utilization and harmless treatment of the heavy metal waste plant (GreenNovo Environmental Technology Co., Ltd., Honghe, China).
Experimental procedures
The experimental conditions were as follows: a total reaction duration of 3 h, the oxygen partial pressure of 0.4 MPa, a seed addition of 20 g/L. These reaction conditions refer to the technical indicators of the plant (Iijima hematite plant, Japan; Yunnan Hualian Zinc and Indium Co., Ltd, Kunming, China) and the results of our previous studies31,32. The reaction temperature range is 150–190 °C, and the agitation speed range is 400–700 rpm. When the optimum temperature and agitation speed were determined, the effect of seed addition amount of 0, 5, 10, 15, and 20 g/L on the hematite process was studied. The procedures of this study are shown in Fig. 1. Before each experiment, the autoclave was carefully cleaned. The specific experimental procedures refer to our previous literature reports32.
Analytical techniques
The concentration of ferrous in the solution was determined by potassium dichromate (K2Cr2O7) titration39,40. The total iron concentration was determined by reducing ferric ions using titanium trichloride (TiCl3) before titrating them with potassium dichromate. The pH of the solution was measured using a S220-K-CN digital pH meter (METTLER TOLEDO, Switzerland) with a glass electrode. X-ray diffraction (XRD; CuKα radiation, D/Max 2200, Rigaku, Japan) was employed to analyze the phase composition of the filter residues. Atomic absorption spectroscopy (ASS) was used to determine the zinc content in the filter residues. The microstructure and surface composition of the iron precipitates was characterized by scanning electron microscopy (SEM, Gemini 300, ZEISS, Germany). The infrared absorption method after combustion in the induction furnace was used to determine the sulfur content in the filter residues. The K and Na contents in the precipitation products was determined using inductively coupled plasma-optical emission spectrometry (ICP-OES, iCAP 7000, Thermo Fisher Scientific, UK).
Results and discussion
Characterization of the leaching solution
The smelting raw materials of the two factories (Iijima Refinery, Japan; Yunnan Hualian Zinc and Indium Co., Ltd, Kunming, China) are respectively sphalerite and high-iron sphalerite. In the combined pyro-hydrometallurgical processes of iron and steelmaking dusts and sludges, the smelting raw material of the hydrometallurgical process phase is secondary zinc oxide powder (SZOP), whose leaching solution has a very different chemical composition from that of the Iijima plant and Hualian plant. The main chemical composition of the leaching solution from the different raw materials is shown in Table 2.
It can be seen from Table 2 that the concentrations of zinc, iron, potassium, and sodium in the leaching solution were very similar between the Iijima and Hualian plants. Unlike the hydrometallurgical zinc process based on sphalerite and high-iron sphalerite, the leaching solution of the SZOP contained about 1.7 times more zinc ions, 6.5 times more potassium irons, and 15 times more sodium ions than the previous two solutions. The high concentration of potassium and sodium ions in the solution may produce jarosite (Na-jarosite, K-jarosite) during the reaction, making it challenging to cleanly separate and recycle zinc and iron resources in the leaching solution of the SZOP using the hematite process. At the same time, the high concentration of zinc ions in the solution may increase the zinc content in hematite residues. In this study, the transformation of jarosite under hydrothermal conditions is the focus, aiming to increase the iron content in the iron removal residues to maximize resource utilization.
Separation of zinc and iron using the hematite process
Comprehensive experiment of temperature and agitation speed
Based on industrial production conditions of the hematite process, a total reaction duration of 3 h, an oxygen partial pressure of 0.4 MPa, and a seed addition of 20 g/L, the hematite behavior of the leaching solution of the SZOP was investigated in the temperature range of 150–190 °C and the agitation speed range of 400–700 rpm. Elemental analysis of the filter residues and supernatant after iron removal is shown in Fig. 2. It can be seen from Fig. 2a–c that at the same temperature, as the agitation speed increased, the Fe content in the filter residues greatly decreased, and the K, Na, and S contents in the filter residues increased. Under a constant agitation speed, the Fe content in the filter residues increased with the temperature, while the K, Na, and S contents changed in the opposite direction. However, when the agitation speed was ≥ 600 rpm, this phenomenon was not obvious. This shows that in addition to temperature, the agitation speed in high concentration potassium ion and sodium ion solution has an important effect on the content of elements in the filter residues.
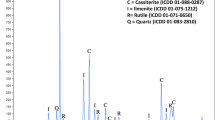
XRD analysis of the filter residue after the iron removal process is shown in Fig. 3. As illustrated in Fig. 3a–d, by increasing the agitation speed from 400 rpm to 700 rpm, the characteristic peaks of KFe3(SO4)2(OH)6 became clearer and sharper, indicating the formation of K-jarosite particles with a better crystal structure. It is clear that increasing the agitation speed promotes the formation of jarosite (Fig. 3a–e). The XRD results also revealed that as the temperature increased, the characteristic peaks of KFe3(SO4)2(OH)6 gradually became weaker when the agitation speed was ≤ 500 rpm (Fig. 3a and b). There was no significant change at 600 rpm and 700 rpm (Fig. 3c and d). The concentration of iron in the supernatant after iron removal was determined by potassium dichromate titration, and the results are shown in Fig. 2d. The iron concentration decreased from 9.37 g/L to 7.50 g/L upon increasing the temperature from 150 °C to 190 °C at 400 rpm. In contrast, the Fe content in the filter residues showed the opposite trend and increased from 56.84 to 64.37% upon increasing the temperature, which is similar to the iron content in pure hematite (69.94%). Consequently, the higher the reaction temperature, the lower the iron concentration in the supernatant and the higher the iron content in the filter residues because reaction (2) shifts to the right (towards products).
When the speed was increased from 400 rpm to 500 rpm, the formation of jarosite was promoted (Eqs. (3), (6)), and the iron concentration in the supernatant and the Fe content of the filter residues sharply decreased from 9.37 g/L to 1.60 g/L and from 56.84 to 45.22% at 150 °C, respectively. As the temperature increased, reactions (3) and (6) shifted to the left (towards reactants) based on the thermodynamic calculations. This resulted in an increase the iron concentration in the supernatant from 3.34 g/L to 4.23 g/L and the Fe content of the filter residues from 48.76 to 61.16%. When the agitation speed exceeded 500 rpm, the iron in the solution precipitated in the form of jarosite, which caused the iron content in the residues to maintain at about 42%, and the iron concentration in the supernatant remained at about 0.5 g/L. This shows that increasing the temperature inhibited the formation of K-jarosite but could not promote the transformation of K-jarosite into hematite when the temperature was increased from 150 °C to 190 °C. It should be noted that the content of sodium in the filter residues was much lower than that of potassium, and X-ray diffraction did not detect the Na-jarosite phase. This may be because Na-jarosite was easily converted into hematite during the hematite process, which is consistent with the thermodynamic calculations. In order to make the point in a dramatic way, the standard free energy changes and standard enthalpy changes, ΔG and ΔH, for the reactions of the hematite process in the leaching solution of the SZOP, were calculated for the temperature range of 0–300 °C using the software HSC Chemistry 8.141.
The hydrolytic reaction of ferric ions becomes complicated when potassium and sodium ions simultaneously exist in the solution under hydrothermal conditions. During the reaction, ferric ions can be hydrolyzed to form different precipitates, such as Fe2O3, NaFe3(SO4)2(OH)6, and KFe3(SO4)2(OH)6. The reaction equations during the iron removal process are given in Eqs. (1)–(6).
The ΔG curves of the formation reactions of Fe2O3, NaFe3(SO4)2(OH)6, and KFe3(SO4)2(OH)6 and the decomposition reactions of NaFe3(SO4)2(OH)6 and KFe3(SO4)2(OH)6 are illustrated in Fig. 4a. Figure 4b illustrates the ΔH curves of the corresponding reactions.
According to basic thermodynamics principles and ignoring, possible kinetic effects aside, one may predict the general likelihood of a chemical reaction based on the most negative ΔG values denoting more favorable reactions. One may determine whether a reaction is endothermic or exothermic based on the enthalpy change. It follows from Fig. 4a that the formation reaction of KFe3(SO4)2(OH)6 from K2SO4 and Fe2(SO4)3 is more favorable than that of NaFe3(SO4)2(OH)6 from Na2SO4 and Fe2(SO4)3, which is more favorable than that of the Fe2O3 from Fe2(SO4)3 in the temperature range of 0–260 °C. Furthermore, the decomposition reaction of NaFe3(SO4)2(OH)6 is more favorable than that of KFe3(SO4)2(OH)6. As the temperature increased, the ΔG values of the formation reactions of NaFe3(SO4)2(OH)6 and KFe3(SO4)2(OH)6 increased, and the ΔG values of the formation reaction of Fe2O3 and ΔG of the decomposition reactions of NaFe3(SO4)2(OH)6 and KFe3(SO4)2(OH)6 decreased. This means that the formation reaction of Fe2O3 and the decomposition reactions of jarosite (Na-jarosite, K-jarosite) were promoted, and the formation reactions of the jarosite were correspondingly inhibited during the heating process. It is observed from Fig. 4b that reactions (2)–(6) are endothermic reactions. However, the ΔH values of reactions (4) and (6) decreased upon increasing the temperature, which means these reactions produced a greater inhibitory effect compared with other reactions during heating.
The morphology of the filter residues formed at different temperatures and agitation speeds was studied using SEM. The chemical composition of the selected particle and the SEM images of the filter residues are presented in Fig. 5. The residues contained two kinds of reaction products with different physical morphologies. One is dispersed hexagonal block-shaped particles (Fig. 5a3) with a large volume, regular structure, and relatively smooth surface, which has a large proportion of K, S, and O, and low Fe content. It is identified as K-jarosite (Spot 1). The other is irregularly-shaped particles that appeared to be aggregates of small spherical crystals (Fig. 5a4), which were determined to be hematite due to their high Fe and O contents and low K and S contents (Spot 2). When the agitation speed was ≤ 500 rpm, the number of irregular particles in the filter residues gradually increased with the temperature, and the change in the hexagonal particles was the opposite (Fig. 5a1,a2,a5–a7 and Fig. 5b1–b3. In other words, the hematite phase increased with the temperature, while the K-jarosite phase showed the opposite trend. When the agitation speed was ≥ 600 rpm, as shown Fig. 5b4–b9, the morphology of the filter residues was mainly hexagonal block-shaped particles upon increasing the temperature. It can be observed that SEM results of the filter residues are in agreement with the elemental analysis.
According to the production process and quality control indicators of the hematite process, the iron content of the hematite residues must be above 55%, the sulfur content lower than 5%, the zinc content not more than 1%, and the iron concentration in the solution after iron removal must be 3–6 g/L42,43. The hematite process parameters of the Yunnan Hualian Zinc and Indium Co., Ltd, Kunming, China and the Iijima hematite plant are given in Table 3. The iron concentration in the supernatant after iron removal, a vital industry parameter, is closely related to whether the supernatant can be returned to the zinc hydrometallurgy system. During the zinc hydrometallurgy process, a large amount of ferric hydroxide was produced during the oxidation and neutralization stage to remove impurities when excess iron was present in the supernatant. It adsorbed many valuable metals, resulting in their loss. Although the iron content in the filter residues exceeded 55% when the rotation speed was 400 rpm, the iron concentration in the supernatant, however, was above 7.5 g/L, which cannot meet actual production requirements. In contrast, the experimental results of 600 rpm and 700 rpm could not be applied to the actual production of the hematite process due to the large amounts of jarosite generated, even though the iron concentration in the solution was only about 0.5 g/L. Therefore, the optimum speed of the leaching solution of the SZOP during the hematite process was 500 rpm.
The iron removal results presented in Table 3 show that the experimental data at 500 rpm and 190 °C met the actual production indexes of the current industrial hematite process42,43. The temperature has a remarkable effect on iron removal and is also closely related to energy cost. In order to reduce energy consumption, we studied the iron removal at 500 rpm and 185 °C. As shown in Table 3, under the temperature of the hematite process, the iron concentration and the Fe content of the filter residues decreased from 4.23 g/L to 3.81 g/L, and 61.16–59.26%, respectively, due to the formation of jarosite when the temperature was decreased from 190 °C to 185 °C at 500 rpm. It can be seen that lowering the temperature also met the requirements of the hematite process.
Effect of seed addition on iron removal
To explore the optimum seed addition, the hematite process of the leaching solution of the SZOP was studied at seed addition of 0 g/L, 5 g/L, 10 g/L, 15 g/L, and 20 g/L. The other reaction conditions were kept constant: a total reaction duration of 3 h, an oxygen partial pressure of 0.4 MPa, a reaction temperature of 185 °C, and an agitation speed of 500 rpm. As shown in Fig. 6a and b, the Fe content in the filter residues increased from 52.35 to 59.26% upon increasing the seed addition from 0 g/L to 20 g/L. In contrast, the iron concentration decreased from 4.70 g/L to 3.75 g/L. The S and K contents decreased from 5.27 to 3.65%, and from 2.30 to 1.31%, respectively, and the Na content remained at about 0.2%. Hence, the higher the seed addition, the higher the iron content in the filter residues and the lower the iron concentration in the supernatant because the seed accelerated the hematite nucleation rate.
The SEM images of the filter residues formed at different seed additions and chemical compositions of the selected particle are presented in Fig. 7, which shows that the seed addition significantly affected the filter residue size and morphology. Figure 7c and d are partially enlarged drawings of the surroundings of spot 1 and spot 2 in Fig. 8a. The filter residue formed at 0 g/L seed consisted of smaller spindle-shaped crystals that were intergrown, and larger hexagonal block-shaped particles. The former wrapped the latter. The main phase of the spindle-shaped particles (spot 1) was hematite due to the high Fe and O contents. The hexagonal block-shaped particle (spot 2) is K-jarosite because of the large proportion of K, S and O, and low Fe content, which is similar to Fig. 5a3. As the seed addition increased, hematite crystals agglomerated into larger irregular particles, and the volume of jarosite was higher because it was not wrapped by fine hematite particles.
When the seed addition amount was 15 g/L, the Fe, Zn, and S contents in the filter residues were 59%, 0.92%, and 3.22%, respectively. The iron concentration in the supernatant is 4.182 g/L, which meets the requirements of the hematite process (Table 3). The iron content in the filter residues merely increased by 0.26% upon increasing the seed addition from 15 g/L to 25 g/L. To reduce the seed consumption, the optimum seed addition should be 15 g/L. The filter residues obtained at 15 g/L seed were characterized by XRD, and the results are shown in Fig. 8. The filter residues were mainly composed of hematite and a minor amount of K-jarosite. Overall, the optimum conditions of the hematite process in the leaching solution of the SZOP were a reaction temperature of 185 °C, an agitation speed of 500 rpm, and a seed addition of 15 g/L.
Sampling analysis of hematite behavior under the optimum conditions
To understand the reaction process under different agitation speeds, the hematite process in the leaching solution of the SZOP was sampled by different sampling methods and analyzed in terms of its pH, elemental analysis and XRD under the optimized temperature and seed addition. In real industry, the temperature of the leaching solution before iron removal is in the range of 90–100 °C, and the oxygen has been pumped into the solution at the beginning of the heating step. In other words, oxygen is introduced into the solution during the heating process prior to the hematite reaction and continues until the end of the reaction. To simulate this process under laboratory conditions, samples were taken during heating. Under laboratory heating conditions, it took 15 min for the solution temperature to rise from 150 °C to 185 °C. Therefore, the “-” of the “-15 min” denotes 15 min before the reaction in Fig. 9, and the temperature of the solution was 150 °C at this time. 185 °C means that the solution was heated to 185 °C, which is the starting point of the reaction time (0 min).
It is observed from formulas (2)–(6) that the formation reactions of hematite and jarosite liberate an acid. As shown in Fig. 10a, the agitation speed significantly influenced the solution pH. When the temperature reached 150 °C, the pH of the solution decreased to 2.990 at 400 rpm, 2.998 at 500 rpm, 4.096 at 600 rpm, and 4.134 at 700 rpm, respectively. Accordingly, the iron concentration of the solution decreased to 5.28 g/L at 400 rpm, 5.44 g/L at 500 rpm, 19.87 g/L at 600 rpm, and 20.33 g/L at 700 rpm (Fig. 10b), respectively. Consequently, the higher the agitation speed, the higher the reaction rate of reaction (1), resulting in a higher ferric ion concentration in the solution and a lower pH due to H+ generated by the hydrolysis of ferric iron. As shown in Fig. 10c, the K and Na contents in the filter residues increased from 0.52 to 4.01% and 0.063–0.47% upon increasing the agitation speed from 400 rpm to 700 rpm at 150 °C, respectively.
XRD analysis of the filter residues showed that their main component was hematite at 400 rpm and 500 rpm, and the main component was K-jarosite when the agitation speed was ≥ 600 rpm (Fig. 9). When the temperature increased to 185 °C, the formation of jarosite was complete because the pH of the solution was 0.584 at 600 rpm and 0.546 at 700 rpm, and it did not significantly change as the reaction progressed. The K and Na contents in the filter residues were the same as those at 150 °C. For the agitation speeds of 400 rpm and 500 rpm, the pH of the solution decreased slowly until the reaction time was 90 min. This indicates that the hematite reaction generally occurred in the early stage of the hematite process. During the hydrolysis of ferric iron in sulfate, jarosite preferentially precipitated at a low pH23, and the formation of jarosite was thermodynamically favorable (Fig. 4a). As illustrated in Fig. 9a and b, by decreasing the pH of the solution from 1.69 to 0.546 at 150 °C and 0.886 to 0.527 at 185 °C, the characteristic peaks of KFe3(SO4)2(OH)6 became clearer and sharper.
In addition, as the temperature increased, the solubility of the ferrous sulfate decreased44. As a result, the difference in the iron concentration between pulp and supernatant increased during heating (Fig. 10d). Increasing the agitation speed accelerated the dissolution of ferrous sulfate crystal and also accelerated the oxidation reaction rate, resulting in a rapid increase in the concentration of ferric ions in the solution. On the other hand, reaction Eq. (2) shows that the hydrolysis of Fe(III) to hematite is an acid-producing reaction. Cheng research shows that the solubility of hematite increases with an increase in free acid concentration for the given temperature, but it decreases as temperature is increased. In other words, the increase in the concentration of ferric ions in the solution may contain the contribution of hematite dissolution45. Previous research results showed that a higher ferric ion concentration is more conducive to the formation of jarosite23, so the jarosite phase in the filter residues gradually increased upon increasing the agitation speed.
Based on the above experimental data, it can be concluded that the reaction speed is very fast for the agitation speeds of 600 rpm and 700 rpm. When the temperature rises to 185 °C, the total iron concentration in the solution is below 1 g/L. Therefore, we did not carry out kinetic studies at the agitation speeds of 600 rpm and 700 rpm. The second-order reaction kinetic equation (Eq. 7) was used to fit the oxidation process of ferrous sulfate at 400 rpm and 500 rpm. Kinetic parameter for the oxidation of ferrous have been widely investigated using the methods based on the general kinetic equation as46,47:
where t is the reaction time, k is the reaction rate constant, \(\:{\text{P}}_{{\text{O}}_{\text{2}}}\) is the oxygen partial pressure.
The ferrous concentration in the supernatant versus time at different agitation speed is given in Fig. 11a. Linear fitting result of 1/[Fe(II)] versus time at different agitation speed is presented in Fig. 11b. The slope represents the rate constant for a certain temperature, the results of slope k are represented in Table 4. It can be seen from Table 4 that under the reaction conditions of 400 rpm and 500 rpm, the reaction rate constant is k1 > k2, which indicates that the oxidation reaction of ferrous is mainly carried out in the early stage of the hematite process. The oxidation reaction rate decreases after about 60 min of reaction time. The reaction rate constant increased more than two times upon increasing the agitation speed from 400 rpm to 500 rpm. The rapid oxidation of ferrous iron leads to an increase in the concentration of ferric ions in the solution, which is more conducive to the formation of jarosite.
Conclusions
The controllable mechanism of hazardous jarosite transformation into recyclable hematite in the leaching solution of secondary zinc oxide powder was systematically elucidated. Temperature and agitation speed were the main factors affecting the process index of iron removal during the hematite process. As the temperature increased, the formation of Fe2O3 and the decomposition reactions of jarosite (Na-jarosite, K-jarosite) were promoted, and the formation reactions of jarosite were correspondingly inhibited during heating. The reaction rate constant increased more than two times upon increasing the agitation speed from 400 rpm to 500 rpm, resulting in a higher ferric ion concentration in the solution and a lower pH of the solution due to the large amounts of H+ generated by the hydrolysis of ferric iron. This was more conducive to the formation of jarosite. Under the optimized conditions, the iron, sulfur, and zinc contents in the filter residues and the iron concentration in the supernatant were 59%, 3.22%, 0.92%, and 4.182 g/L, respectively, which meet the requirements of the industrial hematite process. The research has successfully achieved the controlled and waste-free removal of iron from aqueous solutions in the process of ironmaking, steelmaking dust and sludge hydrometallurgy.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Proctor, D. M. et al. Physical and chemical characteristics of blast furnace, basic oxygen furnace, and electric arc furnace steel industry slags. Environ. Sci. Technol. 34, 1576–1582 (2000).
Gargul, K. et al. Removal of zinc from dusts and sludges from basic oxygen furnaces in the process of ammoniacal leaching. Arch. Civ. Mech. Eng. 15, 179–187 (2015).
Omran, M. & Fabritius, T. Utilization of blast furnace sludge for the removal of zinc from steelmaking dusts using microwave heating. Sep. Purif. Technol. 210, 867–884 (2019).
Binnemans, K. et al. Hydrometallurgical processes for the recovery of metals from steel industry by-products: a critical review. J. Sustain. Metall. 6, 505–540 (2020).
Hamann, C. et al. Recycling of blast-furnace sludge by thermochemical treatment with spent iron (II) chloride solution from steel pickling. J. Hazard. Mater. 40, 123511 (2021).
World Steel Association. Steel Industry By-products Report (World Steel Association, 2020).
World Steel Association, World Steel in Figures, World Steel Association. https://worldsteel.org/ (2021).
Stewart, D. J. et al. The production of high value pig iron nuggets from steelmaking by-products-A thermodynamic evaluation. Resour. Conserv. Recy. 170, 105592 (2021).
Das, B. et al. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recy. 50, 40–57 (2007).
Trung, Z. H. et al. Acidic leaching both of zinc and iron from basic oxygen furnace sludge. J. Hazard. Mater. 192, 1100–1107 (2011).
Van Herck, P. et al. Zinc and lead removal from blast furnace sludge with a hydrometallurgical process. Environ. Sci. Technol. 34, 3802–3808 (2000).
Kretzschmar, R. et al. Speciation of Zn in blast furnace sludge from former sedimentation ponds using synchrotron X-ray diffraction, fluorescence, and absorption spectroscopy. Environ. Sci. Technol. 46, 12381–12390 (2012).
Omran, M. & Fabritius, T. Improved removal of zinc from blast furnace sludge by particle size separation and microwave heating. Min. Eng. 127, 265–276 (2018).
Jha, M. K. et al. Review of hydrometallurgical recovery of zinc from industrial wastes. Resour. Conserv. Recy. 33, 1–22 (2001).
Orhan, G. Leaching and cementation of heavy metals from electric arc furnace dust in alkaline medium. Hydrometallurgy 78, 236–245 (2005).
Stewart, D. J. & Barron, A. R. Pyrometallurgical removal of zinc from basic oxygen steelmaking dust-A review of best available technology. Resour. Conserv. Recy. 157, 104746 (2020).
Al-Harahsheh, M. et al. Microwave treatment of electric arc furnace dust with PVC: dielectric characterization and pyrolysis-leaching. J. Hazard. Mater. 274, 87–97 (2014).
Lundkvist, K. et al. Oxyfines technique for upgrading zinc containing blast furnace sludge-part 1: pilot trials. Metals 10, 1468 (2020).
Jiang, T. et al. Occurrence state and sulfuric-acid leaching behavior of germanium in secondary zinc oxide. Min. Eng. 137, 334–343 (2019).
Ismael, M. R. C. & Carvalho, J. M. R. Iron recovery from sulphate leach liquors in zinc hydrometallurgy. Min. Eng. 16, 31–39 (2003).
Sayilgan, E. et al. A review of technologies for the recovery of metals from spent alkaline and zinc–carbon batteries. Hydrometallurgy 97, 158–166 (2009).
Li, C. X. et al. Production of low-sulfur hematite by hydrothermal oxydrolysis of ferrous sulfate. Hydrometallurgy 178, 294–300 (2018).
Li, C. X. et al. Formation of iron hydroxysulphate phases in the hematite process by hydrolysis of ferric sulphate. Hydrometallurgy 189, 105112 (2019).
Deng, Z. G. et al. Behaviour and characterization of hematite process for iron removal in hydrometallurgical production. Can. Metall. Quart. 58, 223–231 (2019).
Niu, Z. et al. Resource-recycling and energy-saving innovation for iron removal in hydrometallurgy: Crystal transformation of ferric hydroxide precipitates by hydrothermal treatment. J. Hazard. Mater. 416, 125972 (2021).
Wang, K. et al. The effect of iron precipitation upon nickel losses from synthetic atmospheric nickel laterite leach solutions: statistical analysis and modelling. Hydrometallurgy 109, 140–152 (2011).
Mombelli, D. et al. Jarosite wastes reduction through blast furnace sludges for cast iron production. J. Environ. Chem. Eng. 7, 102966 (2019).
Wang, L. S. et al. Recovery of metals from jarosite of hydrometallurgical nickel production by thermal treatment and leaching. Hydrometallurgy 198, 105493 (2020).
Dutrizac, J. E. An overview of iron precipitation in hydrometallurgy. Cryst. Precip. 5, 259–283 (1987).
Cheng, T. C. & Demopoulos, G. P. Hydrolysis of ferric sulfate in the presence of zinc sulfate at 200°C: precipitation kinetics and product characterization. Ind. Eng. Chem. Res. 43, 6299–6308 (2004).
Xing, Y. B. et al. Dissolution behavior of ferrous sulfate in the hematite process. Hydrometallurgy 200, 105561 (2021).
B. Xing, Y. et al. Transformation behavior of hazardous jarosite into recyclable hematite in a solution with high concentrations of K+ and Na+. Sci. Rep. UK 14, 13949 (2024).
Dutrizac, J. E. Converting jarosite residues into compact hematite products. JOM 42, 36–39 (1990).
Dutrizac, J. E. & Sunyer, A. Hematite formation from jarosite type compounds by hydrothermal conversion. Can. Metall. Quart. 51, 11–23 (2012).
Nguyen, V. U. Study of conversion of waste jarosite precipitates to hematite. Inzh. Min. 15, 275–280 (2014).
Zeng, Y. J. et al. Simultaneous recovery of Fe2O3 and PbCl2 from hazardous jarosite residues via hydrothermal phase transformation with NaCl. Hydrometallurgy 221, 106150 (2023).
He, D. D. et al. Recycling of hazardous jarosite residues based on hydrothermal crystal transformation. Waste Manag. 172, 290–298 (2023).
Lugos, C. J. et al. Recovery of silver and lead from jarosite residues by roasting and reducing pyrometallurgical processes. Metals 14, 954 (2024).
Ostadrahimi, M. et al. Determining iron grades of ores or concentrates containing sulfide minerals. Metall. Mater. Trans. B 51, 505–509 (2020).
Ostadrahimi, M. et al. A comparison of Fe(III) to Fe(II) reduction methods in iron analysis via titration. Chem. Pap. 78, 5407–5414 (2024).
Roine, A. & Kobylin, P. HSC Chemistry 8.0. Outotec Research Center (2014).
Arima, H. Autoclave application for zinc leach residue treatment by Akita Zinc Co., Ltd., 34th Annual Hydrometallurgy Meeting, 949–963 (2004).
Deng, Z. G. et al. Recovery of red iron oxide pigment from zinc plant hematite precipitates. Can. Metall. Quart. 62, 24–32 (2021).
Hasegawa, F., Tozawa, K. & Nishimura, T. Solubility of ferrous sulfate in aqueous solutions at high temperatures. Shigen Sozai 112, 879–884 (1996).
Cheng, C. M. Production of hematite in acidic zinc sulphate media. McGill Univ. (Canada), 30–33 (2002).
Akilan, C. & Nicol, M. J. Kinetics of the oxidation of iron(II) by oxygen and hydrogen peroxide in concentrated chloride solutions–A re-evaluation and comparison with the oxidation of copper(I). Hydrometallurgy 166, 123–129 (2016).
Wermink, W. N. & Versteeg, G. F. The oxidation of Fe(II) in acidic sulfate solutions with air at elevated pressures. Part 1. Kinetics above 1 M H2SO4. Ind. Eng. Chem. Res. 56, 3775–3788 (2017).
Acknowledgements
This work was financially supported by the Major Scientific and Technological Projects of Yunnan in China (Grant Nos. 202202AG050008, 202302AB080012), the Science and technology talent programme of Yunnan in China (Grant No. 202405AC350015), the Postdoctoral Targeted Funding of Yunnan Province (Grant No. CG22256E017A), and the Analysis and Test Fund of Kunming University of Science and Technology in China (Grant No. 2020P20181102007).
Author information
Authors and Affiliations
Contributions
Y. X.: Writing-original draft, Methodology, Data curation, Conceptualization. C. W.: Validation, Formal analysis, Visualization.Z. D.: Writing-review & editing, Resources, Formal analysis, Validation.X. L.: Supervision, Data curation.M. L.: Resources, Investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xing, Y., Wei, C., Deng, Z. et al. Controllable mechanism of hazardous jarosite transformation into recyclable hematite in the leaching solution of secondary zinc oxide powder. Sci Rep 14, 24490 (2024). https://doi.org/10.1038/s41598-024-75857-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75857-5