Abstract
Preserving organs at subzero temperatures with halted metabolic activity holds the potential to prolong preservation and expand the donor organ pool for transplant. Our group recently introduced partial freezing, a novel approach in high-subzero storage at -15 °C, enabling 5-day storage of rodent livers through precise control over ice nucleation and unfrozen fraction. However, increased vascular resistance and tissue edema suggested a need for improvements to extend viable preservation. Here, we describe an optimized partial freezing protocol with key optimizations, including an increased concentration of polyethylene glycol (PEG) to enhance membrane stability while minimizing shear stress during cryoprotectant unloading with an acclimation period and a maintained osmotic balance through an increase in bovine serum albumin (BSA). These approaches ensured the viability during preservation and recovery processes, promoting liver function and ensuring optimal preservation. This was evidenced by increased oxygen consumption, decreased vascular resistance, and edema. Ultimately, we show that using the optimized protocol, livers can be stored for 10 days with comparable vascular resistance and lactate levels to 5 days, outperforming the viability of time-matched static cold stored (SCS) livers as the current gold standard. This study represents a significant advancement in expanding organ availability through prolonged preservation, thereby revolutionizing transplant medicine.
Similar content being viewed by others
Introduction
With more than five times the number of patients on the waiting list than those who will receive a donor organ in the USA, the field of transplantation is facing a serious donor shortage crisis1,2. Despite decades of research, a major limitation is the current preservation times for whole organs. The standard method, static cold storage (SCS) at 4 °C, imposes a preservation time limit of 8–10 h3,4. Longer preservation times could have a profound impact on organ allocation, handling, and transplantation in various crucial ways. Firstly, prolonging the preservation duration would shift surgeries from emergency to planned, resulting in reduced transplantation costs and improved matching based on human leukocyte antigen (HLA) compatibility3,5,6. Secondly, the protocols for inducing immune tolerance promise to eliminate rejection and weaning patients off immunosuppressive regimens with numerous side effects, including infection and organ failure, ultimately enhancing recipients’ quality of life and improving patient survival. Thirdly, some organs procured for transplantation are discarded due to the complexity of allocation, which, in theory, could be eliminated with extended preservation duration3,4.
There are two divergent strategies applied in designing preservation methods for donor organs: metabolic support and metabolic depression. Metabolic support involves providing essential nutrients and oxygen to maintain the viability of organs. Machine perfusion, a cutting-edge advancement in this field, has emerged to both extend preservation duration and expand the donor pool of extended-criteria organs (ECD)7,8. This process involves perfusing organs with various cellular and acellular media in a closed-loop, oxygenated circuit. Machine perfusion demonstrated stellar success in the preservation and recovery of potential grafts, increasing viable preservation time from approximately 12 h to up to 7 days9,10,11. Despite this success, limitations with the continuous perfusion method of preservation limit its successes, including high costs, challenging logistics, and labor-intensive equipment12,13. To combat these limitations, the field of cryopreservation aims to prolong liver preservation by lowering storage temperatures and depressing metabolism beyond static cold storage.
Metabolic depression relies on slowing the depletion of energy stores14,15 by lowering temperature. While SCS at 4 °C limits preservation time to 8–10 h, further depression in temperature has the potential to allow indefinite storage (vitrification)3,16,17. Several preservation strategies were developed to take advantage of temperature-induced metabolic depression to extend organ storage18,19,20. For example, high-subzero techniques such as supercooling and isochoric preservation, whereby ice nucleation is avoided through careful maintenance of mechanical stability and volume, can facilitate multi-day storage19,21,22. Alternately, vitrification can stop biological time altogether, reaching temperatures as low as − 196 °C and enabling indefinite storage20. However, these approaches are limited, with supercooling requiring highly stable storage conditions to prevent ice nucleation, isochoric preservation requiring technologically advanced storage systems for maintenance of constant volume, and vitrification requiring high-powered radiofrequency coils for rapid nanowarming to enable the warming rates necessary to avoid ice nucleation23,24.
Partial freezing (PF) is an attractive alternative, taking advantage of subzero preservation with controlled ice formation. Using extracellular ice nucleation mediated through specialized storage solutions, damaging intracellular ice formation can be avoided, enabling deeper storage temperatures than supercooling, as low as − 15 °C25,26. PF is a bioinspired technique derived from hibernation in the Sylvatica wood frog (Rana Sylvatica), which can survive in a frozen state at − 6 °C to − 16 °C for weeks. The wood frog capitalizes on both ice nucleating agents (INA) and endogenous cryoprotective agents (CPAs) to orchestrate freezing and prevent injurious intracellular ice formation27,28,29. The INAs promote ice formation within the vasculature as close to melting point. Studies in freeze-tolerant species showed that INAs control the freezing of extracellular water, which is critical for freezing survival. As extracellular water gradually freezes, it is accompanied by an increase in the osmolality of the non-frozen extracellular fluid. This increase results in cellular dehydration as water is pulled from the intracellular environment. Another important strategy that confers freeze-tolerance is the synthesis of high amounts of carbohydrates, such as glucose. Glucose in the blood and tissues provides colligative resistance to detrimental decreases in cell volume and, together with INAs, restricts intracellular ice formation25,30. Based on these observations, we successfully stored rat livers for 5 days using a combination of pressure-controlled machine perfusion to load and unload CPA/INA. Specifically, livers were gradually loaded with 3-O-methyl-D-glucopyranose (3-OMG; a glucose analog and metabolic depressor), SnoMax (a potent INA), polyethylene glycol 35k (PEG; a membrane stabilizer), trehalose (an extracellular CPA), and propylene glycol (PG; an intracellular CPA)25,31. However, using the current protocol, the extension of storage duration was limited by inadequate suppression of ice formation that results in elevated vascular resistance and graft edema, surrogates of poor outcomes after transplantation.
Here, the study aimed to improve our PF protocol to extend liver preservation up to 10 days. To do so, we adopted the following changes: (i) elevating PEG concentration to improve membrane stability, (ii) introducing a 20-minute acclimation period during the thawing phase to limit shear stress, and (iii) increasing bovine serum albumin (BSA) concentration to reduce edema upon rewarming. Liver viability after 5-day storage was investigated during a 2-hour normothermic machine perfusion (NMP). Next, the efficacy of the improved protocol was tested for 10-day storage and compared to time-matched static cold storage using a blood-based simulated transplantation.
Results
10% PEG shows improvements in liver function during recovery
First, before viability testing during acellular NMP, perfusion metrics during the thaw (hypothermic machine perfusion; HMP) and recovery (subnormothermic machine perfusion; SNMP) phases were collected to compare the original protocol (5% PEG) and the optimized protocol (10% PEG) (Presented in Fig. 1 and Supplementary Table S1). Perfusion pressure during HMP and SNMP were set at 3 mmHg and 5 mmHg, with a maximum flow rate limit of 10 mL/min and 25 mL/min, respectively. During and immediately after the added 20-minute acclimation phase, 5% PEG had an elevated flow rate from 10 min to 28 min compared to 10% PEG; however, 10% PEG reached the same flow rate by 34 min (Fig. 2a-b). Importantly, compared to 5%PEG, 10%PEG led to a significant reduction in the vascular resistance (0.021 mmHg*min/L*g ± 0.005 vs. 0.039 mmHg*min/L*g ± 0.016, p = 0.0054, Fig. 2c), an increase in oxygen uptake (17.5 uL O2/min*g ± 5.0 vs. 9.3 uL O2/min*g ± 6.6, p = 0.015, Fig. 2d), but higher lactate production (1.38 mM ± 1.1 vs. 0.38 mM ± 0.15, p = 0.0034, Fig. 2e). Moreover, end-perfusion weight gain was reduced with the 10% PEG (51.4% ± 11.4 vs. 10.3% ± 5.0, p = 0.0042, Fig. 2f). Bile production was observed similar between the groups (15 uL ± 15 vs. 15 uL ± 17.6, p = 0.7764, Fig. 2g).
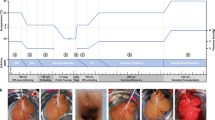
Experimental Design: (a) Schematic overview of rat liver partial freezing protocol showing 9 steps: (1) liver procurement, (2) preconditioning, (3) preloading CPAs, (4) loading of the final storage solution, (5) partial freezing, (6) thawing, (7) unloading CPAs, (8) functional recovery, (9) simulated transplantation or non-blood base NMP. The steps are outlined in the Methods section with more details. The protocol was adapted from Tessier et al.25. (b) Photos of the liver during the consecutive steps of the protocol. Left to right: SNMP preconditioning, CPA loading, partial freezing, CPA unloading, functional recovery during SNMP, simulated transplantation.
10% PEG Shows Improvements in Liver Function During SNMP Recovery: Comparison of liver functionality markers during the recovery phase following storage. Pressure-driven flow led to a more rapid flow rate increase in the 10% PEG group (blue) compared to 5% PEG (red) during the subnormothermic recovery phase (SNMP, 21 °C, blue) (a) potentially driven by the inclusion of a 20-minute acclimation period during the hypothermic thaw phase (HMP, 4 °C, green) (b). Improved vascular health with 10% PEG is shown through decreased resistance early in the SNMP phase (c). Elevated mitochondrial function is observed through an increased oxygen uptake rate in the 10% PEG group throughout the SNMP phase (d). Lactate showed faster elevation in the 10% PEG group (e). Weight gain was markedly reduced at the end of SNMP in the 10% PEG group (f). No difference in bile production was observed between groups (g).
10% PEG partial freezing protocol improves liver viability during acellular NMP following 5 days of storage
Since 10% PEG improved the viability of livers during SNMP, we next investigated viability during a non-blood based, acellular NMP. 10% PEG improved oxygen uptake rate compared to 5% PEG (35.7 uL O2/min*g ± 7.7 vs. 14.3 uL O2/min*g ± 12.4, p < 0.0001, Fig. 3a). Surprisingly, perfusate lactate steadily rose in the 10% PEG group, while 5% PEG remained low (1.10 mM ± 0.82 vs. 0.34 mM ± 0.14, p = 0.0023, Fig. 3b). Resistance was lower in 10% PEG (0.015 mmHg*min/L*g ± 0.004 vs. 0.021 mmHg*min/L*g ± 0.005, p = 0.0022, Fig. 3c). Bile production (Fig. 3d), damage markers alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Fig. 3e/f), and energy charge (Fig. 3h) were similar between both groups (p = 0.7664, 0.5640, 0.3017, and 0.0676 respectively). No significant differences were observed in other bioenergetic markers (Fig. S1). Consistent with improved perfusion, weight gain was reduced in 10% PEG (3.6% ± 8.5 vs. 43.6% ±11.6, p = 0.0008, Fig. 3g). This was confirmed on histological analysis; the lobular structure and liver sinusoidal endothelial cells (LSECs) were better preserved in 10% PEG group (Fig. 4a-b), whereas necrosis was similar between groups (Fig. 4c-d).
10% PEG Partial Freezing Protocol Improves Liver Performance under Normothermic Conditions Following 5 Days of Storage: To evaluate the performance of the partially frozen organs following storage in a more physiologically relevant manner, they were perfused with an acellular media at normothermic conditions (37 C, NMP) for 2 h. Oxygen uptake was greatly elevated in the 10% PEG group at 30, 60, 90, and 120 min (a). Lactate showed no change in 5% PEG, while a steady rise was observed in 10% PEG, resulting in significant elevation at 90 and 120 min (b). Resistance was similar throughout, showing the same dynamic trend; however, 5% PEG was significantly greater at 30 min (c). No difference in bile production was observed (d). ALT (e) and AST (f) showed the same trend in 10% PEG and 5% PEG; however, 10% PEG seemed to show a slight decrease. Weight gain was greatly reduced in 10% PEG, resulting in negligible edema (g). No difference in energy charge was shown (h).
Comparison of the recovery phase in livers partially frozen for 5 and 10 days
To determine the impact of the 10-day storage period, we evaluated liver perfusion during the thaw and SNMP recovery phase. During both thaw and SNMP, 5 mmHg pressure driven flow resulted in no difference in flow rate, with both PF5 and PF10 showing the same flow rate trend, with a slight, non-significant decrease in flow rate in PF10 from 130 min to 145 min (Fig. 5a). When isolating the HMP phase, no difference can be seen in either flow rate of resistance, showing overlapping trends in each, although a slight increase in resistance was seen during the 20-minute acclimation phase in PF10 (Fig. 5b). Resistance during SNMP was significantly higher after 10 days of storage compared to 5 days of storage at 30 min (0.035 mmHg*min/L*g ± 0.002 vs. 0.024 mmHg*min/L*g ± 0.002, p = 0.0024) and 60 min (0.030 mmHg*min/L*g ± 0.002 vs. 0.021 mmHg*min/L*g ± 0.003, p = 0.0153), however, the resistances converged and no difference in average resistance was overserved (0.026 mmHg*min/L*g ± 0.008 for PF10 vs. 0.021 mmHg*min/L*g ± 0.005 for PF5, p = 0.1429, Fig. 5c). A significant decrease in average oxygen uptake was observed in PF10 compared to PF5 (8.05 uL O2/min*g ± 3.01 vs. 17.5 uL O2/min*g ± 5.3, p < 0.0001, Fig. 5d). Perfusate lactate rose steadily throughout recovery, but no difference was observed in both groups (0.011 ± 0.002 vs. 0.005 ± 0.001, p = 0.0503, Fig. 5e). Similarly, potassium (5.13 mM ± 0.26 for PF5 vs. 5.64 mM ± 0.36 for PF10, Fig. 5f) and bile production (15 uL ± 16.08 for PF5 vs. 30 uL ± 10 for PF10, p = 0.6914, Fig. 5g) were similar.
Comparison of the SNMP Recovery Phase in Livers Partially Frozen for 5 and 10 Days: Comparison of perfusion metrics between the recovery phase of rat livers stored using 10% PEG FOR 5- (PF5) and 10-days (PF10). The flow rate in PF5 and PF10 followed the same trend during SNMP (21 °C, blue) (a), and when looking at HMP (4 °C, green), both resistance and flow rate followed the same trend (b). Resistance was elevated in PF10 during the beginning of SNMP at 30 min and 60 min, although it converged to meet PF5 (c). Oxygen uptake rate was decreased in PF10 throughout SNMP, showing significant reduction at 60 min, 120 min, 150 min, and 180 min (d). Lactate showed no difference, although PF5 seemed to show an increase in rate of elevation (e). No difference in potassium was observed (f). No difference in bile production was observed (g).
Partial freezing improves liver function over static cold storage following in simulated transplantation
Having demonstrated that 10% PEG improves liver viability during acellular perfusion, we next compared 10% PEG to the clinical gold standard (SCS) for 10 days during simulated transplant (Fig. 6). Vascular resistance was elevated following static cold storage at (0.032 mmHg*min/L*g ± 0.021 for PF10 vs. 0.185 mmHg*min/L*g ± 0.063 for SCS10, p < 0.0001, Fig. 6a). Average oxygen uptake rate was higher in PF10 compared to SCS10 (17.3 uL O2/min*g ± 6.0 vs. 6.1 uL O2/min*g ± 2.5, p < 0.0001, Fig. 6b). While lactate was lowest at T0 in PEG 10%, no difference in the average was seen over time (0.59 mM ± 0.09 for PF10 vs. 2.44 mM ± 1.84 for SCS10, p = 0.0773, Fig. 6c). Outflow potassium was higher in SCS10 (9.48 mM ± 2.69 vs. 5.20 mM ± 0.65, p < 0.0001, Fig. 6d), suggesting greater cellular damage. Consistently, liver transaminases (ALT and AST) were higher in SCS10 at 60 min (2973.67 U/L ± 869.28 vs. 1078.67 U/L ± 356.23, p = 0.0401) and 120 min (3245.00 U/L ± 886.19 vs. 1082.33 U/L ± 377.89, p = 0.0189, Fig. 6f). AST was also significantly elevated in SCS10 at 60 min (2753.33 U/L ± 869.28 vs. 1150 U/L ± 444.48, p = 0.0031) and 120 min (3151.33 U/L ± 125.34 vs. 1211.33 U/L ± 474.13, p = 0.0007, Fig. 6g). Importantly, edema post-transplant was 3% ± 2 in the PF10 compared to 83% ± 11, (p = 0.0064) in the SCS group (Fig. 6h). Bile production was sustained after PF10 but not SCS (33.33 uL ± 15.28 vs. 0 uL ± 0, p = 0.0634, Fig. 6h). No difference was observed between PF10 and SCS10 in energy charge (0.23 ± 0.048 compared to 0.21 ± 0.022, p = 0.4386) (Fig. S2l). Finally, the analysis of microscopic structure (Fig. 7a-b) showed greater disruption of hepatic lobular architecture in SCS10 compared to PF10. Furthermore, on the macroscopic level, SCS10 exhibited more edema and irregularly perfused regions across the liver, contrasting with the findings in PF10 (Fig. 7c-d).
Partial Freezing Shows Vast Improvement in Simulated Transplantation Performance over Static Cold Storage Following 10 Days of Storage: Comparison of perfusion metrics during simulated transplantation between livers partially frozen (PF10) and static cold stored (SCS10) for 10 days. Resistance was significantly elevated throughout the entire simulated transplantation in SCS10 (a). Oxygen uptake rate was greater in PF10 at 30, 60, 90, and 120 min (b). No difference in lactate was observed; however, SCS10 showed slight elevation at 0 min (c). Potassium was elevated in SCS10 throughout, with a significant increase observed at 120 min (d). Both ALT (e) and AST (f) were significantly elevated at 60 min and 120 min. Weight gain was considerably elevated in SCS10, while PF10 showed no change in weight (g). Bile production was conserved in PF10, while SCS10 resulted in no bile production (h).
Microscopic and Macroscopic Evaluation of Livers Partially Frozen and Static Cold Stored for 10 Days: (a, b) Light microscopy images of parenchymal liver wedges at the end of simulated transplantation (x10, x20). (c) SCS liver, left: After 10-day cold storage, right: During simulated transplantation. (d) PF liver left: After 10-day freezing, right: During simulated transplantation.
The impact of storage time (1 vs. 5 vs. 10 days) and storage solution (5% PEG vs. 10% PEG) on liver viability after partial freezing was demonstrated in Fig. S3, showing significantly enhanced average oxygen uptake following 5-day PF using 10% PEG (15.97 uL O2/min*g ± 10.50 vs. 34.89 uL O2/min*g ± 5.94, p = 0.0118). However, extending storage time to 10 days resulted in a notable decrease in oxygen uptake despite optimizations (16.34 uL O2/min*g ± 4.57, p = 0.350) (Fig. S3a). The average vascular resistance was comparable between groups (Fig. S3b).
Discussion
Although the concept of preserving organs below freezing temperatures with halted metabolic activity is feasible, practical challenges hinder the anticipated level of viability. The storage at high subzero temperatures is limited by the destructive effects of ice formation, toxicity caused by high concentrations of CPA, and challenges with rewarming32,33. The major challenge in unoptimized partial freezing protocol was the excessive tissue edema that occurs during the gradual switch of CPA unloading to recovery solution. Moreover, high initial vascular resistance during the recovery resulted in lower flow rates, which precipitated decreased oxygen delivery to tissues, eventually resulting in lower oxygen uptake rates. Inferred by these outcomes, we hypothesized that mitigating the adverse effects of extracellular ice formation could potentially address the underlying storage damage.
Protecting the integrity of the cell membrane is a critical objective in cold preservation protocols since the temperature alters the physical characteristics of the cell membrane by increasing the rigidity of the lipid layer34. Previous studies have shown that PEG effectively prevents cold-induced lipid peroxidation, thereby preserving cell viability and functionality35,36. Numerous investigations have confirmed that PEG substantially decreases leakage and preserves cellular integrity37,38. Accordingly, we modified our experimental protocol by increasing PEG concentration to intensify its protective effects against membrane damage caused by extracellular ice formation. PEG concentration was increased from 5 to 10% in the final storage solution and from 1 to 2% in other solutions.
A subsequent observation was that the viscosity of the solutions, particularly the final storage solution, seemed to increase notably due to higher PEG concentration, necessitating a reduction in flow rate from 1 ml/min to 0.5 ml/min during the loading stage of the final storage solution (as described in the Methods section). To further mitigate potential membrane damage during unloading, we implemented a 20-minute acclimation period after thawing, maintaining a controlled flow rate of 2 mL/min to gently unload the cryoprotectants and prevent shear stress. This approach differs from the unoptimized protocol, which relied on pressure-based flow rates that increased incrementally up to 10 mL/min with a stable temperature of 4 °C.
Another crucial aspect of freeze survival is the equilibrium of osmotic forces between the intra and extracellular compartments, which must be carefully maintained to prevent damage from osmotic shifts39,40,41. Following the removal of CPAs from the liver after freezing, the intracellular space becomes dehydrated, requiring a counter-balancing agent to restore optimal osmolality between compartments. As a critical regulator of plasma oncotic pressure, albumin helps maintain the precise balance of fluid dynamics between the cell’s interior and exterior, ensuring proper cellular hydration and function. Building on our earlier findings, which showed successful recovery of vascular composite allografts (VCA) from cold injury with increased albumin concentration in the perfusate42, we modified the BSA concentration of the recovery solution. By increasing the albumin concentration from 1 to 7.5%, we were able to optimize liver recovery and minimize edema.
Additionally, we used a non-blood base solution for NMP following the recovery period to rule out possible effects of whole blood on vascular structure while assessing liver function.
Livers stored with this optimized protocol demonstrated a consistent portal pressure reduction while gradually switching from the unloading solution to the recovery solution. This reduction allowed a quicker increase of the flow rates without any peak in the vascular resistance, resulting in significantly lower edema at the end of SNMP. As shown before with supercooled rat livers, the oxygen uptake is one of the indicators of post-transplant survival22. The oxygen uptake improved in 10% PEG group during SNMP and, most significantly, in the NMP phase. Conversely, lactate levels had an increasing pattern in the 10% PEG group compared to steady levels of the 5% PEG group; nevertheless, end NMP lactate levels of both groups were in the normal range (< 2.5 mmol/L)43. In means of hepatocellular injury, ALT levels were comparable between groups, while AST levels were slightly lower in the 10% PEG group. Tissue adenylate energy charge was comparable between groups, while ATP levels were higher in the 10% PEG group. Histologic analysis with H&E staining showed greater cellular edema in the 5% PEG group.
An increased concentration of PEG successfully minimized the adverse effects of extracellular ice formation, while 7.5% BSA maintained the oncotic pressure. This combination led to significant improvements in liver function, showing promising results. Leveraging these findings, we extended the storage duration to 10 days, applying our enhanced protocol. To our knowledge, this is the first study testing 10-day high subzero storage, twice the duration of our group’s initial efforts. The 10-day storage length has been chosen to evaluate the suppression of metabolic activity when stored for more than a week. While we assessed optimized 5-day (10% PEG) livers during acellular NMP phase, to evaluate the impact of longer storage duration, we used simulated transplantation for 10-day livers.
Extending the storage duration from 5 to 10 days led to high initial vascular resistance. However, by the end of SNMP, the vascular resistance in 10-day stored livers had decreased to the normal range and converged with 5-day (10% PEG) livers. The reduction in the oxygen uptake was significant in 10-day livers, and lactate levels were comparable to those in 5-day (10% PEG) livers.
Our final investigation involved a comparative analysis of liver viability between partially frozen livers (stored at -15 °C to -20 °C for 10 days) and livers maintained using static cold storage at 4 °C for the same duration, during a simulated transplantation procedure. We explicitly selected SCS as the comparison group to illustrate its limitations in mitigating metabolic activity over an extended duration at 4 °C. This strategic choice allowed us to contrast the metabolic profiles of PF and SCS livers, providing valuable insights into their distinct properties and acknowledging the temperature-related constraints of SCS as a control. Importantly, PF demonstrated successful preservation of liver viability following the 10-day period, outperforming SCS livers in all aspects. SCS livers gained excessive edema by the end (> 80%). Significantly reduced oxygen uptake and elevated potassium levels indicated cold ischemic damage in the SCS group when compared with the high oxygen uptake level and normal potassium levels in the PF group. Regarding markers of hepatocellular injury, AST and ALT levels were notably lower in the PF group. Even after freezing for 10 days, PF livers produced bile; this was not observed in SCS livers. Tissue adenylate energy charge was comparable between groups. Finally, the SCS group showed more significant destruction of hepatic structure on histology.
While this study has limitations, our results on 5 and 10-day storage using partial freezing (PF) highlight the need for multi-time point assessments to accurately capture metabolic dynamics in future studies. Future research should consider large animal and discarded human liver studies as a valuable alternative to simulate transplant conditions and challenges. Although these studies provide useful insights, actual transplantation procedures are essential for understanding the practical implications of PF. A comparative analysis of PF and machine perfusion technologies can reveal unique approach-specific profiles during identical storage periods, ultimately optimizing transplantation outcomes.
In conclusion, we introduced an optimized partial freezing protocol to mitigate potential storage-related damage by precisely controlled ice formation. With these optimizations, we demonstrated improved viability and functionality of livers up to 10 days of storage.
Methods
Ethical statement
All the experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Massachusetts General Hospital (Boston, MA, USA; 2017N000227). All experiments were performed in accordance with relevant guidelines and regulations. All experiments were performed in accordance with ARRIVE guidelines.
Experimental design
The experimental steps are shown in Figure 1 and adapted from Tessier et al.25;
1) liver procurement, 2) preconditioning during subnormothermic machine perfusion, 3) preloading CPAs during hypothermic machine perfusion, 4) loading of the final storage solution during HMP, 5) freezing, 6) thawing, 7) unloading CPAs during HMP, 8) functional recovery during SNMP, 9) viability assessment during simulated transplantation or non-blood base NMP.
Our initial step was to replicate the same condition that was previously shown by our group, using 5% PEG in the storage solution. Next, we tested the effect of increased PEG concentration to 10% in the storage solution, 2% in preconditioning, loading, and recovery solutions, and BSA concentration in the recovery solution to 7.5%. Additionally, a 20-minute acclimation period is introduced following the thawing phase. For this step, a total number of 10 rat livers were divided into two experimental groups (optimized/unoptimized) for a 5-day storage duration (n = 5 per group). We excluded simulated transplantation to avoid the confounding effect of blood. Once optimized, we evaluated the viability of PF livers stored for 10 days (n = 3) compared to time-matched SCS (n = 3). Viability was evaluated with simulated transplantation using a blood-based solution.
Liver procurement
Livers from healthy, adult female Lewis rats (10-12 weeks old, weighing 175-200 g) (Charles River Laboratories, Wilmington, MA, USA) were used for all experiments to ensure consistency between groups. The animals were housed socially in a temperature and humidity-controlled room and provided unrestricted food and water.
The liver was procured as previously described44. The perfusion was started immediately after procurement, except for the static cold stored livers.
Static cold storage
After procurement, static cold stored livers were flushed with 30 ml of ice-cold Belzer University of Wisconsin (UW) Cold Storage Solution. Then, livers were placed in a petri dish with 30 ml UW and stored at 4°C for 10 days.
Machine perfusion
Machine perfusion provides continuous perfusion with pressure, flow, and temperature control through the portal vein. The perfusion system setup and operation are previously described44. We used a Radnoti (Cat# 130144) oxygenator.
Partial freezing protocol
After procurement, livers were weighed and perfused with 250 ml of preconditioning solution (Supplementary Table S1) at 21°C, starting with the flow of 5 ml/min and gradually increasing to 25 ml/min with a maximum pressure of 5 mmHg. Livers were perfused under this condition for 30 minutes, then the preconditioning solution was gradually switched to preloading with 10% incremental increase in 25 ml volume. During the switch, the temperature was set to 4°C, and the flow rate was decreased to 7 ml/min to keep pressures below 3 mmHg, which perfused for an additional 30 minutes. Next, the preloading solution was gradually switched to storage solution with 10% incremental increase in 1 ml volume. The perfusion temperature was kept at 4°C, and the flow rates were decreased to 1 ml/min for 5% PEG groups and 0.5 ml/min for 10% PEG groups. The storage solution was loaded for 30 minutes, after which livers were placed in a bag with 25 ml of storage solution and immediately stored in a pre-cooled chiller at -15°C.
At the end of the storage period, the livers were removed from the bag and placed into a water bath (Cole Parmer, US) containing 25 ml of thawing solution at 37°C. The heater was turned off when the liver was placed, and the liver was gently swished until thawed, usually 2-3 minutes. Following this, the livers were connected to the perfusion system, beginning a 20-minute period of hypothermic machine perfusion at 4°C, with an initial flow rate of 2 ml/min. In the unoptimized group, the flow rate was gradually increased to 10 ml/min, reaching a maximum pressure of 3 mmHg. In contrast, the optimized group maintained a steady flow rate of 2 ml/min and consistent pressure throughout the 20-minute period (acclimation period). After 20 minutes, the temperature was gradually increased to 21°C with adjustments in flow rates to keep the pressure 4-5 mmHg. When the temperature reached 21°C, the thawing solution gradually switched to recovery solution with a 10% incremental increase in 25 ml volume.
After 100% recovery solution was reached, the liver was perfused with 250 ml of SNMP recovery solution for 3 hours with a maximum pressure of 5 mmHg and a flow of 25 ml/min. Following SNMP, 5-day (10% PEG) and 5-day (10% PEG) livers were removed and weighed, the system was loaded with a fresh 250 ml recovery solution, and the temperature was set to 37°C. The livers were perfused for 2 hours with a maximum pressure of 11 mmHg and flow of 30 ml/min (Fig. 1).
Supplementary Table S2 presents detailed specifications for the reagents required for the protocol.
Simulated transplantation
After SNMP, 10-day (10% PEG) PF livers were removed from the system and weighed. The perfusion system was emptied and loaded with the blood solution consisting of 10% whole blood and 90% recovery solution with a total volume of 100 ml. Whole blood was obtained from one Lewis rat (~10 ml), stored at room temperature (21°C), and used within 4 hours after blood draw. For the control group, 10-day SCS livers were flushed with 30 ml of lactated ringer to rinse out the UW. Then, livers were connected to the perfusion system. All livers were perfused for 2 hours at 37°C with a flow rate of 30 ml/min or a maximum pressure of 11 mmHg.
Viability assessment
To measure edema, a precision pocket scale (MAXUS) was used, with weight measurements being taken immediately after surgery, before storage, after thawing, after recovery, and after NMP. Viability assessment was performed as described before25.
Histology
Wedge biopsies were taken at the end of NMP and stored in 10% formalin, which, after 24 hours, was moved to 70% ethanol. Samples were paraffin-embedded, cut, and stained with Hematoxylin and eosin (H&E) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) as previously described25.
Liquid-chromatography-mass spectrometry
To determine the concentration of various bioenergetic molecules, wedge biopsies of tissue were taken following NMP and were immediately flash-frozen in liquid nitrogen. Samples were stored at -80C until analysis. Liquid-chromatography-mass spectrometry (LC-MS) was performed by the Mass Spectrometry Core Facility at Shriners Hospital (Boston, MA, USA) as previously described44. Briefly, the tissue was homogenized in liquid nitrogen and analyzed with targeted multiple-reaction monitoring.
Statistical analysis
Statistical analysis and graphing were performed using GraphPad Prism 10 software version 10.0.1 (GraphPad Software, San Diego, CA, USA). The two-sided significance was set to 0.05. Unpaired t-tests were performed on time-dependent data (results). Additional statistics (graphed) were performed using two-way analysis of variance (ANOVA) for time-dependent analysis, as well as post-hoc Tukey testing for significance. Statistics for time-independent data were determined using paired t-tests. All metrics are reported as means with standard deviation.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kwong, A. J. et al. OPTN/SRTR 2021 Annual Data Report: liver. Am. J. Transpl. 23(2 Suppl 1), S178–S263 (2023).
Lai, J. C. Defining the threshold for too sick for transplant. Curr. Opin. Organ. Transpl. 21(2), 127–132 (2016).
Giwa, S. et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 35(6), 530–542 (2017).
Arulraj, R. & Neuberger, J. Liver transplantation: filling the gap between supply and demand. Clin. Med. (Lond) 11(2), 194–198 (2011).
Sheetz, K. H. & Waits, S. A. Outcome of a change in allocation of livers for transplant in the United States. JAMA Surg. 156(5), 496–498 (2021).
Reddy, M. S. et al. Matching donor to recipient in liver transplantation: relevance in clinical practice. World J. Hepatol. 5(11), 603–611 (2013).
Zarrinpar, A. & Busuttil, R. W. Liver transplantation: past, present and future. Nat. Rev. Gastroenterol. Hepatol. 10(7), 434–440 (2013).
Haque, O. et al. Evolving utilization of donation after circulatory death livers in liver transplantation: the day of DCD has come. Clin. Transpl. 35(3), e14211 (2021).
Marecki, H. et al. Liver ex situ machine perfusion preservation: a review of the methodology and results of large animal studies and clinical trials. Liver Transpl. 23(5), 679–695 (2017).
Raigani, S. et al. Viability testing of discarded livers with normothermic machine perfusion: alleviating the organ shortage outweighs the cost. Clin. Transpl. 34(11), e14069 (2020).
Eshmuminov, D. et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 38(2), 189–198 (2020).
Matsuno, N. & Kobayashi, E. Challenges in machine perfusion preservation for liver grafts from donation after circulatory death. Transpl. Res. 2(1), 19 (2013).
Bejaoui, M. et al. Emerging concepts in liver graft preservation. World J. Gastroenterol. 21(2), 396–407 (2015).
Lucia, A., Ferrarese, E. & Uygun, K. Modeling energy depletion in rat livers using Nash equilibrium metabolic pathway analysis. Sci. Rep. 12(1), 3496 (2022).
Storey, K. B. & Storey, J. M. Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q. Rev. Biol. 65(2), 145–174 (1990).
A, C. & Fraser, P. K. P. Why does metabolism scale with temperature? 2004: Functional Ecology. pp. 243–251.
Schulte, P. M. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 218(Pt 12), 1856–1866 (2015).
Ozgur, O. S. et al. Current practice and novel approaches in organ preservation. Front. Transpl. 2, 1156845 (2023).
Botea, F. et al. An exploratory study on isochoric supercooling preservation of the pig liver. Biochem. Biophys. Rep. 34, 101485 (2023).
Sharma, A. et al. Cryopreservation of whole rat livers by vitrification and Nanowarming. Ann. Biomed. Eng. 51(3), 566–577 (2023).
Bruinsma, B. G. et al. Supercooling preservation and transplantation of the rat liver. Nat. Protoc. 10(3), 484–494 (2015).
Berendsen, T. A. et al. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat. Med. 20(7), 790–793 (2014).
Chen, P. et al. Nanowarming and ice-free cryopreservation of large sized, intact porcine articular cartilage. Commun. Biol. 6(1), 220 (2023).
Năstase, G. et al. Isochoric supercooling organ preservation system. Bioeng. (Basel) 10(8), 934 (2023).
Tessier, S. N. et al. Partial freezing of rat livers extends preservation time by 5-fold. Nat. Commun. 13(1), 4008 (2022).
Tessier, S. N. et al. The role of antifreeze glycoprotein (AFGP) and polyvinyl alcohol/polyglycerol (X/Z-1000) as ice modulators during partial freezing of rat livers. Front. Phys. 10, 1033613 (2022).
Wolanczyk, J. P., Storey, K. B. & Baust, J. G. Ice nucleating activity in the blood of the freeze-tolerant frog, Rana sylvatica. Cryobiology 27(3), 328–335 (1990).
Lee, M. R. et al. Isolation of ice-nucleating active bacteria from the freeze-tolerant frog, Rana sylvatica. Cryobiology 32(4), 358–365 (1995).
Morris, G. J. & Acton, E. Controlled ice nucleation in cryopreservation–a review. Cryobiology 66(2), 85–92 (2013).
Costanzo, J. P. et al. Hibernation physiology, freezing adaptation and extreme freeze tolerance in a northern population of the wood frog. J. Exp. Biol. 216(Pt 18), 3461–3473 (2013).
Sugimachi, K. et al. Nonmetabolizable glucose compounds impart cryotolerance to primary rat hepatocytes. Tissue Eng. 12(3), 579–588 (2006).
Fahy, G. M. Cryoprotectant toxicity neutralization. Cryobiology 60(3 Suppl), S45-53 (2010).
Wusteman, M., Robinson, M. & Pegg, D. Vitrification of large tissues with dielectric warming: biological problems and some approaches to their solution. Cryobiology 48(2), 179–189 (2004).
Quinn, P. J. Principles of membrane stability and phase behavior under extreme conditions. J. Bioenerg Biomembr. 21(1), 3–19 (1989).
Puts, C. F. et al. Polyethylene glycol protects primary hepatocytes during supercooling preservation. Cryobiology 71(1), 125–129 (2015).
Dutheil, D. et al. Polyethylene glycols interact with membrane glycerophospholipids: is this part of their mechanism for hypothermic graft protection?. J. Chem. Biol. 2(1), 39–49 (2009).
Oltean, M. et al. Intraluminal polyethylene glycol stabilizes tight junctions and improves intestinal preservation in the rat. Am. J. Transpl. 12(8), 2044–2051 (2012).
Pasut, G. et al. Polyethylene glycols: an effective strategy for limiting liver ischemia reperfusion injury. World J. Gastroenterol. 22(28), 6501–6508 (2016).
Xu, X. et al. Effects of osmotic and cold shock on adherent human mesenchymal stem cells during cryopreservation. J. Biotechnol. 162(2–3), 224–231 (2012).
Stadmiller, S. S. et al. Osmotic shock Induced Protein destabilization in living cells and its reversal by Glycine betaine. J. Mol. Biol. 429(8), 1155–1161 (2017).
von Filz, I. et al. Sub-zero non-freezing of vascularized composite allografts in a rodent partial hindlimb model. Cryobiology 104950 (2024).
Lellouch, A. G. et al. Tolerance of a Vascularized Composite Allograft Achieved in MHC Class-I-mismatch swine via mixed chimerism. Front. Immunol. 13, 829406 (2022).
Laing, R. W. et al. Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: study protocol for an open-label, non-randomised, prospective, single-arm trial. BMJ Open 7(11), e017733 (2017).
Bruinsma, B. G. et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci. Rep. 6, 22415 (2016).
Acknowledgements
This material is partially based upon work supported by the National Science Foundation under Grant No. EEC 1941543. Support from the US National Institutes of Health is gratefully acknowledged for the following awards: R01DK114506, R01DK096075, R01EB028782, K99/R00 HL1431149, R01HL157803, R01DK134590, R24OD034189 and from the Swiss National Science Foundation to A.L. (SNSF PZ00P3-185927). We thank the Mass Spectrometry Special Shared Facilities at Shriners Hospitals for Children (Boston) for the quantification of metabolite levels.
Author information
Authors and Affiliations
Contributions
Ozge Sila Ozgur: Concept/design, Conduct experiments, Data collection & analysis, Drafting article.Mclean Taggart: Conduct experiments, Data collection & analysis, Drafting article.Mohammedreza Mojoudi: Data collection & analysis.Casie Pendexter: Concept/design, Data collection & analysis.Irina Filz von Reiterdank: Data collection & analysis.Anil Kharga: Data collection & analysis.Heidi Yeh: Concept/design, Critical revision of article.Mehmet Toner: Concept/design, Critical revision of article.Alban Longchamp: Concept/design, Critical revision of article.Shannon N. Tessier: Concept/design, Critical revision of article. Korkut Uygun: Concept/design, Critical revision of article. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Some authors declare competing interests. Drs. Uygun, Tessier, Yeh and Toner have patent applications relevant to this study. Drs. Uygun, Tessier and Toner have a financial interest in and serve on the Scientific Advisory Board for Sylvatica Biotech Inc., a company focused on developing high subzero organ preservation technology. Competing interests for MGH investigators are managed by the MGH and MGB in accordance with their conflict-of-interest policies. All the remaining authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ozgur, O.S., Taggart, M., Mojoudi, M. et al. Optimized partial freezing protocol enables 10-day storage of rat livers. Sci Rep 14, 25260 (2024). https://doi.org/10.1038/s41598-024-76674-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76674-6
This article is cited by
-
The Effects of Water Migration on Fresh Food Freezing Processing
Food Engineering Reviews (2025)
-
Cryopreservation of biological materials: applications and economic perspectives
In Vitro Cellular & Developmental Biology - Animal (2025)