Abstract
Decompensated cirrhotic patients experience severely increased intestinal permeability and bacterial translocation. Thus, autologous blood salvaged during deceased donor liver transplantation (DDLT) may be contaminated with enteric bacteria. We aimed to evaluate bacterial contamination of autologous blood salvaged during DDLT and its association with post-transplant bacteremia. In 30 patients undergoing DDLT, bacterial culture was performed in salvaged autologous blood samples: one before graft reperfusion (non-leukoreduced) and two after graft reperfusion (non-leukoreduced and leukoreduced). The primary outcome was bacterial contamination of salvaged autologous blood. Seven of 30 patients (23.3%) were positive for bacteria (3 enteric/4 non-enteric) before graft reperfusion while 11 patients (36.7%) were positive (5 enteric/6 non-enteric) after graft reperfusion. Six of 7 patients who were positive for bacteria before graft reperfusion were positive after graft reperfusion with the same bacteria. Only 4 of 11 contaminated blood samples were converted to negative after leukoreduction. Post-transplant bacteremia risk was insignificantly greater in patients who received autologous blood with bacteria than in patients without bacteria (30.0% vs. 5.0%, P = 0.06). We found contamination of salvaged autologous blood with enteric bacteria throughout DDLT and incomplete performance of leukoreduction, indicating high bacterial load. The potential association between contaminated autotransfusion and post-transplant bacteremia warrants further validation in a larger prospective study.
Clinical trial notation: This study was registered at the Clinical Research Information Service (CRiS; https://cris.nih.go.kr; No. KCT0007223; principal registration investigator: Sangbin Han, date of registration: April 25, 2022).
Similar content being viewed by others
Introduction
Blood salvage and autotransfusion technique is widely used during liver transplantation to decrease the requirement of allogeneic red blood cells and avoid transfusion-induced complications, like immuno-modulation and consequent infection1,2. However, salvaged autologous blood has the potential risk of bacterial contamination because the surgical field may be contaminated with bacteria from various sources including the gastrointestinal tract3. Intestinal permeability is increased in cirrhotic patients due to the compromised gut barrier, resulting in bacterial translocation from the gastrointestinal tract to other sites including the liver and bloodstream4. In our recent study, the proportion of salvaged autologous blood unit contaminated with enteric/non-enteric bacteria decreased after graft reperfusion in living donor liver transplantation (LDLT). Particularly, enteric bacteria were not found in autologous blood salvaged after graft reperfusion3. Also, all autologous blood units positive for bacteria were successfully converted to negative by using a single leukoreduction, indicating tolerable bacterial load and there was no post-transplant bacteremia3. However, it is unclear if this is applicable in patients undergoing deceased donor liver transplantation (DDLT) because the gut damage and intestinal permeability are known to increase in relation to decompensated cirrhosis as well as decreased functional status5. Thus, it is necessary to identify whether the aforementioned findings in LDLT recipients are observed in DDLT recipients. We hypothesized that the pattern of bacterial contamination of autologous blood salvaged during DDLT differs from that salvaged during LDLT. Thus, we aimed to investigate (1) bacterial contamination of autologous blood salvaged during DDLT, (2) the effect of leukoreduction on removing bacteria mixed in the autologous blood, and (3) the relationship between transfusion of contaminated autologous blood and post-transplant bacteremia.
Materials and methods
Subjects and ethics
In this prospective observational study, 30 patients undergoing emergency adult-to-adult DDLT (> 19 years old) were recruited between April 2022 and February 2023. Exclusion criteria include co-transplantation, re-transplantation, and any signs/symptoms of infection, including fever or the clues of respiratory infection (i.e., abnormal consolidation or opacity on chest radiographs, cough, and sputum), on operative day. This study was approved by the Institutional Review Board of Samsung medical center (SMC No. 2019-07-105) and was registered at the Clinical Research Information Service (CRiS; https://cris.nih.go.kr; No. KCT0007223; principal registration investigator: Sangbin Han, date of registration: April 25, 2022). The first participant was recruited after being registered at the CRiS, and informed consent was obtained from all participants. This study was conducted according to the principles stated in the Helsinki Convention. Korean Network for Organ Sharing, which was notified by the Korea Organ Donation Agency, allocated the liver according to the transplant waiting list in Korea through a computer matching program. The name of institutes from where the organs were obtained are as follows: Ajou medical center, Daegu Fatima hospital, Ewha womans university Mokdong hospital, Gacheon university Gill medical center, Gyeongsang national university hospital, Hallym university medical center, Inha university hospital, Jeonbuk national university hospital, Korea university medical center, Pusan national university hospital, Samsung medical center, Seoul national university hospital, and Soonchunhyang university hospital. No organs/tissues were procured from prisoners.
Autologous blood sampling time points
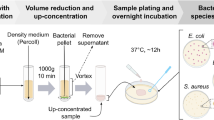
Blood culture was performed for three samples of each patient: one from autologous blood salvaged before graft reperfusion and two from autologous blood salvaged after graft reperfusion (one was sampled before leukoreduction and another was sampled after leukoreduction)6. Leukoreduction was performed using a leukocyte depletion filter (RCEZIT; Pall Biomedical Co, NY, USA)3. All samples were obtained by a dedicated cell salvage technician (S.R.Y.) with highly sterile precaution after the shed blood completed the in-device process (centrifugation, washing, and filtering) of blood salvage device (Cell Saver 5, Haemonetics, Braintree, MA) and was recollected in a sterile infusion bag2. Leukoreduction of salvaged autologous blood was performed for patients with hepatocellular carcinoma to remove cancer cells7,8. In patients without hepatocellular carcinoma, leukoreduction was performed only for the amount required for this study to perform blood culture3.
Bacteria culture process of sampled autologous blood
Bacterial culture was performed as described elsewhere3. Sampled autologous blood was immediately inoculated in one pair of culture bottles (BacT/Alert bottles, aerobic and anaerobic, BioMérieux, Marcy l’Etoile, France), 10 mL for aerobic culture and 10 mL for anaerobic culture, and transferred to the in-house central bacterial culture laboratory within 10 min after sampling. To prevent unintentional contamination of blood samples, the inlet of culture bottles was sterilized with 70% isopropyl alcohol prior to the inoculation. Blood culture was performed until bacterial growth was detected for a maximum of 5 days using a dedicated instrument (BacT/Alert 3D®, BioMérieux, Durham, USA). After gram staining, aerobic positive blood cultures were subcultured on three agar plates: 5% sheep blood agar, chocolate blood agar and MacConkey agar. These plates were incubated at 35 °C in atmosphere containing 5% CO2. For anaerobic positive blood culture bottles, aliquots of the broth were subcultured onto 5% sheep blood agar, MacConkey agar, and brucella agar. The brucella agar was incubated in an anaerobic chamber (Bactron II & IV, Sheldon Manufacturing Inc., Cornelius, OR, USA). Each isolated organism was identified using the Vitek 2 or Vitek MS system (BioMérieux, Marcy l’Etoile, France). Medical staff members conducting blood culture were not informed about the purpose of the study.
Patients underwent preoperative bacterial culture test for blood and other body fluids including sputum, urine, and ascites before surgery while postoperative blood culture test was performed at postoperative day 1, 3, and 5 according to routine clinical protocol. Blood or other body fluids were additionally tested when there are concern for infection as clinically indicated.
Anesthetic and surgical managements
Intraoperative anesthetic management was performed based on the standardized institutional protocol described elsewhere8,9. A central venous catheter (Multi-Lumen Access Catheters, MAC™, Arrow international, Wayne, PA, USA) was inserted at the internal jugular vein and angiocatheters were placed in the radial artery, femoral artery, and femoral vein while all procedures were performed by using maximal sterile barrier precautions technique based on the Center for Disease Control and Prevention guidelines10. In-house surveillance for central line-associated bloodstream infection (CLABSI) was routinely performed for all patients undergoing central line insertion in our hospital by the Center for Infection Prevention of our institution11. The amount/rate of fluid infusion was managed based on the pulse pressure variation and vasoactive agents were administered to maintain the mean arterial pressure ≥ 70 mmHg.
The positive pressure using laminar ventilation system was applied in our operating room and air was filtered over at least 50 change per hour12. Surgical management was performed based on the standardized institutional protocol described elsewhere13. Before laparotomy, surgical site was scrubbed with betadine soap and cleaned by using 2% chlorhexidine. After that, an antimicrobial drape film (Ioban™, 3 M™, Minnesota, USA) was applied on the abdomen from the xiphoid process to the anterior superior iliac spines. Piggyback technique was used for graft implantation. After the completion of the portal vein anastomosis, the graft was reperfused. Subsequently, the hepatic artery and bile duct were anastomosed. Immunosuppression was performed based on basiliximab, methylprednisolone, tacrolimus, and mycophenolate mofetil, as described elsewhere8.
Transfusion and blood salvage protocol
The transfusion policy of our transplant team is characterized by restrictive and prophylactic use of blood products while each blood component was separately transfused based on its respective indication, as described elsewhere7,8,14,15. In particular, the trigger for allogeneic red blood cell transfusion was hemoglobin concentration of 8.0 g/dL and only prestorage leukoreduced red blood cell units were used when allogeneic red blood cell transfusion was required15. Once peritoneal washing and ascites removal were completed following laparotomy, blood salvage was initiated. No medications, including antibiotics, were added to the salvaged blood. Based on the results of our recent research evaluating bacterial contamination of salvaged autologous blood in LDLT3, we changed our protocol and blood salvage and autotransfusion technique was continued even after bile duct anastomosis until the end of surgery. Accordingly, all patients in the current study received autologous blood salvaged before graft reperfusion as well as that salvaged after graft reperfusion.
Perioperative antibiotics treatment
Within 1 h prior to laparotomy, ampicillin/sulbactam 3 g (Ubacsin Inj., Jeil Pharmaceutical Co., Ltd., Seoul, Korea) and cefotaxime 2 g (C.K.D. Cefotaxime Inj., Chong Kun Dang Pharmaceutical Corp., Seoul, Korea) were administered to the recipients without a history of any infection. If antibiotics were prescribed before surgery due to infection (e.g. spontaneous bacterial peritonitis, cholangitis, or sepsis), the antibiotics were continuously used. Recipients who were using antibiotics due to pre-existing infection underwent an additional blood culture test before surgery. There were no recipients with active infection at the time of liver transplantation. Preoperative and postoperative antibiotics used for patients in the current study were described in Supplementary Table S1 and S2.
Outcome variables and statistics
The primary outcome was bacterial contamination defined as the isolation of Gram-positive cocci, Gram-positive bacilli, Gram-negative rods, or any kind of bacterial species during culture. The secondary outcome was postoperative bacteremia within 2 weeks after transplantation. Because there have been no reference studies with the same purpose, the sample size (n = 30) was determined based on the Roscoe’s simple rules of thumb and also for the comparable evaluation with our recent study of 29 LDLT recipients demonstrating bacterial contamination of salvaged autologous blood3,16. The McNemar’s test was used to analyze paired data. Continuous variables were described as median (interquartile range). Categorical variables were described as frequency (%) and compared between different subjects using chi-square test or Fisher’s exact test. The odds ratio (OR) and 95% confidence intervals (CI) of the incidence of post-transplant bacteremia according to autologous blood transfusion was also analyzed. Statistical analysis was performed using SPSS 25.0 (SPSS Inc., Chicago, IL, USA) or SAS version 9.4 (SAS Institute, Cary, NC, USA). P < 0.05 was considered as statistically significant.
Presentation
2022 Korean Society of Transplantation Anesthesiologists Conference.
Results
Patient characteristics
Thirty patients who met the inclusion criteria were consecutively enrolled in the study without dropout case. None of the patients underwent ductoplasty, hepaticojejunostomy, or bile duct stent insertion. None of the patients experienced any major surgical events (e.g., portocaval shunt, venoveno bypass, splenectomy, massive bleeding, or refractory postreperfusion syndrome). No patients were diagnosed with CLABSI. Three autologous blood samples were successfully drawn from all 30 recipients (Fig. 1). The indication for transplantations were alcohol-associated cirrhosis (n = 15), hepatocellular carcinoma (n = 12), cryptogenic cirrhosis (n = 2), and toxic acute hepatic failure (n = 1). Recipients’ characteristics and intraoperative data are presented in Table 1.
Bacterial contaminations of autologous blood samples
Table 2 presented detailed information on bacterial strain identified in the 3 autologous blood samples. There was a trend toward a higher proportion of autologous blood samples with bacterial contamination after graft reperfusion compared to before graft reperfusion (11/30 [36.7%] vs. 7/30 [23.3%], P = 0.25). All 7 patients with positive culture before graft reperfusion were also positive after graft reperfusion and 6 of the 7 patients showed the same bacteria at before and after graft reperfusion. One patient showed 2 different bacterial strains in one autologous blood sample salvaged before graft reperfusion while 2 patients showed 2 different bacterial strains in one autologous blood sample salvaged after graft reperfusion. Seven of the 11 contaminated autologous blood salvaged after graft reperfusion were still positive even after leukoreduction with a conversion ratio of only 36.4% (36.7% [11/30] to 23.3% [7/30], P = 0.25) (Table 2).
As shown in Tables 2 and 3, enteric strains detected in autologous blood salvaged before graft reperfusion were Escherichia coli (n = 1), Enterococcus faecium (n = 1), and Klebsiella pneumoniae (n = 1), while non-enteric flora were Staphylococcus epidermidis (n = 3), Stenotrophomonas maltophilia (n = 1), and Cutibacterium acnes (n = 1). Enteric strains detected in non-leukoreduced autologous blood salvaged after graft reperfusion were Escherichia coli (n = 1), Enterococcus faecium (n = 2), Enterococcus faecalis (n = 1), Streptococcus mitis/Streptococcus oralis (n = 1), and Klebsiella pneumoniae (n = 1) while non-enteric strains were Staphylococcus epidermidis (n = 4), Stenotrophomonas maltophilia (n = 1), Staphylococcus capitis (n = 1), and Acinetobacter baumannii (n = 1). The proportion of autologous blood samples contaminated with enteric bacteria were not significantly different between before and after graft reperfusion (10.0% [3/30] vs. 20.0% [6/30], P > 0.99) and the proportion of autologous blood samples contaminated with non-enteric bacteria were not significantly different, either (16.7% [5/30] vs. 23.3% [7/30], P > 0.99). Among the 5 samples positive at enteric bacteria, 2 samples were converted to negative after leukoreduction (conversion ratio = 40.0%) while enteric strains which remained in the 3 samples were Escherichia coli (n = 1), Enterococcus faecium (n = 2), and Streptococcus mitis/ Streptococcus oralis (n = 1). Among the 7 samples positive at non-enteric bacteria, 2 samples were converted to negative after leukoreduction (conversion ratio = 28.6%) while non-enteric strains which remained in the 5 samples were Staphylococcus epidermidis (n = 2), Stenotrophomonas maltophilia (n = 1), Staphylococcus capitis (n = 1), and Acinetobacter baumannii (n = 1).
Salvaged autologous blood transfusion and post-transplant bacteremia
Four of 30 patients (13.3%) developed bacteremia within 2 weeks after transplantation (Table 2). Among the 4 patients with bacteremia, 3 patients intraoperatively received salvaged autologous blood contaminated with bacteria and the causative bacteria strain of bacteremia and the bacteria found in autologous blood were identical (2 Enterococcus faecium and 1 Acinetobacter baumannii). Among the 11 patients with autologous blood contamination, 8 patients received the contaminated blood without leukoreduction and 3 patients with hepatocellular carcinoma received it after leukoreduction (one was converted to negative and the other two were still positive) while 2 of the 8 patients (25.0%) without leukoreduction and 1 of the 3 patients (33.3%) with leukoreduction, the one whose sample was still positive with Enterococcus faecium after leukoreduction, developed bacteremia (Table 2). As shown in Fig. 2, the risk of post-transplant bacteremia is greater in patients who received autologous blood positive at bacteria than in patients who received autologous blood negative at bacteria with marginal significance (30.0% [3/10] vs. 5.0% [1/20], OR: 8.14, 95% CI: -2/08, 4.08, P = 0.06).
Discussion
This study of patients undergoing DDLT (n = 30) was conducted as a series to our previous study investigating bacterial contamination of autologous blood salvaged during LDLT (n = 29)3. In line with the previous LDLT study, salvaged autologous blood was contaminated from the dissection and anhepatic phases. Of importance, the current study showed three findings different from the findings observed in LDLT3. First, enteric bacteria were consistently found even after graft reperfusion. The number of samples positive for enteric bacteria did not decrease after graft reperfusion in DDLT, whereas no enteric bacteria were found after graft reperfusion in LDLT. Second, leukoreduction was not as effective as in LDLT. Only 36.4% of contaminated autologous blood samples were converted to negative for bacteria after a single leukoreduction, although it was 100% in LDLT using the same leukocyte depletion filter. The unexpected poor conversion rate indicated that bacterial load in autologous blood salvaged during DDLT is very high, while the bacterial strains were similar to LDLT. Third, the potential association between contaminated autotransfusion and early post-transplant bacteremia was found with a marginal significance, whereas none of 13 LDLT patients with contaminated autotransfusion developed postoperative bacteremia in our previous study3, again indicating relatively high bacterial load in DDLT. These differences suggest the need to modify perioperative antibacterial strategy, including autotransfusion strategy, for patients undergoing DDLT.
In this study, bacteria classified as skin flora were frequently identified both before and after graft reperfusion, as shown in patients undergoing LDLT3. This finding clearly showed a limitation of the current skin disinfection strategy including 2% chlorhexidine and Ioban17. In terms of enteric bacteria, in contrast to LDLT3, the proportion of patients with contaminated salvaged blood was increased after graft reperfusion. In the LDLT study, we deduced that the amount of bacterial translocation from the gut to the extraintestinal areas already starts to decrease immediately after graft reperfusion during LDLT when the transplanted liver normally functions and the intestinal permeability recovers3. Because intestinal permeability is normalized with the newly transplanted liver, the amount of bacterial translocation after graft reperfusion may be determined by the balance between the amount of baseline mesenteric damages and the recovery rate of the damaged mesentery after graft reperfusion18. Thus, this conflicting result between LDLT and DDLT might be primarily attributable to more severe liver disease status and consequent severe mesenteric damages in DDLT recipients. In addition, we found that early post-transplant bacteremia occurred more frequently in DDLT. Jafarpour et al. demonstrated that renal replacement therapy, mechanical ventilation for more than 48 h, and elevated bilirubin levels were highly correlated with postoperative infections in liver transplant recipients19. In our studies, there were differences in these factors between DDLT and LDLT recipients. None of the LDLT recipients received preoperative renal replacement therapy or mechanical ventilation. In contrast, more than half of the DDLT recipients received preoperative renal replacement therapy or mechanical ventilation. Also, DDLT recipients and LDLT recipients showed significant differences in preoperative total bilirubin and creatinine levels. Reticuloendothelial system is also thought to play a role for the different findings between DDLT and LDLT. That is, DDLT recipients with decompensated liver function have severely impaired reticuloendothelial system, including Kupffer cells and liver sinusoidal endothelial cells20. Reticuloendothelial system has the function of removing various foreign substances or harmful substances including bacteria from circulating blood or interstitial space through phagocytosis. Thus, severely impaired phagocytosis in DDLT recipients can increase bacterial load in salvaged blood as well as the circulation, leading to greater bacteremia risk.
In this study, blood culture test results from recipients were investigated under the assumption that bacteremia that occurred within 2 weeks after DDLT can be related to contaminated autotransfusion. Hego investigated the types and frequency of infections occurring within 3 months after pediatric LT and found that bacterial infections occurred most frequently within the first 2 weeks21. In addition, According to Wißmann et al., some gram-negative bacteria, such as Acinetobacter calcoaceticus, Acinetobacter spp., and Escherichia coli, survived for up to 14 days on glass slides22. Since the 2000s, gram negative bacteria have been reported to be the most frequent cause of infection after LT23. Acinetobacter spp. and Escherichia coli were found in salvaged autologous blood in the current study; thus, it was considered reasonable to observe 2 weeks post-transplant. Although there are various sources of bacteremia, like indwelling intravascular catheters24 and pulmonary causes25, our findings clearly showed that autotransfusion is an important source of post-transplant bacteremia particularly in DDLT. Risk factors for bacteremia include old age, diabetes, pre- and post-transplant renal dysfunction, and hypoalbuminemia24,26. Further research may evaluate how these factors interact with contaminated autotransfusion and develop post-transplant bacteremia.
Only 4 of 11 blood samples positive for bacteria were converted to negative after leukoreduction. This unexpected finding was surprising and very different from the findings in LDLT where all 22 contaminated autologous blood samples were converted to negative after single leukoreduction3. The pore size of the finest layer of modern leukocyte depletion filters is 7 − 10 um and the reduction ratio for leukocyte reaches to 3 log (99.9% removal of leukocytes). Aside from the original purpose, leukocyte depletion filter also removes other cells in the blood (red blood cells, platelets, cancer cells27 and bacteria6,28) and the reduction ratio for different cells is determined by the size of the cell as well as the degree of adherence between the cells and filter fibers29. For instance, the reduction ratio for red blood cells (5 − 7 um) is about 15% and that for solid tumor cells (> 20 um) reaches > 7 log30. In other words, there should be an upper limit of bacterial load to be converted to negative by a leukocyte depletion filter and contaminated blood cannot be converted to negative by single leukoreduction if the bacterial load surpasses the capacity of leukocyte depletion filter, particularly considering that all bacterial strains observed in this study are smaller than the finest pores of leukocyte depletion filter. The biggest one observed in this study was Klebsiella pneumoniae with 6 − 7 um in length while other bacteria are < 2.5 um. In addition, we found that a same bacteria strain (Staphylococcus epidermidis and Staphylococcus capitis) was converted to negative after leukoreduction in some cases while being still positive even after leukoreduction in other cases, also suggesting that negative conversion depends upon bacterial load. We speculate that bacterial load in autologous blood salvaged from the abdominal cavity during DDLT is greater than LDLT and the bacterial load of DDLT surpasses the filtering capacity of modern leukocyte depletion filter. The greater bacterial load may be attributable to greater mesentery damages, greater bacterial translocation, lower functional capacity, and immune function of DDLT recipients31,32,33.
These findings of the current study different from observed findings in LDLT further suggest the need to apply a more advanced anti-bacterial strategy dedicated to DDLT, being differentiated from LDLT. First, the kind, dose, or treatment timings of perioperative antibiotics can be modified. For instance, additional antibiotics can be considered along with routine antibiotics when massive blood loss occurs and a large amount of fluid/blood is injected because both serum and tissue concentrations of antibiotics are significantly diluted34. Second, double-filtered leukoreduction can be considered, although it significantly delays the autotransfusion timing8,35. Future research is warranted to weigh the respective risk and benefit of delayed sterile autotransfusion vs. timely contaminated autotransfusion as well as the effects of double leukoreduction. Third, adding antibiotics in salvaged autologous blood before reinfusion can be considered. Lasko et al. reported that only 2% of antibiotics mixed in red blood cell unit remained after cell saver processes36, suggesting that most of prophylactic antibiotics injected to the blood circulation is washed out during the cell salvage process. Fourth, Enterococci species are particularly resistant to general antibiotics, and since there is concern about the emergence of multidrug-resistant bacteria such as vancomycin-resistant37, adequate broad spectrum antibiotics are required38. These suggestions for the dedicated anti-bacterial strategy for DDLT recipients are thought to be applicable considering the possible association between contaminated autotransfusion and early post-transplant bacteremia found in the current study and the known association between early postoperative infection and post-transplant morbidity/mortality39. In the current study, 3 of 4 patients with bacteremia received autologous blood contaminated with the bacteria identical with the bacteria found in the body blood after transplantation while Enterococcus faecium was found in the 2 of the 3 patients, being in line with a study reporting that Enterobacteriaceae and Enterococci were the most common strains responsible for post-transplant infection40.
We acknowledge several limitations in this study. First, the sample size was small and analyses were not adequately powered. Nonetheless, in combination to our recent study of LDLT recipients with almost same sample size but totally different findings3, the current study gives new insight regarding autologous blood contamination and its association with bacteremia after LT. Second, this study was not to compare the risk/benefit between allogeneic red blood cell transfusion and salvaged autologous transfusion. Blood salvage and autotransfusion technique is widely accepted in LT community especially for patients without hepatocellular carcinoma because the side effects, some of them are fatal8,15,41, of allogeneic red blood cell transfusion have long been elucidated. The findings in the current study should not directly connect to exclusion of blood salvage in DDLT. Third, different results could be obtained depending on the organ allocation system outside of Korea42. Lastly, dedicated fungal culture was not obtained in the current study while none of the bacterial cultures were positive for fungi and none of the 30 recipients developed post-transplant fungemia.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- LDLT:
-
Living donor liver transplantation
- DDLT:
-
Deceased donor liver transplantation
- CLABSI:
-
Central line-associated bloodstream infection
References
Klein, H. G., Spahn, D. R. & Carson, J. L. Red blood cell transfusion in clinical practice. Lancet. 370, 415–426. https://doi.org/10.1016/s0140-6736(07)61197-0 (2007).
Ashworth, A. & Klein, A. A. Cell salvage as part of a blood conservation strategy in anaesthesia. Br. J. Anaesth. 105, 401–416. https://doi.org/10.1093/bja/aeq244 (2010).
Kim, D. et al. Bile duct anastomosis does not promote bacterial contamination of autologous blood salvaged during living donor liver transplantation. Liver Transpl. 28, 1747–1755. https://doi.org/10.1002/lt.26525 (2022).
Alexopoulou, A., Agiasotelli, D., Vasilieva, L. E. & Dourakis, S. P. Bacterial translocation markers in liver cirrhosis. Annals Gastroenterol. 30, 486 (2017).
Nicoletti, A. et al. Intestinal permeability in the pathogenesis of liver damage: from non-alcoholic fatty liver disease to liver transplantation. World J. Gastroenterol. 25, 4814–4834. https://doi.org/10.3748/wjg.v25.i33.4814 (2019).
Dzik, W. Use of leukodepletion filters for the removal of bacteria. Immunol. Invest. 24, 95–115. https://doi.org/10.3109/08820139509062765 (1995).
Han, S. et al. Safety of the use of blood salvage and Autotransfusion during Liver Transplantation for Hepatocellular Carcinoma. Ann. Surg. 264, 339–343. https://doi.org/10.1097/sla.0000000000001486 (2016).
Kwon, J. H. et al. Blood salvage and autotransfusion with single leukoreduction does not increase the risk of tumor recurrence after liver transplantation for advanced hepatocellular carcinoma. Ann. Surg. 276, e842–e850. https://doi.org/10.1097/SLA.0000000000004866 (2022).
Han, S. et al. Bioreactance is not interchangeable with thermodilution for measuring cardiac output during adult liver transplantation. PloS One. 10, e0127981. https://doi.org/10.1371/journal.pone.0127981 (2015).
Cho, S. Y. et al. Impact of targeted interventions on trends in Central Line-Associated Bloodstream infection: a single-center experience from the Republic of Korea. Crit. Care Med. 45, e552–e558. https://doi.org/10.1097/ccm.0000000000002306 (2017).
Choi, J. Y. et al. Trends in the distribution and antimicrobial susceptibility of causative pathogens of device-associated infection in Korean intensive care units from 2006 to 2013: results from the Korean nosocomial infections Surveillance System (KONIS). J. Hosp. Infect. 92, 363–371. https://doi.org/10.1016/j.jhin.2015.12.012 (2016).
Gruenberg, M. F., Campaner, G. L., Sola, C. A. & Ortolan, E. G. Ultraclean air for prevention of postoperative infection after posterior spinal fusion with instrumentation: a comparison between surgeries performed with and without a vertical exponential filtered air-flow system. Spine (Phila Pa. 1976). 29, 2330–2334 (2004).
Lee, K. W. et al. Higher risk of posttransplant liver graft failure in male recipients of female donor grafts might not be due to anastomotic size disparity. Transplantation. 102, 1115–1123. https://doi.org/10.1097/TP.0000000000002118 (2018).
Han, S. et al. Association between intraoperative platelet transfusion and early graft regeneration in living donor liver transplantation. Ann. Surg. 264, 1065–1072. https://doi.org/10.1097/SLA.0000000000001526 (2016).
Kwon, J. H. et al. Decrease in the risk of posttransplant hepatocellular carcinoma recurrence after the conversion to prestorage leukoreduction for transfused red blood cells. Transplantation. 105, 577–585. https://doi.org/10.1097/TP.0000000000003265 (2021).
Hill, R. What sample size is enough in internet survey research. Interpers. Comput. Technology: Electron. J. 21st Century. 6, 1–10 (1998).
Johnston, D. H., Fairclough, J. A., Brown, E. M. & Morris, R. Rate of bacterial recolonization of the skin after preparation: four methods compared. Br. J. Surg. 74, 64. https://doi.org/10.1002/bjs.1800740121 (1987).
Ponziani, F. R. et al. Effect of liver transplantation on intestinal permeability and correlation with infection episodes. PLoS One. 15, e0235359. https://doi.org/10.1371/journal.pone.0235359 (2020).
Jafarpour, Z., Pouladfar, G., Malek Hosseini, S. A., Firoozifar, M. & Jafari, P. Bacterial infections in the early period after liver transplantation in adults: a prospective single-center cohort study. Microbiol. Immunol. 64, 407–415. https://doi.org/10.1111/1348-0421.12785 (2020).
Cousens, L. P. & Wing, E. J. Innate defenses in the liver during Listeria infection. Immunol. Rev. 174, 150–159. https://doi.org/10.1034/j.1600-0528.2002.017407.x (2000).
Nafady-Hego, H., Elgendy, H., Moghazy, W. E., Fukuda, K. & Uemoto, S. Pattern of bacterial and fungal infections in the first 3 months after pediatric living donor liver transplantation: an 11-year single-center experience. Liver Transpl. 17, 976–984. https://doi.org/10.1002/lt.22278 (2011).
Wißmann, J. et al. (ed, E.) Persistence of pathogens on Inanimate surfaces: a narrative review. Microorganisms 9 https://doi.org/10.3390/microorganisms9020343 (2021).
Singh, N. et al. Bacteremias in liver transplant recipients: shift toward gram-negative bacteria as predominant pathogens. Liver Transpl. 10, 844–849. https://doi.org/10.1002/lt.20214 (2004).
Kim, S. I. et al. Epidemiology and risk factors for bacteremia in 144 consecutive living-donor liver transplant recipients. Yonsei Med. J. 50, 112–121. https://doi.org/10.3349/ymj.2009.50.1.112 (2009).
Iida, T. et al. Posttransplant bacteremia in adult living donor liver transplant recipients. Liver Transpl. 16, 1379–1385. https://doi.org/10.1002/lt.22165 (2010).
Singh, N., Paterson, D. L., Gayowski, T., Wagener, M. M. & Marino, I. R. Predicting bacteremia and bacteremic mortality in liver transplant recipients. Liver Transpl. 6, 54–61. https://doi.org/10.1002/lt.500060112 (2000).
Gwak, M. S. et al. Can a leukocyte depletion filter (LDF) reduce the risk of reintroduction of hepatocellular carcinoma cells? Liver Transpl. 11, 331–335. https://doi.org/10.1002/lt.20346 (2005).
Waters, J. H., Hobson, T. M. & Procop, D. F. Bacterial reduction by cell salvage washing and leukocyte depletion filtration. Anesthesiology. 99, 652–655 (2003).
Callaerts, A. J., Gielis, M. L., Sprengers, E. D. & Muylle, L. The mechanism of white cell reduction by synthetic fiber cell filters. Transfusion. 33, 134–138 (1993).
Kongsgaard, U. E., Wang, M. Y. & Kvalheim, G. Leucocyte depletion filter removes cancer cells in human blood. Acta Anaesthesiol. Scand. 40, 118–120 (1996).
Pinzone, M. R., Celesia, B. M., Di Rosa, M., Cacopardo, B. & Nunnari, G. Microbial translocation in chronic liver diseases. Int J Microbiol 694629, doi: (2012). https://doi.org/10.1155/2012/694629 (2012).
Wiest, R. & Rath, H. C. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut. Best Pract. Res. Clin. Gastroenterol. 17, 397–425. https://doi.org/10.1016/s1521-6918(03)00024-6 (2003).
Finlayson, N. D., Krohn, K., Fauconnet, M. H. & Anderson, K. E. Significance of serum complement levels in chronic liver disease. Gastroenterology. 63, 653–659 (1972).
Swoboda, S. M., Merz, C., Kostuik, J., Trentler, B. & Lipsett, P. A. Does intraoperative blood loss affect antibiotic serum and tissue concentrations? Arch. Surg. 131, 1165–1171. https://doi.org/10.1001/archsurg.1996.01430230047009 (1996). discussion 1171 – 1162.
Chun, S. et al. Double-filtered leukoreduction as a method for risk reduction of transfusion-associated graft-versus-host disease. PLoS One. 15, e0229724. https://doi.org/10.1371/journal.pone.0229724 (2020).
Lasko, M. J., Conelius, A. M., Serrano, O. K., Nicolau, D. P. & Kuti, J. L. Impact of intraoperative cell salvage on concentrations of antibiotics used for Surgical Prophylaxis. Antimicrob. Agents Chemother. 64 https://doi.org/10.1128/aac.01725-20 (2020).
Hollenbeck, B. L. & Rice, L. B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence. 3, 421–433. https://doi.org/10.4161/viru.21282 (2012).
Wallace, M. J., Fishbein, S. R. S. & Dantas, G. Antimicrobial resistance in enteric bacteria: current state and next-generation solutions. Gut Microbes. 12, 1799654. https://doi.org/10.1080/19490976.2020.1799654 (2020).
Kim, S. I. Bacterial infection after liver transplantation. World J. Gastroenterol. 20, 6211–6220. https://doi.org/10.3748/wjg.v20.i20.6211 (2014).
Ferrarese, A. et al. Management of bacterial infection in the liver transplant candidate. World J. Hepatol. 10, 222–230. https://doi.org/10.4254/wjh.v10.i2.222 (2018).
Han, S. et al. Perioperative fresh red blood cell transfusion may negatively affect recipient survival after liver transplantation. Ann. Surg. 267, 346–351. https://doi.org/10.1097/SLA.0000000000002062 (2018).
Ha, H. S. et al. Deceased donor liver transplantation under the Korean model for end-stage liver disease score-based liver allocation system: 2-year allocation results at a high-volume transplantation center. Korean J. Transpl. 33, 112–117. https://doi.org/10.4285/jkstn.2019.33.4.112 (2019).
Funding
This study was supported by a grant provided by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Health and Welfare (HI22C189500).
Author information
Authors and Affiliations
Contributions
DK conducted the study, helped with designing the study, collected and analyzed data, writing the manuscript, and manuscript preparation. SH designed the study protocol, conducted the study, contributed to data collection, the interpretation of data, and revised the manuscript. JDY helped data interpretation, writing, and revision.JHK helped with conducting the study and data collection.GSC helped with conducting the study, data collection, and data interpretation, and manuscript preparation.JMK helped with data collection and interpretation. YJC helped with conducting the study and data collection.CC helped with conducting the study and data collectionJSK helped with data interpretation.MSG helped with data interpretation.JWJ helped with data interpretation.GSK contributed to the concept of the study and interpretation of data.All authors contributed to data analysis, drafting the article, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, D., Han, S., Yang, J.D. et al. Bacterial contamination of autologous blood salvaged during deceased donor liver transplantation: a prospective observational study. Sci Rep 14, 26785 (2024). https://doi.org/10.1038/s41598-024-76476-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76476-w