Abstract
Sickle cell anemia (SCA) is a monogenic blood disease with complex and multifactorial pathophysiology. The endocannabinoid system (ECS) could be a candidate for modulating SCA complications, such as priapism, as it has demonstrated an essential role in hematopoiesis, platelet aggregation, and immune responses. We evaluated the association of ECS-related single nucleotide polymorphisms (SNP) (FAAH rs324420, MAGL rs604300, CNR1 rs7766029, and CNR2 rs35761398) with priapism in a Brazilian SCA cohort. The study involved 138 SCA patients (n = 80 with priapism and n = 58 without priapism). SCA was detected with HPLC, and the Hb SS genotype was confirmed with PCR-RE. Alpha thalassemia mutations were detected with Multiplex-PCR, and SNP genotyping was performed using TaqMan genotyping assays. We observed a lower frequency of -α3.7kb-thalassemia mutation in patients with priapism than in patients without this complication (p < 0.001), and in adjusted multivariate analyses TT-CC genotype of CNR2 rs35761398 was associated with a lower chance of developing priapism (OR = 0.386 [0.175–0.854], p = 0.019) and a lower risk of it over time (HR = 0.634 [0.402–0.987], p = 0.049). The SCA ischemic priapism is related to unbalanced vasodilation/vasoconstriction pathways, such as decreased RhoA/Rho-kinase (ROCK) signaling. Since activating the type 2 cannabinoid receptor (CB2) decreases RhoA activation, we suggest a novel approach to SCA priapism involving CB2.
Similar content being viewed by others
Introduction
Sickle cell disease (SCD) is an inherited group of monogenic disorders characterized by a mutation in the beta-globin gene (HBB) that encodes hemoglobin (Hb) S, either in the homozygous state (sickle cell anemia, SCA) or compound heterozygous with another HBB mutation1,2. The primary determinant of SCA severity is the Hb S polymerization level, which generates rigid fibers of Hb S that distort and damage the membrane and cytoskeleton of erythrocytes, inducing sickling1,3,4. Erythrocyte injury results in both extra- and intravascular hemolysis, leading to endothelial dysfunction, vasculopathy, occlusion of small and large blood vessels, and consequently, tissue ischemia/reperfusion injury and inflammation5. Over time, this complex pathophysiology can lead to clinical acute events and chronic complications6.
Among the SCA clinical complications, priapism is a pathological condition defined as a prolonged and painful penile erection not associated with sexual drive7,8. Ischemic priapism, marked by penile pain and rigidity, is caused by blood entrapment in the corpora cavernosa sinusoids during erection7,9. Due to the critical hemolytic event in SCA, nitric oxide (NO) depletion results in persistent relaxation of the cavernosal tissue, preventing penile detumescence10,11.
Since Hb S mutation alone is insufficient to explain the SCA clinical subphenotypes, several studies have proposed the identification of genetic and biochemical modulators related to the disease’s pathophysiological mechanisms, establishing more accurate prognoses and guiding the development of more specific and effective therapies12,13. In this context, the endocannabinoid system (ECS) emerges as a potential modulator of clinical complications in SCA.
Endocannabinoids (EC), such as anandamide (AEA), are endogenous signaling lipids involved in several molecular activities in cells through the activation of G protein-coupled receptors (GPCRs) - cannabinoid receptors (CBs) type 1 and 2 (CB1 and CB2, respectively)14. The EC, their specific receptors, and the enzymes responsible for their metabolism constitute the ECS15. Recent studies revealed that EC interact with other GPCRs, peroxisome proliferator-activated nuclear receptors (PPARs), and selected ion channels, such as transient receptor potential vanilloid 1 (TRPV1)16,17,18. This pleiotropic action plays a key role for ECS in many physiological pathways and pathological states19.
In the SCA context, ECS participates in cellular and physiological processes of interest. AEA can stimulate hematopoiesis, inhibit platelet aggregation and aggregate formation in the blood underflow, and play a role in erythrocyte survival and physiology20,21,22. Furthermore, activation of CB2 suppresses the expression of adhesive molecules and modulates leukocyte adhesion and macrophage chemotaxis to inflammation and injury sites23,24. Therefore, mutations in genes involved in the signaling and metabolism of EC might contribute to developing specific subphenotypes in SCA. In this study, we aimed to evaluate the association of ECS single nucleotide variants (SNV) with priapism, an SCA hemolytic subphenotype, in a northeast Brazilian cohort.
Methods
Patients
The observational, case-control, non-paired analytic study was performed with 138 male SCA patients (median age 27 years, range 18 to 56 years). Hematologists and other health professionals regularly follow up with patients at the outpatient clinic of the Hemocentro de Pernambuco (HEMOPE), a reference center for SCD in the Pernambuco state, following the guidelines of the Clinical Protocol and Therapeutic Guidelines for Sickle Cell Disease (PCDT) of the Brazilian Ministry of Health25.
The clinical history of 440 SCA male patients was evaluated through medical records. Patients without regular outpatient follow-up at HEMOPE, patients under 18 years old, patients using hydroxyurea, and patients who presented clinical complications such as acute chest syndrome (ACS), stroke, leg ulcers, and osteonecrosis were excluded from the study. The priapism group was composed of 80 patients with a current history of painful penile erection lasting 1 to 4 h in their medical records. Priapism episodes were confirmed by physical examination when patients sought medical care. The control group included 58 SCA patients without the clinical complications described above (ACS, stroke, leg ulcers, and osteonecrosis). The clinical definition and diagnostic criteria for clinical complications in SCA followed the protocols established in the literature26,27. Demographic and laboratory data of age, total hemoglobin, reticulocyte count, indirect bilirubin (IB), and lactate dehydrogenase (LDH) were obtained close to the date of medical record analysis.
According to Brazilian Regulations, the Data Safety Monitoring Board (DSMB) approved this study (CAAE: 58863316.6.3001.5195). Following the Declaration of Helsinki, informed consent forms were obtained from all patients, their parents, or legal guardians, when applicable. All methods were carried out in accordance with relevant guidelines and regulations.
SCA diagnosis confirmation and alpha thalassemia analysis
Peripheral blood samples were collected from all patients, and genomic DNA was extracted using the phenol-chloroform protocol (UltraPure™ Buffer-Saturated Phenol, 15513039, Thermo Fisher Scientific)28. The Hb SS phenotype was diagnosed by high-performance liquid chromatography (HPLC) (Premier Resolution, Trinity Biotech). The SCD genotype was confirmed by polymerase chain reaction (PCR) using Taq DNA polymerase enzyme (11615036, Thermo Fisher Scientific) and thermal cycler (SimpliAmp, Thermo Fisher Scientific), followed by restriction analysis with DdeI (ER1882, Thermo Fisher Scientific)29. Alpha-thalassemia mutations were detected with Multiplex-PCR using the Multiplex PCR Kit (206143, Qiagen) and thermal cycler (SimpliAmp, Thermo Fisher Scientific)30. Primers and other reagents for the reactions are specified in the references.
Single nucleotide polymorphisms (SNPs) selection and genotyping
Relying on the functional impact or minor allele frequencies (MAF) > 10%, we selected four SNPs in the ECS that could be related to SCA. The rs324420 is one of the most-studied SNPs of the FAAH gene, which encodes the fatty-acid amide hydrolase (FAAH), an enzyme responsible for AEA hydrolysis and inactivation. This SNP is characterized by a cytosine (C) > adenine (A) substitution, and the A allele affects FAAH activity due to increased sensitivity to proteolytic degradation, resulting in higher AEA peripheral concentration31,32. The rs604300 [A > guanine (G) substitution] is an intronic SNP located just downstream of an enhancer site of the MAGL gene, which encodes the monoacylglycerol lipase (MAGL), responsible for the hydrolysis of another EC, 2-arachidonoylglycerol (2-AG). Since this enhancer site is heavily influenced by epigenetic modification, this SNP may affect MAGL expression in an epigenetically-dependent way, affecting 2-AG concentration as well33.
Regarding the CBs, rs7766029 [C > tyrosine (T) substitution] is located in the 3’-untranslated region (3’-UTR) of CNR1 exon 4, involved in gene expression regulation and, thus, CB1 production34. Finally, the CNR2 rs35761398 (TT > CC substitution) is a common variant that affects the response of CB2 to cannabinoids, reducing cannabinoid immune modulation and differently regulating the EC-induced inhibition of lymphocyte proliferation35,36,37. We performed genotyping analyses using TaqMan SNP assays (4351379, Thermo Fisher Scientific) [C_1897306_10 (FAAH rs324420), C_2095673_20 (MAGL rs604300), C_28979971_20 (CNR1 rs7766029) and C_33555412_20 (CNR2 rs35761398)], with TaqMan Genotyping Master Mix (4371355, Thermo Fisher Scientific) and QuantStudio5 Real-Time PCR (Thermo Fisher Scientific) following manufacturer’s instructions.
Statistical analyses
We performed statistical analyses using SPSS Statistics 26.0 (IBM Corporation, Somers, NY, USA) and R 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). At the same time, graphs and figures were produced with GraphPad Prism 9.0 (GraphPad Software, CA, USA). Continuous variables were expressed as medians and were tested for normal distribution using Lilliefor’s test. We compared the medians between groups using the Mann-Whitney test. We assessed the distribution of genotypes for each SNP for deviation from the Hardy–Weinberg equilibrium (HWE). Moreover, we applied Fisher’s exact and Pearson’s Chi-square tests for genotype and allele frequency comparisons between groups. The SNP inheritance models were analyzed using the R package “SNPassoc.”
Cumulative incidence curves reflecting time to complication development were constructed using the Kaplan–Meier method and the log-rank test to compare the curves. Multivariate binary logistic regression and Cox proportional hazard regression were performed for SNP genotypes and potential prognostic factors for the priapism subphenotype. All p-values were two-sided, with a significance level of 0.05.
Results
The association analysis of genetics, demography, and laboratory data between groups is represented in Table 1. We observed a lower frequency of -α3.7kb-thalassemia mutation in patients with priapism than in patients without this complication (p < 0.001), and we did not observe statistical differences between other parameters.
The distribution of genotypes for the four polymorphisms studied herein showed no deviation from HWE (all p > 0.05; Supplementary Table S1). Considering the genotype and allele frequencies, we did not observe statistical differences between priapism and control groups (Supplementary Table S1). Also, the allele frequencies in our population were not statistically different from the MAF for the Latin America 1 subpopulation (NCBI’s Allele Frequency Aggregator [ALFA] dataset, dbSNP database, BioSample: SAMN10492699; Sample name: Latin American 1)38.
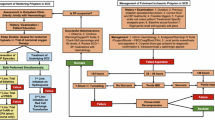
We analyzed all SNPs’ co-dominant, dominant, recessive, and over-dominant inheritance models. Due to a better data fit, we considered the dominant model for MAGL rs604300 (p = 0.049) and the overdominant model for FAAH rs324420 (p = 0.049), CNR1 rs7766029 (p = 0.025) and CNR2 rs35761398 (p = 0.038). Adopting these models, we did not observe statistical differences between the SNPs’ genotype frequencies in priapism and control groups (Fig. 1), nor a statistical impact of all possible SNPs’ inheritance models in the laboratory markers (total Hb, reticulocyte count, IB, and LDH), except for a lower level of total Hb related to the TT-CC genotype of CNR2 (Supplementary Tables S2-5).
Genotype frequencies in priapism and control groups for rs324420, rs604300, rs7766029, and rs35761398. Pearson’s Qui-square test was performed for FAAH, CNR1, and CNR2 frequencies comparisons, and Fisher’s exact test for MAGL frequencies comparison. FAAH, fatty-acid amide hydrolase; MAGL, monoacylglycerol lipase; CNR1, cannabinoid receptor type 1; CNR2, cannabinoid receptor type 2.
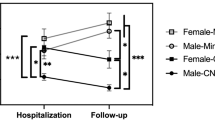
Since the -α3.7kb-thalassemia mutation frequencies significantly differed between the groups (Table 1), we performed multivariate analyses adjusted by this variable as a covariate. In binary logistic regression analysis, we found that the TT-CC genotype for CNR2 rs35761398 conferred a protective effect on priapism occurrence (OR = 0.386 [0.175–0.854], p = 0.019), regardless of the -α3.7kb-thalassemia mutation (OR = 0.148 [0.059–0.375], p < 0.001) (Fig. 2). We did not observe statistical differences between priapism and the other SNPs.
Forest plot of multiple binary logistic regression analysis of selected SNPs and priapism vs. control group. The horizontal lines correspond to the study-specific OR and 95% CI. Each of the SNPs was included separately in different models adjusted by -α3.7kb-thalassemia mutation as a covariate. FAAH, fatty-acid amide hydrolase; MAGL, monoacylglycerol lipase; CNR1, cannabinoid receptor type 1; CNR2, cannabinoid receptor type 2; OR, odds ratio; CI, confidence interval.
Considering the binary logistic regression results, we performed cumulative incidence and Cox proportional hazards regression analyses only for CNR2 rs35761398. Adopting the Kaplan-Meier method with the log-rank test, we did not observe a statistical difference for patients with TT-CC genotype compared to patients with TT-TT/CC-CC genotypes (p = 0.203) (Supplementary Fig. S1). In Cox proportional hazards regression analysis, the CNR2 rs35761398 TT-CC genotype was associated with a lower risk of priapism over time (HR = 0.634 [0.402–0.987], p = 0.049), regardless of the -α3.7kb-thalassemia mutation (HR = 0.348 [0.177–0.682], p = 0.002) (Fig. 3). The cumulative incidence curve for priapism development based on the Cox proportional hazards regression model is also represented in Fig. 3.
Cox proportional hazards regression and cumulative incidence curve for priapism development considering -α3.7kb-thalassemia mutation as a covariate. Hazard ratio and cumulative incidence of priapism in patients with SCA according to the CNR2 rs35761398 polymorphism, adjusted by -α3.7kb-thalassemia mutation as a covariate, using Cox proportional hazards regression analysis. HR, hazard ratio; CI, confidence interval.
Discussion
Association studies with SCA clinical outcomes and genetic variants have the potential to help overcome several challenges that arise from this remarkable phenotypic heterogeneity39. Moreover, comprehending the causes of variability leads to identifying new pathological mechanisms and their association with well-known pathophysiology pathways, suggesting new therapeutic approaches6. Here, we investigated, for the first time to our knowledge, the association of ECS polymorphisms and priapism, an SCA hemolytic subphenotype.
Notably, alpha-thalassemia is one of the central modulators of SCA severity outcomes5. Since it attenuates hemolysis by reducing mean cell Hb concentration and cell density, thereby reducing Hb S polymerization rate, co-inheritance with SCA is associated with lower chances of developing clinical complications40,41,42. Our data agrees with these findings, considering -α3.7kb-thalassemia mutation presented a protective effect on priapism occurrence, giving a lower chance of developing this outcome and a lower risk over time. Studies have shown that the frequency of heterozygous α-thalassemia in SCD is 30 to 35% and 3 to 5% for the homozygous form, both for the -α3.7kb mutation13. Another review shows that at least 40% of patients have some form of α-thalassemia, in which approximately 35% are heterozygous for -α3.7kb and 5% are homozygous43. In our study, the control group presented a frequency of 41.4% of heterozygous α-thalassemia (-α3.7kb), that is, a value close to the references cited previously, which demonstrates that α-thalassemia is a factor that mitigates SCA severity, such as reducing the occurrence of priapism and the absence of other complications such as stroke, leg ulcer, osteonecrosis, and ACS.
Regarding the inheritance SNP models, the dominant for MAGL and the overdominant for FAAH, CNR1, and CNR2 were statistically significant. However, we did not find a statistical difference among the genotype frequencies in the control and priapism groups. When we analyzed the impact of such inheritance models in the patients’ laboratory markers, we did not find statistical differences, except for a lower level of total Hb related to the TT-CC genotype of CNR2. Despite that, all patients are in a pathophysiological state marked by lower Hb levels than expected, and this result could be related to other SCA pathological mechanisms. Since no studies relate the ECS SNPs analyzed here to SCA, we cannot compare our findings. Allele frequencies were also not significantly different between our groups, nor with the MAF of each SNP, considering our population’s ethnicity.
So, our study provides a first-time inheritance model for ECS SNPs in SCA and characterizes these SNPs in a Brazilian cohort. Moreover, the inheritance model of an SNP in a disease can be vital to unraveling the genetic characteristics of complex diseases such as SCA and linking disease phenotypes to specific genotypes. In our study, we demonstrated for the first time that the rs35761398 CNR2 polymorphism presented a protective effect in priapism occurrence, with a lower chance and a lower risk over time of its development.
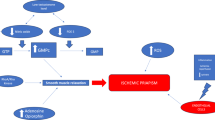
SCA patients with the highest hemolysis rates are more likely to develop SCA hemolytic subphenotypes, such as priapism44,45. Ischemic priapism is caused by blood entrapment in the corpora cavernosa sinusoids during erection. Recent studies revealed a probable molecular basis of ischemic priapism involving nitric oxide (NO) depletion, resulting in persistent cavernosal tissue relaxation10,11.
Normal erectile physiology is regulated by the NO/cyclic guanosine monophosphate (cGMP) pathway46. Endothelial NOS (eNOS) and neuronal NOS (nNOS) convert L-arginine in L-citrulline, releasing NO that binds to guanylate cyclase and converts guanosine-5’-triphosphate (GTP) to cGMP. After recruiting the cGMP-dependent protein kinase G (PKG), the cascade promotes the relaxation of corpora cavernosal smooth muscle and vasodilation of blood vessels, leading to penile erection47. The cGMP-specific type 5 phosphodiesterase (PDE5) inactivates cGMP, converting it to 5’-GMP, terminating erection48.
The dysregulation of the NO/cGMP/PKG pathway is the primary molecular mechanism of ischemic priapism in SCA49. Hemolysis releases free Hb and arginase, which scavenges NO and degrades L-arginine5. eNOS functional uncoupling, decreased phosphorylation, and increased oxidative/nitrosative stress are also involved in the reduced activity of eNOS in SCA-related ischemic priapism50,51,52,53,54. The chronic decrease in endothelial NO results in reduced cGMP production and a compensatory reduction in PDE5 expression and activity. Thus, cGMP accumulates under erectogenic stimulation, resulting in priapism11.
The dysregulated RhoA/Rho-kinase (ROCK) vasoconstrictor pathway also contributes to the pathophysiology of priapism48. In normal physiological conditions, RhoA agonist activation of ROCK mediates vasoconstriction of the penile vasculature and regulates eNOS expression and activation, maintaining the penis’ flaccid state55. In sickle cell mice, both RhoA and total ROCK activities are significantly reduced, and further studies confirmed a dysregulated Rho signaling with reduced RhoA expression in human SCA penile tissue56,57. Such dysregulation leads to reduced vasoconstriction in the penis in SCA, maintaining cavernous relaxation and increasing susceptibility to priapism.
In the ECS context, a study demonstrated that a CB2 agonist decreased TNF-α-induced RhoA activation in human coronary artery endothelial cells (HCAECs), and a CB2 antagonist attenuated this effect58. CB2 receptors are mainly expressed in immune cells, with the highest levels in macrophages, B cells, NK cells, monocytes, and polymorphonuclear neutrophils59. Endothelial cells and smooth muscle also express CB260. It potently modulates immune responses, positively or negatively, and its expression is highly induced by tissue injury or inflammation61. According to our findings, we hypothesize that a reduced, yet basal, level of CB2 activation in endothelial cells of penile vasculature, related to the TT-CC genotype for the CNR2 SNP analyzed, might play a role in maintaining regular levels of RhoA/ROCK pathway recruitment. Therefore, it would decrease the chances of developing priapism. Interestingly, repeated priapism episodes have been reported in SCA patients following recreative Cannabis use62. Such anecdotal finding reinforces the hypothesis of cannabinoid receptor activation and decreased RhoA/ROCK recruitment in penile vascular modulation in such cases.
The rs35761398 SNP is a missense mutation of the second and third bases at codon 63 of the CNR2 gene, leading to a glutamine (Q)/arginine (R) substitution, which causes a different polarization state of the protein35. The signal intensity caused by 63R activation seems weaker than the signal by 63Q activation, and a molecular docking study showed that the predicted structures of 63R could not bind to the G-protein at the correct position, with decreased signaling transduction mechanisms36,37,63. As the TT-CC genotype may allow the expression of both 63Q/63R CB2 receptors, with varying levels among different cell types or physiological states, that could explain to some extent the protective effect of such genotype in priapism occurrence.
In conclusion, we provide the first association of ECS SNPs in SCA and report the characterization of these SNPs in a Brazilian population. Finally, our data suggest a novel approach to the pathophysiology of priapism in SCA involving CB2. Nevertheless, the exact functional consequences of CNR2 rs35761398 in CB2 activity in the SCA context still need to be well-established, so our findings should be considered cautiously, and validations in independent cohorts could significantly strengthen our study. Also, evaluating other biochemical and molecular markers involving the RhoA/ROCK pathway could contribute to the elucidation of CB2, inflammation, and vasoconstriction in ischemic priapism. Considering recent moves towards using cannabinoids in SCD64, our results also pave the way to the further characterization of ECS genetic polymorphisms in this population to guide future safety and efficacy trials.
Data availability
This published article (and its Supplementary Information files) includes all data generated or analyzed during this study.
References
Kato, G. J. et al. Sickle cell disease. Nat. Rev. Dis. Prim. 4, 18010. https://doi.org/10.1038/nrdp.2018.10 (2018).
Ware, R. E., de Montalembert, M., Tshilolo, L. & Abboud, M. R. Sickle cell disease. Lancet. 390 (10091), 311–323. https://doi.org/10.1016/S0140-6736(17)30193-9 (2017).
Rees, D. C., Williams, T. N. & Gladwin, M. T. Sickle-cell disease. Lancet. 376, 2018–2031. https://doi.org/10.1016/S0140-6736(10)61029-X (2010).
Barabino, G. A., Platt, M. O. & Kaul, D. K. Sickle cell biomechanics. Annu. Rev. Biomed. Eng. 12, 345–367. https://doi.org/10.1146/annurev-bioeng-070909-105339 (2010).
Kato, G. J., Steinberg, M. H. & Gladwin, M. T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Invest. 127 (3), 750–760. https://doi.org/10.1172/JCI89741 (2017).
Williams, T. N. & Thein, S. L. Sickle cell anemia and its phenotypes. Annu. Rev. Genomics Hum. Genet. 19, 113–147. https://doi.org/10.1146/annurev-genom-083117-021320 (2018).
Burnett, A. L. Priapism pathophysiology: Clues to prevention. Int. J. Impot. Res. 15 (5), 80–85. https://doi.org/10.1038/sj.ijir.3901077 (2003).
Nolan, V. G., Wyszynski, D. F., Farrer, L. A. & Steinberg, M. H. Hemolysis-associated priapism in sickle cell disease. Blood. 106 (9), 3264–3267. https://doi.org/10.1182/blood-2005-04-1594 (2005).
Broderick, G. A. Priapism and sickle-cell Anemia: diagnosis and nonsurgical therapy. J. Sex. Med. 9 (1), 88–103. https://doi.org/10.1111/j.1743-6109.2011.02317.x (2012).
Ahuja, G. et al. Priapism and Sickle Cell Disease: Special considerations in Etiology, Management, and Prevention. Urology. 156, 40–47. https://doi.org/10.1016/j.urology.2021.06.010 (2021).
Anele, U. A., Morrison, B. F. & Burnett, A. L. Molecular Pathophysiology of Priapism: emerging targets. Curr. Drug Targets. 16 (5), 474–483. https://doi.org/10.2174/1389450115666141111111842 (2015).
Belini-Júnior, E. et al. The severity of Brazilian sickle cell disease patients: severity scores and feasibility of the bayesian network model use. Blood Cells Mol. Dis. 54 (4), 321–327. https://doi.org/10.1016/j.bcmd.2015.01.011 (2015).
Rees, D. C., Brousse, V. A. M. & Brewin, J. N. Determinants of severity in sickle cell disease. Blood Rev. 56, 100983. https://doi.org/10.1016/j.blre.2022.100983 (2022).
Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 17, 623–639. https://doi.org/10.1038/nrd.2018.115 (2018).
Cristino, L., Bisogno, T. & Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 16 (1), 9–29. https://doi.org/10.1038/s41582-019-0284-z (2020).
Di Marzo, V. & De Petrocellis, L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop New Endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 17 (14), 1430–1449. https://doi.org/10.2174/092986710790980078 (2010).
Iannotti, F. A., Di Marzo, V. & Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism, and role in neurological disorders. Prog Lipid Res. 62, 107–128. https://doi.org/10.1016/j.plipres.2016.02.002 (2016).
Iannotti, F. A. & Vitale, R. M. The Endocannabinoid System and PPARs: Focus on their Signalling Crosstalk, Action and Transcriptional Regulation. Cells. 10, 586. https://doi.org/10.3390/cells10030586 (2021).
Dziemitko, S., Harasim-Symbor, E. & Chabowski, A. How do phytocannabinoids affect cardiovascular health? An update on the most common cardiovascular disease. Ther. Adv. Chronic Dis. 14, 1–18. https://doi.org/10.1177/20406223221143239 (2023).
Bentzen, P. J. & Lang, F. Effect of Anandamide on Erythrocyte Survival. Cell. Physiol. Biochem. 20, 1033–1042. https://doi.org/10.1159/000110714 (2007).
De Angelis, V. et al. Endocannabinoids control platelet activation and limit aggregate formation under Flow. PLoS ONE. 9 (9). https://doi.org/10.1371/journal.pone.0108282 (2014). e108282.
Gasperi, V. et al. Downstream effects of endocannabinoid on blood cells: implications for health and disease. Cell. Mol. Life Sci. 72, 3235–3252. https://doi.org/10.1007/s00018-015-1924-0 (2015).
Raborn, E. S., Marciano-Cabral, F., Buckley, N. E., Martin, B. R. & Cabral, G. A. The Cannabinoid Delta-9-tetrahydrocannabinol mediates inhibition of Macrophage Chemotaxis to RANTES/CCL5: linkage to the CB2 receptor. J. Neuroimmune Pharmacol. 3, 117–129. https://doi.org/10.1007/s11481-007-9077-z (2008).
Zhao, Y. et al. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis Associated with suppression of Adhesion molecules. J. Cardiovasc. Pharmacol. 55, 292–298. https://doi.org/10.1097/FJC.0b013e3181d2644d (2010).
Brasil Ministério da Saúde. Secretaria de Atenção à Saúde. Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Portaria Conjunta SAS/SCTIE/MS nº 05–19/02/2018. Protocolo Clínico e Diretrizes Terapêuticas da Doença Falciforme. (2018). https://www.gov.br/conitec/pt-br/midias/protocolos/pcdt_doencafalciforme_2018-1.pdf
Montague, D. K. et al. American Urological Association guideline on the management of priapism. J. Urol. 170, 1318–1324. https://doi.org/10.1097/01.ju.0000087608.07371.ca (2003).
Ballas, S. K. Sickle cell disease: classification of clinical complications and approaches to preventive and therapeutic management. Clin. Hemorheol Microcirc. 68 (2–3), 105–128. https://doi.org/10.3233/CH-189002 (2018).
Sambrook, J. & Russell, D. W. Molecular Cloning: A Laboratory Manual 1st edn (Cold Spring Harbor Laboratory Press, 2001).
Saiki, R. K. et al. Enzymatic amplification of Beta-globin genomic sequences and restriction site analysis for diagnosis of sickle-cell Anemia. Science. 230, 1350–1354. https://doi.org/10.1126/science.2999980 (1985).
Chong, S. S., Boehm, C. D., Higgs, D. R. & Cutting, G. R. Single-tube multiplex-PCR screen for common deletional determinants of α-thalassemia. Blood. 95 (1), 360–362. https://doi.org/10.1182/blood.V95.1.360 (2000).
Chiang, K. P., Gerber, A. L., Sipe, J. C. & Cravatt, B. F. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum. Mol. Genet. 13 (18), 2113–2119. https://doi.org/10.1093/hmg/ddh216 (2004).
Spohrs, J., Ulrich, M., Grön, G., Plener, P. L. & Abler, B. FAAH polymorphism (rs324420) modulates extinction recall in healthy humans: an fMRI study. Eur. Arch. Psychiatry Clin. Neurosci. 272 (8), 1495–1504. https://doi.org/10.1007/s00406-021-01367-4 (2022).
Carey, C. E. et al. Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: evidence from an endocannabinoid system-level analysis. J. Abnorm. Psychol. 124 (4), 860–877. https://doi.org/10.1037/abn0000079 (2015).
Loureiro, C. M. et al. Lifetime cannabis use and childhood trauma increase risk of psychosis in carriers of CNR1 genetic variants: findings from the STREAM study. Braz J. Psychiatry. 45 (3), 226–235. https://doi.org/10.47626/1516-4446-2022-2882 (2023).
Carrasquer, A., Nebane, N. M., Williams, W. M. & Zhao-Hui, S. Functional consequences of nonsynonymous single nucleotide polymorphisms in the CB2 cannabinoid receptor. Pharmacogenet Genomics. 20 (3), 157–166. https://doi.org/10.1097/FPC.0b013e3283367c6b (2010).
Tahamtan, A. et al. Cannabinoid CB2 receptor functional variation (Q63R) is Associated with multiple sclerosis in Iranian subjects. J. Mol. Neurosci. 70 (1), 26–31. https://doi.org/10.1007/s12031-019-01395-9 (2020).
Rastegar, M. et al. Functional variation (Q63R) in the cannabinoid CB2 receptor may affect the severity of COVID-19: A human study and molecular docking. Arch. Virol. 166 (11), 3117–3126. https://doi.org/10.1007/s00705-021-05223-7 (2021).
dbSNP [Internet]. Bethesda (MD). National Library of Medicine (US), National Center for Biotechnology Information; 1999 – [cited 2024 Mar 12] https://www.ncbi.nlm.nih.gov/snp/
Pincez, T., Ashley-Koch, A. E., Lettre, G. & Telen, M. J. Genetic modifiers of Sickle Cell Disease. Hematol. /Oncol Clin. N Am. 36 (6), 1097–1124. https://doi.org/10.1016/j.hoc.2022.06.006 (2022).
Belisário, A. R., Rodrigues, C. V., Martins, M. L., Silva, C. M. & Viana, M. B. Coinheritance of α-thalassemia decreases the risk of cerebrovascular disease in a cohort of children with sickle cell anemia. Hemoglobin. 34 (6), 516–529. https://doi.org/10.3109/03630269.2010.526003 (2010).
Lamarre, Y. et al. Alpha Thalassemia protects sickle cell anemia patients from macro-albuminuria through its effects on red blood cell rheological properties. Clin. Hemorheol Microcirc. 57 (1), 63–72. https://doi.org/10.3233/CH-131772 (2014).
Santos, B. et al. Co-inheritance of alpha-thalassemia and sickle cell disease in a cohort of Angolan pediatric patients. Mol. Biol. Rep.47 (7), 5397–5402. https://doi.org/10.1007/s11033-020-05628-8 (2020).
Hoss, S. E., Nemer, W. E. & Rees, D. C. Precision Medicine and Sickle Cell Disease. Hemasphere. 6 (9), E762. https://doi.org/10.1097/HS9.0000000000000762 (2022).
Kato, G. J., Gladwin, M. T. & Steinberg, M. H. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 21 (1), 37–47. https://doi.org/10.1016/j.blre.2006.07.001 (2007).
Nader, E., Conran, N., Romana, M. & Connes, P. Vasculopathy in Sickle Cell Disease: from Red Blood Cell Sickling to Vascular Dysfunction. Compr. Physiol. 11 (2), 1785–1803. https://doi.org/10.1002/cphy.c200024 (2021).
Hurt, K. J. et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc. Natl. Acad. Sci. USA. 99(6), 4061–4066 (2002). https://doi.org/10.1073/pnas.052712499
Bivalacqua, T. J., Musicki, B., Kutlu, O. & Burnett, A. L. New insights into the pathophysiology of Sickle Cell Disease-Associated Priapism. J. Sex. Med.Bold">9 (1), 79–87. https://doi.org/10.1111/j.1743-6109.2011.02288.x (2012).
Champion, H. C., Bivalacqua, T. J., Takimoto, E., Kass, D. A. & Burnett, A. L. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc. Natl. Acad. Sci. USA. 102 (5), 1661–1666. https://doi.org/10.1073/pnas.0407183102 (2005).
Musicki, B. & Burnett, A. L. Mechanisms underlying priapism in sickle cell disease: targeting and key innovations on the preclinical landscape. Expert Opin. Ther. Targets. 24 (5), 439–450. https://doi.org/10.1080/14728222.2020.1745188 (2020).
Musicki, B., Champion, H. C., Hsu, L. L., Bivalacqua, T. J. & Burnett, A. L. Post-translational inactivation of endothelial nitric oxide synthase in the transgenic sickle cell mouse Penis. J. Sex. Med. 8 (2), 419–426. https://doi.org/10.1111/j.1743-6109.2010.02123.x (2011).
Musicki, B., Liu, T., Sezen, S. F., Burnett, A. L. & Targeting NADPH oxidase decreases oxidative stress in the transgenic sickle cell mouse Penis. J. Sex. Med.9 (8), 1980–1987. https://doi.org/10.1111/j.1743-6109.2012.02798.x (2012).
Bivalacqua, T. J. et al. Sildenafil citrate-restored eNOS and PDE5 regulation in Sickle Cell Mouse Penis prevents Priapism Via Control of Oxidative/Nitrosative stress. PLoS ONE. 8 (7), 3–10. https://doi.org/10.1371/journal.pone.0068028 (2013).
Musicki, B., Bivalacqua, T. J., Champion, H. C. & Burnett, A. L. Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis. J. Sex. Med. 11 (2), 424–430. https://doi.org/10.1111/jsm.12391 (2014).
Silva, F. H. et al. Sympathetic hyperactivity, increased tyrosine hydroxylase and exaggerated corpus cavernosum relaxations associated with oxidative stress plays a major role in the penis dysfunction in Townes sickle cell mouse. PLoS ONE. 11 (12), 1–17. https://doi.org/10.1371/journal.pone.0166291 (2016).
Sopko, N., Hannan, J. & Bivalacqua, T. Understanding and targeting the rho kinase pathway in erectile dysfunction. Nat. Rev. Urol. 11 (11), 622–628. https://doi.org/10.1038/nrurol.2014.278 (2014).
Bivalacqua, T. J. et al. Attenuated rhoA/rho-kinase signaling in penis of transgenic sickle cell mice. Urology. 76(2), 510.e7-510.e12 (2010). https://doi.org/10.1016/j.urology.2010.02.050
Lagoda, G., Sezen, S. F., Cabrini, M. R., Musicki, B. & Burnett, A. L. Molecular Analysis of Erection Regulatory Factors in Sickle Cell Disease Associated Priapism in the human penis. J. Urol. 189 (2), 762–768. https://doi.org/10.1016/j.juro.2012.08.198 (2013).
Rajesh, M. et al. CB2-receptor stimulation attenuates TNF-α-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am. J. Physiol. Heart Circ. Physiol. 293, H2210–H2218. https://doi.org/10.1152/ajpheart.00688.2007 (2007).
Atwood, B. K. & Mackie, K. CB2: a cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 160, 467–479. https://doi.org/10.1111/j.1476-5381.2010.00729.x (2010).
Dowie, M. J., Grimsey, N. L., Hoffman, T., Faull, R. L. M. & Glass, M. Cannabinoid receptor CB2 is expressed on vascular cells, but not astroglial cells in the post-mortem human Huntington’s disease brain. J. Chem. Neuroanat. 59, 62–71. https://doi.org/10.1016/j.jchemneu.2014.06.004 (2014).
Atwood, B. K., Straiker, A. & Mackie, K. CB2: therapeutic target-in-waiting. Prog Neuro-Psychopharmacol Biol. Psychiatry. 38, 16–20. https://doi.org/10.1016/j.pnpbp.2011.12.001 (2012).
Matta, A., Tandra, P. K. & Berim, L. Priapism in a patient with sickle cell trait using marijuana. BMJ Case Rep. bcr2014204199 (2014). https://doi.org/10.1136/bcr-2014-204199
Wang, J. et al. Genetic variant Q63R of cannabinoid receptor 2 causes Differential ERK Phosphorylation in Human Immune cells. Genet. Test. Mol. Biomarkers. 22 (5), 320–326. https://doi.org/10.1089/gtmb.2018.0005 (2018).
Argueta, D. A. et al. Considerations for Cannabis Use to treat Pain in Sickle Cell Disease. J. Clin. Med. 9 (12), 3902. https://doi.org/10.3390/jcm9123902 (2020).
Acknowledgements
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, grant 88887.659679/2021-00) and Fundect (TO number 155/2024) for financially supporting this study. This study was funded in part by the Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES) - Finance Code 001" and "Support from the Federal University of Mato Grosso do Sul.
Author information
Authors and Affiliations
Contributions
A.C.M.B. conceived the study, performed experiments and statistical analyses, interpreted data, and prepared the manuscript. V.S.R.C. performed experiments and reviewed the paper. G.S.A., A.S.A., A.R.L.A., and M.A.C.B. performed experiments and collected the clinical data. L.G. supported statistical analyses and reviewed the paper. D.G.H.S. and E.B.J. designed the study, reviewed the paper, and gave the final approval of the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Federal University of Pernambuco Ethics Committee and Hemotherapy Foundation of Pernambuco (CAAE: 58863316.6.3001.5195).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Berti, A.C.M., de Castro, V., Arcanjo, G.S. et al. The endocannabinoid system’s genetic polymorphisms in sickle cell anemia patients. Sci Rep 14, 31562 (2024). https://doi.org/10.1038/s41598-024-76480-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76480-0