Abstract
Thrombospondin 2 (TSP2) plays a vital role in collagen/fibrin formation, bone growth, vascular density regulation, hemostasis, and cell adhesion. Close associations of serum TSP2 with histological severity in non-alcoholic fatty liver disease and chronic hepatitis C were reported. The present study investigated the significance of circulating TSP2 in chronic hepatitis B patients. Eighty-seven biopsy-proven chronic hepatitis B patients were analyzed in cross-sectional Study 1 to search for correlations between serum TSP2 levels prior to liver biopsy and clinicopathological parameters. In longitudinal Study 2, 51 chronic hepatitis B patients with long-term follow-up (mean: 7.5 years) were examined for changes in serum TSP2 levels during nucleos(t)ide analog (NA) therapy along with trends in hepatocarciongenesis. In Study 1, serum TSP2 levels were not significantly associated with portal inflammation or fibrosis. Study 2 revealed that serum TSP2 was significantly decreased after 48 weeks of NA therapy (P < 0.001). Notably, TSP2 levels at 48 weeks of NA administration (TSP2-48W) were significantly higher in the hepatocellular carcinoma (HCC) (+) group than in the HCC (−) group (P = 0.043). Kaplan–Meier analysis showed that higher TSP2-48W (≥ 24 ng/mL) was associated with future HCC development (P = 0.030). Serum TSP2 levels may be a potential predictor of HCC development in hepatitis B patients receiving NA therapy. Longitudinal prospective studies are necessary to validate our findings.
Similar content being viewed by others
Introduction
Hepatitis B virus (HBV) infection is a widespread health issue that leads to frequent morbidity and mortality in afflicted patients. It is estimated that over 350 million individuals globally carry the HBV, causing roughly 600,000 deaths yearly1. HBV infection outcomes range from spontaneous clearance to viral persistence that may advance to liver cirrhosis and hepatocellular carcinoma (HCC)2,3. Nuleos(t)ide analog (NA) have greatly improved the treatment of HBV by suppressing viral replication and reducing liver-related complications4,5,6. Despite these advancements, patients on NA therapy still face a residual risk of HCC, with an annual incidence rate of around 1%6,7. Identifying HBV-infected patients at higher risk for HCC remains crucial for improving monitoring and implementing effective prevention strategies.
The THBS2 gene encodes the thrombospondin 2 (TSP2) protein, which is involved in the synthesis of collagen and fibrin, bone development, hemostasis, and cell adhesion8. Previous study showed that TSP2 was a valuable serum biomarker reflecting pathological activity in patients with non-alcoholic fatty liver disease (NAFLD) and hepatitis C9,10. These findings were replicated in multiple studies using a comprehensive genetic analysis approach in NAFLD patient livers and in a large investigation of NAFLD patients with diabetes mellitus11,12. Thus, regardless of the background liver disease, TSP2 may play a prominent role in its pathogenesis. However, the precise involvement of TSP2 in hepatitis B patients remains unknown. This study evaluated the clinicopathological characteristics of serum TSP2 in HBV-infected patients, its quantitative changes with NA therapy, and its usefulness in predicting carcinogenesis.
Methods
Patients
Patients visiting Shinshu University Hospital (Matsumoto, Japan) for the management of HBV infection were included in this study. The exclusion criteria were patients with other causes of chronic liver disease, including hepatitis C infection, NAFLD, primary biliary cholangitis, autoimmune hepatitis, and alcoholic liver disease. Alcoholic liver disease was defined as an average daily ethanol consumption of > 60 g. The racial background of all individuals was Japanese. Serum levels of HBV-DNA and hepatitis B surface antigen (HBsAg) were quantified using the COBAS AmpliPrep-COBAS TaqMan HBV test (Roche Diagnostics, Basel, CH, Switzerland) and Lumipulse Presto HBsAg-HQ assay (Fujirebio, Tokyo, Japan; cut-off value: 0.005 IU/mL), respectively.
Study 1
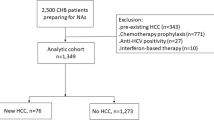
A total of 87 treatment-naïve HBV patients who had undergone liver biopsy between 1998 and 2013 were retrospectively targeted for cross-sectional examination of histopathological findings.
Study 2
Fifty-one patients receiving NA therapy for at least 48 weeks between 2011 and 2021 with available stored serum samples were enrolled in a longitudinal study of quantitative changes in TSP2 and other clinical findings in addition to HCC development.
Measurement of TSP2 levels
Serum TSP2 levels were measured by a commercially available enzyme-linked immunoassay kit (QuantikineⓇ ELISA, #DTSP20, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions using patient serum samples stored at − 30 °C after collection10. In Study 1, TSP2 levels were determined with serum samples obtained just prior to liver biopsy. In Study 2, TSP2 levels were quantified at a baseline point just before NA commencement and at 48 weeks of NA therapy.
Definition of HCC development
HCC was diagnosed based on imaging characteristics, arterial hypervascularity, and venous or delayed phase washout on contrast-enhanced dynamic computed tomography and/or magnetic resonance imaging This diagnosis was made when a nodular lesion was detected by ultrasonography or when tumor markers such as α-fetoprotein and des-γ-carboxyprothrombin were elevated during follow-up, in accordance with the American Association for the Study of Liver Diseases practice guidelines for HCC surveillance and diagnosis13.
Histological findings
Liver specimens of at least 1.5 cm in length were obtained from segments 5 or 8 using a 14-gauge needle and immediately fixed in 10% neutral formalin14. Sections of 4 μm in thickness were cut and stained using the hematoxylin and eosin and Azan-Mallory methods. The histological activity was independently assessed by expert pathologists in a blinded manner according to the METAVIR scoring system15. Disease activity grade was scored as follows: A0, none; A1, minimal activity; A2, moderate activity; and A3, severe activity. Fibrosis stage was scored as follows: F0, none; F1, perisinusoidal or periportal fibrosis; F2, perisinusoidal and portal/periportal fibrosis; F3, bridging fibrosis; and F4, cirrhosis15.
Ethics
This study was reviewed and approved by the Institutional Review Board of Shinshu University School of Medicine (approval number: 5797). All researchers involved in this study conducted the investigation in accordance with the Declaration of Helsinki (revised in 2013 by Fortaleza) and the Ethical Guidelines for Medical Research Involving Human Subjects (partially revised on February 28, 2017). Informed consent for the use of the serum used in this study was obtained from the subjects. In addition, an opt-out system is in place at our institution. All information on the protocol and conduct of the study, including its purpose, is available on the Department of Medicine, Shinshu University School of Medicine website (http://www.shinshu-u.ac.jp/faculty/medicine/chair/i-2nai/). If patients did not wish to participate in the research, they were freely able to opt out of the study.
Statistical analysis
Statistical analysis and data visualization were carried out using StatFlex ver. 7.0.11 software (Artech Co., Ltd., Osaka, Japan). Continuous baseline data are expressed as the median and interquartile range (IQR) and statistically evaluated by means of the Mann–Whitney U test. Categorical variables are presented as the frequency (percentage) and analyzed using the chi-square test. The Wilcoxon signed-rank test was employed to analyze the differences in continuous variables between the immediately pre-treatment baseline and 48 weeks of NA time points. The diagnostic accuracy was assessed by calculating the area under the receiver operating characteristic (ROC) curve (AUROC). All statistical tests were two-sided and evaluated at the 0.05 level of significance.
Results
Patient characteristics in Study 1
The cohort’s clinicopathological features are summarized in Table 1. Median age was 48 years, and 58.6% of subjects were male. Of the 87 patients, 50 (57.5%) were hepatitis B e-antigen (HBeAg) positive. The study population included 2 asymptomatic carriers, 2 inactive carriers, 64 patients with chronic hepatitis, and 19 patients with liver cirrhosis. Median serum TSP2 was 21.0 ng/mL (IQR: 14.2–31.4). The respective number of patients with fibrosis stage F0-1, F2, F3, and F4 was 31, 22, 15, and 19. The number of patients exhibiting activity grade A0, A1, A2, and A3 was 4, 24, 42, and 17, respectively.
Correlation between serum TSP2 levels and clinicopathological features of HBV patients
Table 2 presents the correlation analysis findings for clinical parameters with serum TSP2 levels. TSP2 did not show significant correlations with any clinical parameters, including age (r = 0.166, P = 0.123), platelet count (r = 0.011, P = 0.919), alanine aminotransferase (r = 0.003, P = 0.981), Wisteria floribunda agglutinin-positive human Mac-2-binding protein (M2BPGi) (r = 0.073, P = 0.501, Supplementary Fig. 1), or HBV-DNA (r = − 0.209, P = 0.094, Supplementary Fig. 2A). Furthermore, the presence or absence of HBe antigen had no impact on TSP2 concentration (P = 0.871, Supplementary Fig. 2B). TSP2 was also not associated with fibrosis grade (r = − 0.059, P = 0.587) or activity grade (r = 0.024, P = 0.825) (Fig. 1).
Patient characteristics in Study 2
The demographic and clinical features of the 51 patients treated with NA therapy for at least 48 weeks in Study 2 are summarized in Table 3. Median age was 56 years, and 52.9% of subjects were male. Twenty-eight patients (54.9%) were HBeAg positive at baseline. The median follow-up period, defined as the last observation date from 48 weeks after the start of NA treatment, was 6.3 years.
Changes in serum TSP2 and other laboratory findings during NA therapy
Aspartate aminotransferase (AST) levels (from 35 to 24 U/L, P < 0.001), alanine aminotransferase (ALT) levels (from 32 to 17 U/L, P < 0.001), and HBsAg levels (from 3.34 to 3.26 log IU/L, P = 0.005) decreased significantly from baseline to 48 weeks in HBV patients receiving NA therapy (Table 3). HBV-DNA levels in most patients also decreased from a median of 5.8 log IU/mL to below detection sensitivity. Interestingly, TSP2 levels (from 32.8 to 24.1 ng/mL, P < 0.001) also fell significantly (Fig. 2).
Association of TSP2 with HCC development
Among the 51 patients in Study 2, 8 (15.7%) experienced complicating HCC during NA treatment. Table 4 presents clinical comparisons between the HCC (−) and HCC (+) groups at 48 weeks after the start of NA therapy. Serum TSP2 levels measured 48 weeks after NA administration (TSP2-48W) were significantly higher in the HCC (+) group (P = 0.043) (Fig. 3). In addition, we observed significant differences in age, platelet count, and HBsAg between the groups (all P < 0.05). However, no significant correlation was observed between age and TSP2 levels (r = − 0.056, P = 0.693).
Supplementary Fig. 3 presents the ROC curve for TSP2-48W in predicting HCC, with an AUROC of 0.727. Using a cutoff value of 24.0 ng/dL, which corresponds to the median TSP2-48W, the sensitivity was 0.875, and the specificity was 0.628. Kaplan–Meier analysis revealed higher TSP2-48W (≥ 24 ng/mL) to be significantly associated with development of HCC (P = 0.030, Fig. 4). The cumulative incidence of HCC development over 5 years was 23.3% in the higher TSP2-48W group. In contrast, the cumulative incidence in the lower TSP2-48W (< 24 ng/mL) group was 7.1%, with only one of the 25 patients developing HCC during the follow-up period.
Discussion
Chronic HBV infection has been associated with an increased risk of HCC16. Since HCC can develop even in patients with chronic HBV but without cirrhosis17, it is clinically important to predict HCC onset on a patient-by-patient basis. In addition to disease progression to cirrhosis, several prognostic HCC factors have been reported to date, including high HBV-DNA levels, high HBsAg levels, male gender, genotype C, and family history of HCC18. With advances in HBV treatment, biomarkers for predicting HCC development that are useful during NA therapy are also of particular interest.
This study investigated serum TSP2 patterns in patients with hepatitis B in both cross-sectional and longitudinal under NA therapy settings to reveal 3 major findings. First, serum TSP2 did not correlate significantly with histopathological results, including liver fibrosis, in the cross-sectional cohort. Second, serum TSP2 levels were significantly decreased after 48 weeks of NA therapy. Third, patients with higher TSP2-48W were associated with HCC onset in patients receiving NA therapy (Supplementary Fig. 4). These results raise the novel possibility of TSP2 as a predictor of HCC development in NA therapy of HBV-infected patients.
Thrombospondins are glycoproteins that interact with other components of the extracellular matrix to facilitate angiogenesis, tissue healing, and connective tissue organization19. TSP2 is widely recognized as a secreted protein20. Experimental data have demonstrated that transforming growth factor beta (TGF-β) induces an increase in TSP2 concentrations not only in cell lysates but also in the cell supernatant21. Given its characteristics as a secreted protein, we recently proposed serum TSP2 as a novel biomarker for detecting advanced fibrosis in patients with NAFLD10. Furthermore, our recent analysis using liver biopsy samples and microarray datasets also demonstrated that serum TSP2 levels correlate with fibrosis and disease activity in HCV-infected patients9. Kimura et al. reported that TSP2 is predominantly expressed in hepatic stellate cells in patients with NAFLD and regulates collagen gene activation and hepatic fibrosis21.
Based on accumulating evidence, we hypothesized that a correlation between TSP2 and liver disease activity could also be present in hepatitis B. However, unlike findings in patients with NAFLD or hepatitis C9,10, Study 1 did not reveal significant correlations between pathological fibrosis or inflammation and TSP2 levels. In addition to its role in liver fibrosis and inflammation, TSP2 has been linked to hepatocyte ballooning in NAFLD patients, indicating that TSP2 may play various roles in the progression of liver disease10. Differences in immune responses and mechanisms of fibrosis progression between hepatitis B and other conditions such as NAFLD or hepatitis C may also explain these discrepancies22,23. Additionally, the complexity of the unique natural history of hepatitis B, and the limited sample size may have influenced our findings, and so future studies of larger cohorts are needed to clarify the relationship between TSP2 and the pathological features of hepatitis B.
In Study 2, TSP2 levels decreased following NA treatment. Two potential hypotheses might explain this observation. First, when NA treatment suppresses HBV replication and reduces liver damage, it is possible that the expression of TSP2, which is involved in tissue remodeling processes such as regeneration and wound healing, will be suppressed24. Second, NA treatment might alter the liver microenvironment, activating pathways that regulate TSP2 expression independently of fibrosis or inflammation. Further investigation, including in vivo experiments, is needed to validate these hypotheses.
The observation that high TSP2-48W could predict HCC development was noteworthy in Study 2. Since patients with histopathological fibrosis are particularly susceptible to HBV-related HCC, it is important to accurately assess liver fibrosis and determine the appropriate therapeutic strategy for patients with HBV, including the optimal timing for antiviral therapy. Several non-invasive methods have been established for estimating liver fibrosis progression and disease activity. These include FIB-4 index, M2BPGi, autotaxin, and magnetic resonance elastography25,26,27,28. However, the risk of HCC development independently of liver fibrosis has not been sufficiently investigated. In this context, serum TSP2 could represent a new predictive marker for HCC development in HBV patients.
TSP2 has been reported to be associated with the progression of various solid cancers, such as pancreatic cancer, bile duct cancer, and gastric cancer29,30. The reported mechanism involves its effects on cell proliferation signaling and the extracellular matrix, which are crucial for tumor cell invasion and migration within the tumor microenvironment29. Therefore, a similar mechanism may exist in the progression of HCC. Matsumae et al. reported that TSP2 was useful for predicting the development of HCC after HCV eradication with direct-acting antivirals, independently of the FIB-4 index31. This finding suggests that TSP2 may contribute to tumor progression in HCC independently of liver fibrosis.
This study had several limitations. First, it was conducted at a single center with a retrospective design. Second, the number of patients was relatively small, which precluded multivariate Cox regression analysis. Larger multi-center analyses are needed to confirm the clinical utility of TSP2 in HBV.
In conclusion, higher TSP2-48W of ≥ 24 ng/mL may be a predictive biomarker of HCC occurrence in HBV patients receiving NA therapy. Following the quantitative changes in serum TSP2 may be of clinical benefit during NA treatment and warrants further study.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to contain personal patient information but are available from the corresponding author on reasonable request. Please note that data must be de-identified when shared.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AUROC:
-
Area under the receiver operating characteristic curve
- ETV:
-
Entecavir
- HBeAg:
-
Hepatitis B e-antigen
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- IQR:
-
Interquartile range
- M2BPGi:
-
Mac-2 binding protein glycan isomer
- NA:
-
Nucleos(t)ide analog
- NAFLD:
-
Non-alcoholic fatty liver disease
- ROC:
-
Receiver operating characteristic
- TAF:
-
Tenofovir alafenamide
- TDF:
-
Tenofovir disoproxil fumarate
- TSP2:
-
Thrombospondin 2
- TSP2-48W:
-
Thrombospondin 2 levels measured 48 weeks after NA administration
References
Tawada, A., Kanda, T. & Yokosuka, O. Current and future directions for treating hepatitis B virus infection. World J. Hepatol. 7, 1541–1552. https://doi.org/10.4254/wjh.v7.i11.1541 (2015).
Yim, H. J. & Lok, A. S. Natural history of chronic hepatitis B virus infection: What we knew in 1981 and what we know in 2005. Hepatology 43, S173-181. https://doi.org/10.1002/hep.20956 (2006).
Ganem, D. & Prince, A. M. Hepatitis B virus infection–Natural history and clinical consequences. N. Engl. J. Med. 350, 1118–1129. https://doi.org/10.1056/NEJMra031087 (2004).
European Association for the Study of the Liver. Electronic address, e. e. e. & European Association for the Study of the, L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 67, 370-398, https://doi.org/10.1016/j.jhep.2017.03.021 (2017).
Chang, T. T. et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 52, 886–893. https://doi.org/10.1002/hep.23785 (2010).
Wong, G. L. et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 58, 1537–1547. https://doi.org/10.1002/hep.26301 (2013).
Huang, Z. H. et al. Risk of hepatocellular carcinoma in antiviral treatment-naive chronic hepatitis B patients treated with entecavir or tenofovir disoproxil fumarate: A network meta-analysis. BMC Cancer 22, 287. https://doi.org/10.1186/s12885-022-09413-7 (2022).
Calabro, N. E., Kristofik, N. J. & Kyriakides, T. R. Thrombospondin-2 and extracellular matrix assembly. Biochim. Biophys. Acta 2396–2402, 2014. https://doi.org/10.1016/j.bbagen.2014.01.013 (1840).
Iwadare, T. et al. Circulating thrombospondin 2 levels reflect fibrosis severity and disease activity in HCV-infected patients. Sci Rep 12, 18900. https://doi.org/10.1038/s41598-022-23357-9 (2022).
Kimura, T. et al. Serum thrombospondin 2 is a novel predictor for the severity in the patients with NAFLD. Liver Int 41, 505–514. https://doi.org/10.1111/liv.14776 (2021).
Kozumi, K. et al. Transcriptomics identify thrombospondin-2 as a biomarker for NASH and advanced liver fibrosis. Hepatology 74, 2452–2466. https://doi.org/10.1002/hep.31995 (2021).
Lee, C. H. et al. Circulating thrombospondin-2 as a novel fibrosis biomarker of nonalcoholic fatty liver disease in type 2 diabetes. Diabetes Care 44, 2089–2097. https://doi.org/10.2337/dc21-0131 (2021).
Singal, A. G. et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology https://doi.org/10.1097/hep.0000000000000466 (2023).
Kimura, T. et al. Mild drinking habit is a risk factor for hepatocarcinogenesis in non-alcoholic fatty liver disease with advanced fibrosis. World J. Gastroenterol. 24, 1440–1450. https://doi.org/10.3748/wjg.v24.i13.1440 (2018).
Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 20, 15–20 (1994).
Tsukuma, H. et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N. Engl. J. Med. 328, 1797–1801. https://doi.org/10.1056/nejm199306243282501 (1993).
Chayanupatkul, M. et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J. Hepatol. 66, 355–362. https://doi.org/10.1016/j.jhep.2016.09.013 (2017).
Tseng, T. C. et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology 142, 1140–1144. https://doi.org/10.1053/j.gastro.2012.02.007 (2012).
Adams, J. C. & Lawler, J. The thrombospondins. Cold Spring Harb. Perspect Biol. 3, a009712. https://doi.org/10.1101/cshperspect.a009712 (2011).
Bornstein, P. Thrombospondins as matricellular modulators of cell function. J. Clin. Invest. 107, 929–934. https://doi.org/10.1172/JCI12749 (2001).
Kimura, T. et al. Thrombospondin 2 is a key determinant of fibrogenesis in non-alcoholic fatty liver disease. Liver Int. 44, 483–496. https://doi.org/10.1111/liv.15792 (2024).
Tourkochristou, E., Assimakopoulos, S. F., Thomopoulos, K., Marangos, M. & Triantos, C. NAFLD and HBV interplay: Related mechanisms underlying liver disease progression. Front Immunol 13, 965548. https://doi.org/10.3389/fimmu.2022.965548 (2022).
Saraceni, C. & Birk, J. A review of hepatitis B virus and hepatitis C virus immunopathogenesis. J. Clin. Transl. Hepatol. 9, 409–418. https://doi.org/10.14218/JCTH.2020.00095 (2021).
Kyriakides, T. R. & Maclauchlan, S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J. Cell Commun. Signal 3, 215–225. https://doi.org/10.1007/s12079-009-0077-z (2009).
Tamaki, N. et al. Magnetic resonance elastography for the prediction of hepatocellular carcinoma in chronic hepatitis B. JGH Open 8, e13067. https://doi.org/10.1002/jgh3.13067 (2024).
Hiyama, Y. et al. Value of autotaxin for hepatocellular carcinoma risk assessment in chronic hepatitis B patients treated with nucleos(t)ide analogs. Hepatol Res https://doi.org/10.1111/hepr.14042 (2024).
Ichikawa, Y. et al. Serum Wisteria floribunda agglutinin-positive human Mac-2 binding protein may predict liver fibrosis and progression to hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Hepatol Res 47, 226–233. https://doi.org/10.1111/hepr.12712 (2017).
Suh, B. et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology 61, 1261–1268. https://doi.org/10.1002/hep.27654 (2015).
Liao, X., Wang, W., Yu, B. & Tan, S. Thrombospondin-2 acts as a bridge between tumor extracellular matrix and immune infiltration in pancreatic and stomach adenocarcinomas: An integrative pan-cancer analysis. Cancer Cell Int 22, 213. https://doi.org/10.1186/s12935-022-02622-x (2022).
Byrling, J., Hilmersson, K. S., Ansari, D., Andersson, R. & Andersson, B. Thrombospondin-2 as a diagnostic biomarker for distal cholangiocarcinoma and pancreatic ductal adenocarcinoma. Clin. Transl. Oncol. 24, 297–304. https://doi.org/10.1007/s12094-021-02685-8 (2022).
Matsumae, T. et al. Thrombospondin-2 as a predictive biomarker for hepatocellular carcinoma after hepatitis C virus elimination by direct-acting antiviral. Cancers https://doi.org/10.3390/cancers15020463 (2023).
Acknowledgements
We thank Asami Yamazaki for her technical assistance and Trevor Ralph as the Senior Editor of Impact Language Services for his English editorial assistance. This work was supported by a grant from the Japan Agency for Medical Research and Development (AMED) and Japan Society for the Promotion of Science (JSPS), and the joint research program of the Research Center for GLOBAL and LOCAL Infectious Diseases, Oita University.
Funding
K.T., T.N., and Um.T. were supported by a grant from the Japan AMED (JP23fk0210125, JP24fk0210125) for conducting this research. K.T. was supported by JSPS KAKENHI (JP22K20884, JP24K11087). K.T. and Uc.T. were supported by the joint research program of the Research Center for GLOBAL and LOCAL Infectious Diseases, Oita University (2024B11).
Author information
Authors and Affiliations
Contributions
K.T. and O.T. designed the research; K.T., O.T., I.Y., I.T., W.S., K.H., and Y.Y. collected clinical data; O.T., I.T., Uc.T., and K.T. performed the assays; O.T., K.T., and Uc.T. analyzed the data; O.T., K.T., and T.N. wrote the paper; S.P. and Um.T. supervised this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Okumura, T., Kimura, T., Ichikawa, Y. et al. Circulating thrombospondin 2 as a predictor of hepatocellular carcinoma in hepatitis B patients undergoing nucleos(t)ide analog therapy. Sci Rep 14, 25584 (2024). https://doi.org/10.1038/s41598-024-76532-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76532-5