Abstract
Bladder cancer treatments are highly aggressive and have strong side effects. Safer and more effective treatments are needed. In this study, Dibenzolium (DIB), a potent NADPH oxidase inhibitor, was evaluated for its anti-tumor effects. KK-47 (non-invasive), T24 and 5637 (invasive) cells were used in experiments. Cell proliferation, apoptosis and wound healing assays and western blotting were conducted. In addition, DIB was intratumorally administered to mice bearing KK-47, T24 and 5637 tumors, and tumor size and weight were observed over time. After removing tumors, immunohistochemistry (IHC) staining was conducted. Cell proliferation was significantly suppressed in all cell lines, and apoptotic cells increased in the KK-47 and T24 cell lines after DIB. Wound healing was suppressed in all cell lines by DIB. In KK-47 and T24, DIB increased the protein expression of the epithelial marker E-cadherin. In vivo, DIB safely suppressed tumor growth in all cell lines-bearing mice. Cleaved-Caspase-3 and E-cadherin expression increased in KK-47 and T24 tumors after DIB. In conclusion, DIB inhibited tumor growth by inducing apoptosis through the Caspase-3 pathway and reduced migration and invasion by suppressing epithelial mesenchymal transition (EMT) in bladder cancer similarly shown as our previous study of prostate cancer.
Similar content being viewed by others
Introduction
Bladder cancer is divided into non-muscle invasive and invasive types, and treatment differs greatly between the two types. Currently, total cystectomy, immune-check point inhibitor, antibody therapies,and chemotherapy with anticancer drugs are selected for muscular invasive cancer in Japan, but therapy is highly invasive and may cause strong side effects1. To develop more effective and minimally invasive molecular-targeted therapies, drugs showing specific antitumor effects against cancer are being developed.
The mechanisms of cancer progression include avoidance of programmed cell death and infiltration into the surrounding tumor environment. Programmed cell death mechanisms such as apoptosis have key roles in ultimate cancer cell fate2,3. Apoptosis is inhibited by some oncogenic mutations, leading to tumor initiation, progression or metastasis. Thus inducing apoptosis can suppress cancer progression4. Epithelial-mesenchymal transition (EMT) is one of the main mechanisms by which cancer cells acquire the ability to infiltrate and spread. EMT is associated with a variety of tumor functions including tumor development, tumor stem cell modifications, migration, intravascular invasion, metastasis, and resistance to treatment when cells lose epithelial features and acquire treatment tolerance5. EMT is also correlated with carcinogenesis, decreased survival, cancer cell invasion, and metastasis in breast and prostate cancer6,7.
Therefore, inhibiting EMT may lead to the development of anti-metastatic agents. Compared with other cancers, bladder cancer in the later stages tends to have high metastatic ability acquired through EMT8,9,10.
Dibenzolium (DIB) is a potent NADPH oxidase inhibitor and analog of the antidepressant Imipramine, which suppresses the growth and metastasis of cancer cells by inhibiting the activity of the protooncogene FoxM1 and associated signaling in DNA repair via homologous apoptosis in breast cancer cell lines11,12. Our author, Jack Arbiser, who is an inventor of Imipramine blue, assures us that Imipramine blue and dibenzolium are the same compound. DIB is a new name more consistent with chemical naming conventions, as in our previous study13.
In this study, we evaluated the anti-tumor effect of DIB derivative, using non-invasive and muscle invasive bladder cancer cell lines to investigate this novel molecular-targeted treatment method for bladder cancer.
Materials and methods
Cells
Human bladder cancer non-muscle layer invasive KK-47 cells and invasive T24 cells and 5637 cells were used. RPMI-1640 medium (10% fetal bovine serum and 1% penicillin / streptomycin solution added) was used to culture these three bladder cancer cell lines14.
We obtained human urothelial carcinoma cell lines, non-invasive KK47 (Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer, Tohoku University, Miyagi, Japan) and invasive T24 and 5637 (American Type Culture Collection, Manassas, VA) as in our previous study15,16.
DIB treatments
DIB (combining a triphenylmethane dye with the antidepressant imipramine17) was synthesized at 98% purity by Jack L Arbiser of Emory University (one author). DIB was dissolved in dimethyl sulfoxide (DMSO) and used in the experiment.
Cell proliferation assays
MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2 H-tetrazolium) (Promega, Wisconsin, USA) assays were performed to examine the inhibitory effect of DIB on cell proliferation. KK-47, T24 and 5637 cells were seeded on 96-well plates at 2.0 × 103 cells/well (n = 3), and after 24 h under conditions of 37ºC, 5% CO2, 0.1, 0.5, 1.0 µM DIB and DMSO at the same concentration were added and absorbance (490 nm) was measured over time.
Apoptosis assays
Apoptosis detection experiments were performed to verify the apoptosis-inducing effect of DIB. KK-47 and T-24 cells were seeded at 1.0 × 10⁶ cells/well (n = 3) and 5637 cells were seeded on 1.5 × 106 cells/well (n = 3) on 6-well plates. After 24 h under conditions of 37ºC, 5% CO2, 10 nM DIB and DMSO at the same concentration were added. After 48 h, apoptotic cells were analyzed by flow cytometry using the Annexin V-FITC Apoptosis Detection Kit (Nacalai Tesque, Kyoto, Japan). Apoptotic cells were determined by flow cytometry using BD FACSVerse™ Flowcytometer (Japan Becton Dickinson, CO, Ltd, Tokyo, Japan). In the apoptosis assay, DIB and DMSO were administered at a concentration of 0.01 μm each.
Wound healing assays
Wound healing analysis was performed to evaluate the infiltration ability and migration ability of the cells and investigate the EMT inhibitory effect of DIB. KK-47, T24 and 5637 cells were seeded on 12-well plates at 1.0 × 105 cells/well (n = 3). After 24 h, 5 ng/mL transforming growth factor-β (TGF-β) was added (TGF-β is inducer of EMT and invasiveness in bladder cancer cells18), and the cells were cultured for 24 h under conditions of 37ºC, 5% CO2. Alternately, 50 nM DIB and DMSO at the same concentration were added, and 48 h later the cells were injured and observed over time using the EOS utility (Canon, Ota, Japan). We calculated the wound area using ImageJ (https://imagej.net/ij/index.html) for wound healing analysis. The healing area (%) was shown by calculating the wound area after 24 h, 48 h, and 72 h from the wound closure proceeding. We added the software used for the analysis.
Western blotting
Western blotting was performed to investigate the mechanism of action of DIB at the protein level by evaluating the expression of EMT markers E-cadherin, N-cadherin and Vimentin, and protooncogene FoxM1. KK-47, T24 and 5637 cells were seeded on 6-well plates at 5.0 × 105 cells/well, and after 48 h 50 nM, 100 nM DIB and DMSO were added and cultured for 48 h. Western blotting was conducted as in the previous study15. In this study, anti-E-cadherin (Biolegend, California, USA), anti-N-cadherin (Proteintech Group Inc, Illinois, USA), anti-Vimentin (Proteintech Group Inc, Illinois, USA), anti-FoxM1 (Proteintech Group Inc, Illinois, USA), and anti-β-actin (Santa Cruz Biotechnology, Texas, USA) were used.
For the quantitative analyses of wound healing assays and western blots, we have used.
ImageJ software: https://imagej.net/ij/index.html19.
Animal experiments
Animal experiments were performed using the KK-47, T24 and 5637 cells-bearing mice model to investigate the antitumor effects of DIB in vivo. Cells (1.0 × 106 cell/mouse) and Matrigel were mixed and transplanted into 6–8-week-old Balb/c nu nu mice (KK-47: n = 3, T24 and 5637: n = 5). After 35 days for T24 cells and 83 days for 5637 and KK-47 cells after transplantation of the tumor, these mice were divided into a treatment group randomly with DIB (0.3 µg/mice) and control (DMSO) groups after tumors had grown to the ideal size. DIB was administered intratumorally. The tumor diameter was measured over time, and the tumor volume was calculated as (major diameter) x (minor diameter)2 × 0.5. Mice were also weighed for safety assessment. The drug treatments started when the tumor size exceeded 10 mm in major axis. Tumors with a major axis of 20 mm were set for euthanasia and no mice had tumors with a major axis exceeding 20 mm.
Ethical approval
All aspects of the experimental design and procedures were reviewed and approved by the institutional ethics and animal welfare committees of Kobe University (approval number: P210308). All procedures in animal experiments were performed in accordance with the ethical standards for animal testing facilities and were conducted in compliance with ARRIVE guidelines and related guidelines.
IHC staining
IHC staining was conducted as in the previous study15. In this study, we used anti-E-cadherin (Biolegend, California, USA) and anti-cleaved-Caspase-3 (Cell Signaling Technology, Massachusetts, USA) as primary antibodies. The titers of Cleaved-Caspase-3 and E-cadherin was each 1:400 dilution and amount of 150µL diluted 1st antibody was submitted.
IHC analysis
Stained immunohistochemical slides were observed under a BZ-X710 microscope (Keyence, Osaka, Japan) at x20 magnification (n = 5). The intensity of staining was evaluated as 0 (negative), 1 (weak), 2 (intermediate), and 3 (strong). The percentage of positive cells was evaluated on a three-point scale of 1 (0–10%), 2 (11–50%), and 3 (50% or more). The IHC score was calculated by multiplying the intensity and the percentage of positive cell scores.
Statistical analysis
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics (https://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/manual.html). Comparisons between two different groups were performed using Student’s T-test. Statistical differences among means were considered significant when p < 0.05.
Results
DIB suppressed cell proliferation in KK-47, T24 and 5637
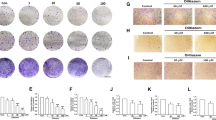
The absorbance every 24 h up to 72 h in KK-47, T24 and 5637 is shown in Fig. 1. The DIB group showed significantly suppressed cell proliferation at each concentration in all cells except 0.1 and 0.5 µM in T24, with a concentration-dependent effect (p < 0.05, or p < 0.01, or p < 0.001).
DIB increased apoptosis in KK-47 and T24
The percentage of detected apoptotic cells is shown separately for the early and late stages (Fig. 2). The rate of late apoptosis cells in KK-47 increased about 2 times by DIB compared to control (early: p < 0.001, late: p < 0.001). The rate of early apoptosis cells in T24 increased about 1.3 times by DIB compared to control, but there was no significant difference at the late stage (early: p = 0.0029). DIB had no significant apoptosis-inducing effect on 5637 cells.
Apoptosis-inducing effect of DIB by flow cytometry analysis. The percentage of detected apoptosis is shown separately for early and late stages. The rate of late apoptosis cells in KK-47 significantly increased by DIB compared to control even though the rate of early apoptosis cells on KK-47 halved). The rate of early apoptosis cells in T24 increased by DIB compared to control but DIB had no significant apoptosis-inducing effect on 5637 cells.
DIB suppressed wound healing in KK-47, T24 and 5637
The change over time in the healing area ratio after making the wound is shown in Fig. 3. In KK-47, 50 nM DIB suppressed wound healing to approximately 50% of control after 24 h (24 h: p = 0.0411, 48 h: p = 0.0148, 72 h: p = 0.0111) (Fig. 3A). In T24, 10 nM DIB suppressed wound healing after 72 h (p = 0.0478) and 50 nM DIB suppressed healing to approximately 40% of control after 6 h (6 h: p < 0.001, 24 h: p < 0.001, 48 h: p = 0.0093, 72 h: p = 0.0027) (Fig. 3B). In 5637, after 24 h 10 nM DIB suppressed wound healing to less than 30% of control (24 h: p = 0.0279, 48 h: p < 0.001, 72 h: p = 0.058) and 50 nM (24 h: p = 0.0326, 48 h: p = 0.0019, 72 h: p < 0.001) (Fig. 3C).
Wound healing inhibitory effect of DIB. The change over time in the healing area ratio after making the wound is shown, calculated from the healing area divided by the wound area at 0 h. Panel A. the KK-47 cell lines, significant differences between the control and DIB treatments were seen with 50nM DIB. Panel B. In T24, 10 nM DIB significantly inhibited wound healing after 72 h (p = 0.0478) and 50 nM after 6 h. Panel C. In 5637, after 24 h 10 nM DIB (24 h: p = 0.0279, 48 h: p < 0.001, 72 h: p = 0.058) and 50 nM DIB significantly inhibited wound healing. The change over time in the healing area ratio after making the wound is shown, calculated from the healing area divided by the wound area at 0 h. Regarding the KK-47 cell lines, significant differences between the control and DIB treatments were seen with 50nM DIB.
Western blotting
In KK-47, DIB decreased the expression of N-cadherin at 50 nM and 100 nM and increased the expression of E-cadherin at 100 nM. In T24, DIB increased the expression of E-cadherin at 50 nM and 100 nM. In 5637, DIB decreased the expression of N-cadherin and Vimentin at 50 nM and 100 nM (Fig. 4A). In KK-47 and T24, DIB decreased the expression of FoxM1 at 50nM and 100nM, but in 5637 there was no significant difference between control and DIB (Fig. 4B). And these findings were supported by quantification analyses divided by β-actin (Supplementary Fig. 1).
Protein expression of E-cadherin, N-cadherin, Vimentin and FoxM1. Panel A. In KK-47, DIB decreased the expression of N-cadherin and increased the E-cadherin. In T24, DIB increased the expression of E-cadherin at. In 5637, DIB decreased the expression of N-cadherin and Vimentin. Panel B. In KK-47 and T24, DIB decreased the expression of FoxM1.
Animal experiments
The tumor volume change rate was calculated, with the tumor volume before drug administration in each group set as 1 (Fig. 5). In KK-47-bearing mice, a significant tumor growth inhibitory effect was observed after the 3rd day (3rd: p = 0.0229, 4th: p = 0.0075, 5th: p = 0.0047, 6th: p = 0.0041, 7th: p < 0.001, 8th: p = 0.0013, 9th: p = 0.0014, 10th: p = 0.0018, 11th: p = 0.0019). In T24-bearing mice, a significant tumor growth inhibitory effect was observed on the 2nd day (p = 0.0472) and after the 6th day (6th: p = 0.0447, 7th: p = 0.0335, 8th: p = 0.0300, 9th: p = 0.0324, 10th: p = 0.0292, 11th: p = 0.0282). In 5637-bearing mice, a significant tumor growth inhibitory effect was observed after the 3rd day (3rd: p = 0.0251, 4th: p = 0.0124, 5th: p = 0.0073, 6th: p = 0.0066, 7th: p = 0.0048, 8th: p = 0.0044, 9th: p = 0.0014, 10th: p = 0.0013, 11th: p < 0.001). Finally, tumor growth was suppressed to about 30% in KK-47 and T24, and 50% in 5637 versus control.
Anti-tumor effects of DIB in mice. The tumor volume change rate was calculated with the tumor volume before drug administration in each group set as 1. In KK-47-bearing mice, a significant tumor growth inhibitory effect was observed after the 3rd day of treatments initiation. In T24-bearing mice, a significant tumor growth inhibitory effect was observed on the 2nd day and after the 6th day after treatments initiation. In 5637-bearing mice, a significant tumor growth inhibitory effect was observed after the 3rd day after treatments initiation.
IHC analysis
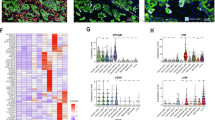
The IHC score of cleaved-Caspase-3 increased about 5 times in KK-47-bearing mice (p = 0.0101) and 2.5 times in T24 (p = 0.0457) after DIB treatment, but did not show a significant difference in 5637-bearing mice (Fig. 6A). The IHC score for E-cadherin increased about twice in KK-47-bearing mice (p = 0.0233) and about 1.5 times in T24-bearing mice (p = 0.0109) after DIB treatment, but did not show a significant difference in 5637 (Fig. 6B).
Expression of cleaved-Caspase-3 and E-cadherin in IHC. Panel A. The IHC score of cleaved-Caspase-3 increased in KK-47-bearing mice and T24-baring mice by DIB treatment (p < 0.05), but not in 5637-bearing mice. Panel B. The IHC score for E-cadherin increased in KK-47-bearing mice and T24-bearing mice (p = by DIB treatment, but not in 5637-bearing mice.
Discussion
DIB inhibited cell proliferation in KK-47 non-muscle layer invasive cells and T24 and 5637 invasive cells, and significantly increased apoptosis in KK-47 and T24 both in vitro and in vivo. Furthermore, DIB inhibited wound healing in KK-47, T24, and 5637 cells, suggesting that DIB reduces migration and invasion in these cell lines.
Apoptosis, a genetic program, is the predominant form of cell death triggered when DNA damage is irreparable4. Apoptosis has been frequently demonstrated in spontaneously regressing tumors and tumors treated with cytotoxic anticancer agents20, suggesting that apoptosis is involved in cell loss in malignant tumors and tumor progression4. In our study, apoptosis was not significantly induced by DIB treatment in 5637 cells in vitro, but was significantly induced in KK-47 and T24 cells. DIB treatment also inhibited tumor growth in vivo in all three cell lines. DIB significantly increased the expression of cleaved-Caspase-3 in KK-47 and T24 in vivo, suggesting that induction of apoptosis via caspases plays a major role in the inhibition of cancer cell growth by DIB. As to the difference of apoptosis-induction among cell lines, T24 and 5637 cells are both invasive cell lines, but there are genetic differences are related to the differences in drug sensitivity of in these cells. Specifically, T24 has a Ras mutation, but 5637 does not.
Previous studies have shown that differences in Ras mutations are associated with differences in drug sensitivity, and similar results have been reported in DIB11,21,22. In this study, we believe that the difference in drug sensitivity between T24 and 5637 is due to the presence or absence of Ras mutations. As to the DIB doses, in addition, although the effect of 50 nM DIB/72 hours was not examined in this experiment, bladder cancer cell death was induced at 10 nM DIB/24 hours in the apoptosis assay. And our cell proliferation and wound healing experiments had different DIB concentration because of the intension of wound healing assay is not killing cells.
Caspases are key mediators of apoptosis. In particular, Caspase-3 is a frequently activated death protease that catalyzes the specific cleavage of many important proteins. Caspase-3 is required for several classic hallmarks of apoptosis and is essential for chromatin condensation and DNA fragmentation leading to loss of cell viability23,24. In KK-47 and in T24, DIB increased the expression of cleaved-Caspase-3 in vivo. DIB appeared to induce apoptosis by promoting activation of Caspase-3 in both non-invasive and invasive cell lines, suppressing cell proliferation. Previous studies have shown that DIB treatment significantly increases caspase-3 cleavage and selectively induces cell death by apoptosis in acute myeloid leukemia cells25. DIB induces caspase activation and apoptosis by triggering the release of Ca2+ localized to the lysosome25,26. Ca 2+ release can occur through inhibition of calcium re-uptake channels or through disruption of cellular organelles that accumulate Ca2+, such as endoplasmic reticulum, Golgi apparatus, and lysosomes27,28. Considering the effect of DIB in acute myeloid leukemia cells, the mechanism of DIB activation of caspases in bladder cancer cells may be related to Ca2+ release or the destruction of cellular organelles. As to our selection of Caspase-3 in this study, since apoptosis was found to be involved in cell growth suppression, Caspase, an enzyme essential in the apoptotic pathway, might be involved. Therefore, we decided to first look at Caspase-3, an important effector caspase in caspase-related apoptosis. We obtained positive results with Caspase-3, and in the future we plan mechanistic studies to see which parts of the protein related to Caspase 3 DIB acts specifically on.
In addition, previous studies investigating the association between caspase-3 and the suppression of bladder cancer growth; for instance, as the caspace-3 for promising indicators of early recurrence in bladder cancer29 and apoptosis by Shikonin via caspase-3 in bladder cancer30. In keeping with the literature, this study also focuses on caspase-3 as a pivotal factor in bladder cancer apoptosis.
EMT is a crucial mechanism associated with cancer cell invasion and migration. DIB significantly suppressed wound healing in KK-47, T24, and 5637 cells. The effect was greater in T24 and 5637, which are invasive cell lines.
At the molecular level, E-cadherin is an epithelial marker and N-cadherin and Vimentin are mesenchymal markers. In EMT inhibition, E-cadherin increases and N-cadherin and Vimentin decrease31,32. In KK-47, DIB decreased the expression of N-cadherin in vitro and increased E-cadherin expression both in vitro and in vivo. In T24, DIB increased E-cadherin expression both in vitro and in vivo. In 5637, DIB decreased N-cadherin and Vimentin in vitro. These results suggest an EMT inhibitory effect of DIB in both non-invasive and invasive bladder cell lines. FoxM1, which is overexpressed in most human cancers, including bladder cancer, is also involved in cell migration, invasion, angiogenesis and metastasis33,34. In lung cancer cells, FoxM1 induces tumor cell EMT via activation of the AKT/p70S6K pathway35. Western blotting results showed that DIB treatment decreased FoxM1 expression in KK-47, T24 and 5637 cells. In sum, DIB suppressed cell proliferation and EMT by reducing FoxM1 expression in both non-invasive and invasive bladder cell lines. We investigated the association between DIB administration and FoxM1 expression by western blot. In T24 and KK47 cells, administration of DIB reduced the expression of FoxM1. Taken together, as to relationship between EMT and apoptosis and FOXM1, EMT is one of mechanisms for avoiding apoptosis36 and FOXM1 is a target of DIB, that is, inhibition of FoxM1-mediated DNA repair by DIB suppressing breast cancer growth and metastasis37.
In addition, many previous studies describing EMT inhibition found increased expression of epithelial markers, especially EMT markers15,31,35. Our results here suggest that EMT was suppressed in KK-47 and T24 due to increased expression of E-cadherin, a marker of epithelial cells. In 5637, the expression of E-cadherin was not increased by administration of DIB. Antitumor effects on 5637 need to be investigated further.
Regarding the difference of DIB drug concentrations among the in vitro assays, although the concentrations used on bladder cancer cell lines in this study were not examined in advance, our research team has already confirmed the antitumor effect of DIB in prostate cancer13, where cell proliferation was significantly suppressed at 0.5 μm and 1.0 μm. The proliferation test in this study is based on these results. In the wound healing and western blot assays, it was necessary to conduct the test with a certain number of cells still alive after administration of DIB, so the concentration was set based on the results of the proliferation test in this study.
In vivo, intratumoral administration of DIB was shown to significantly inhibit tumor growth in both non-invasive and invasive bladder cell lines without significant differences in body weight and physical appearance between control and DIB treated groups. Another study has already shown that DIB has no overt toxicity and no administration-related weight loss or infection11. The dosages are well tolerated by host animals. Thus, DIB may be an attractive candidate for preclinical studies as an antitumor agent. Compared to intraperitoneal injection, direct injection of the drug is thought to shorten the time to significant anti-tumor effects. For example, it was reported that after 2 days of anti-cancer peptide treatment, tumor suppression was 20% for intraperitoneal injection, but 60% for intratumoral injection [38].
We think the sensitivity to DIB of T24 and 5637 cells, which are invasive bladder cell lines, is related to genetic mutations in these cells. In T24, the RAS gene is mutated and possesses active Ras, while 5637 cells do not39,40. The Ras gene is frequently mutated in human cancers, such as breast and lung cancer, and is involved in cancer growth and metastasis41,42. In general, Ras mutant cancer cells are highly resistant to most chemotherapy, but our study showed that Ras mutations may confer sensitivity to DIB, thus meeting a large unmet needs 22. In addition, a previous study examining the antitumor effects of DIB reported that breast cancer cells MDA-MB-231 with Ras mutations were highly sensitive to DIB, with significantly inhibited cancer cell growth and metastasis in vitro and in vivo 11. It is possible that the Ras mutations of T24 cells explains their higher sensitivity to DIB than 5637 cells without Ras mutation.
The limitations of this study include its limited duration and the small number of mice in the in vivo experiment. The mechanisms of growth inhibition in 5637 cells should be further investigated to clarify which pathways are responsible for the antitumor effect of DIB. Next, we only examined the caspase-3 for apoptosis detection but no caspase-3 inhibiting test performed. Third, the data of DIB treatment and FoxM1 signaling pathway are missing. Forth, oxidative stress was not addressed in this study. We have missed the data of DIB IC50. Fifth, In vivo test, we had no tumor photos taken and no control group (normal bladder cells group). These limitations need to be addressed in future studies.
Conclusions
DIB exerts a cytostatic effect on both non-invasive and invasive cell lines by inducing apoptosis through the Caspase-3 pathway and reducing bladder cancer cell migration and invasion by suppressing EMT. Since DIB inhibited the growth and invasion of high grade cancer cells, it is expected to have anti-tumor effects against malignant muscle-invasive bladder cancer.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Choi, E. O., Park, C., Hwan, H. J., Hong, S. H. & Kim, G. Y. 5637 cells. Int. J. Oncol. 49(3), 1009–1018 (2016).
Carneiro, B. A. & EI- Deiry, W. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 17(7), 395–417 (2020).
Morana, O., Wood, W. & Gregory, C. D. The apoptosis paradox in cancer. Int. J. Mol. Sci. 25(3), 1328 (2022).
Xu, X. & Lai, Y. Zi- Chun Hua. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 39(1), BSR20180992 (2019).
Levgenia & Pastushenko Cédric Blanpain. EMT transition states during tumor progression and metastasis. Trends Cell. Biol. 29(3), 212–226 (2019).
Karsten Gravdal, Ole, J., Halvorsen, S. A., Haukaas, Lars, A. & Akslen A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin. Cancer Res. 13(23), 7003–7011 (2007).
Eloïse, M. et al. Triple-negative breast cancer metastasis involves complex epithelial-mesenchymal transition dynamics and requires vimentin. Sci. Transl Med. 14(656), eabn7571 (2022).
Amy, N., Abell, Gary, L. & Johnson Implications of mesenchymal cells in cancer stem cell populations: Relevance to EMT. Curr. Pathobiol. Rep. 2(1), 21–26 (2014).
Cao, R. et al. An EMT-related gene signature for the prognosis of human bladder cancer. J. Cell. Mol. Med. 24(1), 605–617 (2020).
Toshiro Migita, A. et al. Epithelial-mesenchymal transition promotes SOX2 and NANOG expression in bladder cancer. Lab. Invest. 97, 567–576 (2017).
Subapriya Rajamanickam, S. et al. Inhibition of FoxM1-mediated DNA repair by imipramine blue suppresses breast cancer growth and metastasis. Clin. Cancer Res. 22(14), 3524–3536 (2016).
Tobey, J. M. D. et al. Liposome-Imipramine Blue inhibits sonic hedgehog medulloblastoma in vivo. Cancers (Basel) 13(6), 1220 (2021).
Yura Jotatsu, K. Young-Min Yang, Masato Fujisawa. et al. Intralesional chemotherapy for prostate cancer: In vivo proof of Principle. Oncology 101(10), 645–654 (2023).
Li, X. et al. Srebp-2 promotes stem cell-like properties and metastasis by transcriptional activation of c- myc in prostate cancer. Oncotarget 12869–12884 (2016).
Koichi Kitagawa, K. et al. Nanaomycin K inhibited epithelial mesenchymal transition and tumor growth in bladder cancer cells in vitro and in vivo. Sci. Rep. 11(1), 9217 (2021).
Adhim, Z. et al. In vitro and in vivo inhibitory effect of three Cox-2 inhibitors and epithelial-to-mesenchymal transition in human bladder cancer cell lines. Br. J. Cancer 105(3), 393–402 (2011).
Jennifer, M. et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci. Transl. Med. 4(127), 127ra36 (2012).
Ganglong Yang, W. et al. Quantitative analysis of differential proteome expression in epithelial-to-mesenchymal transition of bladder epithelial cells using SILAC method. Molecules 21(1), 84 (2016).
Caroline, A., Schneider, W. S., Rasband, Kevin, W. & Eliceiri NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9(7), 671–675 (2012).
Ramzi, M. et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 35(Suppl (0), S78–S103 (2015).
Murugan, A. K., Grieco, M. & Tsuchida, N. RAS mutations in human cancers: Roles in precision medicine. Semin Cancer Biol. 59, 23–35 (2019).
Sreevidya Santha, X. et al. Mutant Kras as a Biomarker plays a favorable role in FL118-Induced apoptosis, reactive oxygen species (ROS) production and modulation of Survivin, Mcl-1 and XIAP in human bladder Cancer. Cancers 12(11), 3413 (2020).
Ashley & Boice Lisa Bouchier-Hayes. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta Mol. Cell. Res. 1867(6), 118688 (2020).
Kavanagh, E., Rodhe, J., Burguillos, M. A., Venero, J. L. & Joseph, B. Regulation of caspase-3 processing by cIAP2 controls the switch between pro-inflammatory activation and cell death in microglia. Cell. Death Dis. 5(12), e1565 (2014).
Jonathan Metts, H. L. et al. Imipramine blue sensitively and selectively targets FLT3-ITD positive acute myeloid leukemia cells. Sci. Rep. 7(1), 4447 (2017).
Carlotta Giorgi, F. et al. Mitochondrial ca (2+) and apoptosis. Cell. Calcium 52(1), 36–43 (2012).
Patergnani, S. et al. (2020) Paolo various aspects of calcium signaling in the regulation of apoptosis, autophagy, cell proliferation, and cancer. Int .J. Mol. Sci. 21(21), 8323
Danese, A. et al. Cell death as a result of calcium signaling modulation: A cancer-centric prospective. Biochim. Biophys. Acta Mol. Cell. Res. 1868(8), 119061 (2021).
Wang, J. et al. Livin, Survivin and Caspase 3 as early recurrence markers in non-muscle-invasive bladder cancer. World J. Urol. 32(6), 1477–1484 (2014).
Yeh, C. C. et al. Shikonin-induced apoptosis involves caspase-3 activity in a human bladder cancer cell line (T24). Vivo 21(6), 1011–1019 (2007).
Loh, C. Y. et al. The E-Cadherin and N-Cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 8(10), 1118 (2019).
Saima Usman, Naushin, H. et al. Vimentin is at the heart of epithelial mesenchymal transition (EMT) mediated metastasis. Cancers 13(19), 4985 (2021).
Marianna Halasi, Andrei, L. & Gartel Targeting FOXM1 in cancer. Biochem. Pharmacol. 85(5), 644–652 (2013).
Xinping Yang, Y. & Shi, J. Downregulation of FoxM1 inhibits cell growth and migration and invasion in bladder cancer cells. Am. J. Transl Res. 10(2), 629–638 (2018).
Jianguo Song. EMT or apoptosis: A decision for TGF-β. Cell Res. 17(4), 289–290 (2007).
Rajamanickam, S. et al. Inhibition of FoxM1-Mediated DNA repair by imipramine blue suppresses breast cancer growth and metastasis. Clin. Cancer Res. 22(14), 3524–3356 (2016).
Kong, F. F. et al. Overexpression of FOXM1 is associated with EMT and is a predictor of poor prognosis in non-small cell lung cancer. Oncol Rep. 14(6), 2660–2668 (2014).
Surachai Maijaroen, Sompong Klaynongsruang, Somrudee Reabroi, Arthit Chairoungdua, Sittiruk Roytrakul, Jureerut Daduan. Proteomic profiling reveals antitumor effects of RT2 peptide on a human colon carcinoma xenograft mouse model. Eur J Pharmacol. 917, 174753 (2022).
Swiss Institute of Bioinformatics. [cited 2023 Oct 16]. https://www.cellosaurus.org/CVCL_0554
Swiss Institute of Bioinformatics. [cited 2023 Oct 16]. https://www.cellosaurus.org/CVCL_0126
Shian-Ren & Lin Ntlotlang Mokgautsi Andyen-Nien Liu. Ras and wnt Interaction Contribute in prostate Cancer bone metastasis. Molecules 25(10), 2380 (2020).
Leanna, R., Gentry, T. D., Martin, D. J., Reiner & Channing, J. Dera. Ral small GTPase signaling and oncogenesis: More than just 15 minutes of fame. Biochim. Biophys. Acta 1843(12), 2976–2988 (2014).
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, JA and KS; methodology, JA and KS; validation, YJ, MM, IT and KS; formal analysis, YJ, MM, YH, KC and SS; investigation, YJ, MM, YH, ST, and KS; resources, KS and JA; data curation, YJ, MM, YH, ST, IT and KS ; writing—original draft preparation, YJ and MM; visualization, YJ , MM, KC, SS and KS; supervision, JA and KS ; project administration, KS.All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Emory University has a related patent (US20100160296A1) (Inventor: Jack L. Arbiser). All other authors declare that they do not have any competing interest.
Ethical approval
All aspects of the experimental design and procedures were reviewed and approved by the institutional ethics and animal welfare committees of Kobe University (approval number: P210308).
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jotatsu, Y., Arbiser, J.L., Moriwaki, M. et al. Dibenzolium induces apoptosis and inhibits epithelial-mesenchymal transition (EMT) in bladder cancer cell lines. Sci Rep 14, 25501 (2024). https://doi.org/10.1038/s41598-024-75908-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75908-x