Abstract
Since 2011, holopelagic Sargassum have been massively stranding in the coastal environments of the Caribbean Islands inducing damages to coastal ecosystems, public health and the economy. To limit the risks associated with Sargassum stranding, floating barriers with nets can be placed in front of sensitive areas, to divert Sargassum away from the coast. To evaluate the potential transfer of metallic trace element (MTE) from Sargassum to adjacent marine life, seagrasses (Halophila stipulacea, Thalassia testidinum) and urchin (Lytechinus variegatus) were sampled, both close (0 m) and far (200 m) from barriers installed during 4 years in two bays: Baie Cayol (BC) and Cap Est (CE) in Martinique (FWI). A bay without barriers Baie-Tresor (BT) was also sampled in order to compare the effects of Sargassum accumulated in a natural environment versus an environment with floating barriers. The short-term effects of barriers were evaluated by measuring the evolution of MTE after four days, in the algae (Dictyota spp.), located close to Sargassum accumulations. All sampling was realized during two periods of active (July 2021) and reduced (January 2022) Sargassum stranding. The measured concentrations of 19 metal(loid)s trace elements revealed that the proximity of Sargassum to the barriers did not increase MTE concentration. The absence of increase in MTE was observed all sites (BT, BC and CE) and during periods of limited and important Sargassum stranding. Similarly, translocations of Dictyota close to Sargassum accumulations did not reveal any increase in MTE concentrations in the algae after 4 days. The present study suggests that the use of barriers to manage Sargassum stranding would not constitute an important threat of MTE contamination of marine environments.
Similar content being viewed by others
Introduction
One of the most diverse genera among brown algae is the genus Sargassum including over 350 recognized benthic species1. Among this genus, only two species are holopelagic, having their entire life cycle floating in the Atlantic Ocean and reproducing by vegetative fragmentation: Sargassum fluitans and Sargassum natans2,3,4,5. Morphological and molecular studies have differentiated three genotypes: S. fluitans III and S. natans I and VIII6. Holopelagic Sargassum was first reported during the 15th Century in the Sargasso Sea7. Since 2011, Sargassum has formed the Great Atlantic Sargassum Belt extending from the West African coasts to Brazil, the Caribbean Sea and the Gulf of Mexico. Caribbean islands have to face massive stranding of pelagic Sargassum algae (Gower and King, 2011; Széchy et al., 2012) causing several significant issues: (i) ecological damages that threaten endangered species such as turtles8 and lead to the disappearance of coastal ecosystems9 (ii) Sargassum pose health risk to humans, including respiratory diseases, neurological problems and digestive cardiovascular lesions due to \(\:{\text{H}}_{2}\)S emitted from decomposed algae10 (iii) economic issues arise due to the impact on tourism, and obstruction of boat circulation impacting marine trade and fisheries11. Pernicious effects can also be associated with MTE due to the ability of Sargassum algae to absorb MTE from their environment12. In the field, Sargassum exhibits high concentrations of metals, especially with arsenic (As) being one of the most consistently abundant elements found across offshore and coastal environments13,14,15 throughout the year16,17. Sargassum has also been shown to rapidly accumulate As in experimental conditions18. Once they arrive in tropical coastal environments, Sargassum quickly releases their metalloid As into mangroves, seagrasses and coral reefs19. The metalloid As released by Sargassum could be transferred to marine organisms, posing a potential contamination risk for marine life and seafood consumers.
To mitigate the impact of massive accumulations of stranded algae, Sargassum can either be harvested from nearshore areas and harbors while they are floating, or once they become stranded20. Artificial barriers can also be used to redirect floating algae, preventing their accumulation in sensitive areas. These barriers consist of floating buoys equipped with dragging nets that intercept the path of the floating Sargassum. Typically, these devices are installed parallel to the coast, utilizing natural marine currents to guide the algae along these barriers and prevent their massive accumulations.
The aim of the present study is to investigate the potential transfer of metals from Sargassum accumulated in artificial barriers to adjacent marine organisms. We analyzed the metal composition of seagrasses (Halophila stipulacea, Thalassia testudinum) and urchin (Lytechinus variegatus) collected both far and close to Sargassum accumulated in barriers, in two different bays, as well as in a natural bay without barriers in Martinique (French West Indies). Measurements were conducted during two periods with Sargassum stranding activities are active (July 2021), and reduced (January 2022). During both periods, the short-term effects of barriers was assessed by placing the algae Dictyota spp. close to accumulated Sargassum and measuring changes in their MTE concentrations after four days.
Materials and methods
Study sites
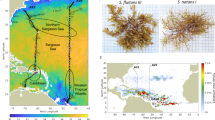
The sampling was conducted on the “windward coast” of Martinique (FWI) which is located on the East side of the Island and is frequently exposed to Sargassum strandings (Fig. 1). To assess the characteristics of MTE accumulation attributed to barriers, sampling was carried out in two bays with barriers installed for 4 years: Baie Cayol (BC) and Cap Est (CE). Additionally, a bay without barriers, Baie-Trésor (BT) was sampled. Artificial barrier used in our study allowed small boats to collect Sargassum continuously in case of accumulation. According to local and episodic currents, accumulated Sargassum can punctually return off shore. Exchange rates of algae have not been quantified and information about resident time of Sargassum in barrier is not available.
Sample collection
Sampling took place in July 2021 and June 2022 during periods of respectively significant and reduced stranding. Samples were obtained at different distance from Sargassum accumulation (i) in artificial barrier (0 m and 400 m) and (ii) in natural bay (0 m and 400 m). Those distance were chosen arbitrarily in order to be (i) large enough to maximize probabilities to observe different effect of Sargassum accumulation and (ii) small enough to limit bias due to different local environmental conditions.
Phanerogams (Halophila stipulacea (n = 30) and Thalassia testidinum) (n = 21) and urchins (Lytechin us variegatus) (n = 27) were sampled closed to Sargassum accumulations (within 10 m) in barriers (CE and BC) and along the seashore (BT).
A similar sampling approach was implemented at locations far from from Sargassum accumulations, more than 200 m away, in each site (Fig. 1). In addition, Dictyota spp. algae (n = 37) were sampled far from the Sargassum accumulations (more than 200 m away) and placed in cages attached one meter above the water surface. These cages were positioned close to Sargassum accumulations (within 1 m) and far from them (more than 200 m) at each site. After a duration of four days, Dictyota spp from each cage were collected for analysis. Sargassum accumulated in artificial barrier and natural were not sampled for metal analysis.
Laboratory analysis
Sample preparation
Immediately after collection, the urchins (L. variegatus) were dissected and their gonads were collected and extracted. Algae (Dictyota spp.) and all parts of the phanerogams (H. stipulacea and T. testidinum) were gently shaken to remove attached particles and biofilm. All samples were then freeze-dried. Algae and phanerogams were then ground and homogenized using a vibro-grinder with 5 mm zirconium for three min with a frequency of 30 beats per second (Restch© MM200).
Heavy metal analysis
An inductively Coupled Plasma Optical Emission Spectrometer (Spectrometer ICP-OES 700®, Agilent Technologies) was used to analyze a series of 19 elements (Ag, Al, As, Ba, Cd, Co, Ca, Cr, Cu, Fe, Gr, Mn, Mo, Ni, Pb, Se, Sr, V, and Zn) For each sample, a fixed amount of algae (70-80 mg) or fauna (20-30 mg) was placed in a plastic tube containing 1 mL of nitric acid (HN\(\:{O}_{3}\) 67%). The powder sample was then mineralized for 3 h at 100 °C using an Environmental EXPRESS HotBlock® − 54. After mineralization, 5 mL of deionized water was added to each sample. The certified reference materials (DOLT-5, TORT-3) were also analyzed following the same process. The metal concentrations in all samples are expressed in µg.g− 1 (ppm) dry weight. A total of 115 samples were analyzed, for each sites CE (33 samples), BC (36 samples) and BT (46 samples).
Data analysis
A Principal Component Analysis (PCA) was performed with RStudio® and R 4.3.1 (R Core Team 2023 21, using the following packages: FactoMiner (Husson et al., 2020), factoextra (Kassambara and Mundt, 2020), ggplot (Wickham et al., 2020) and corrplot (Wei et al., 2021). The aim was to identify MTE with the most structuring influence among the 19 elements (Al, As, B, Ba, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Pb, S, Sb, Sc, Se, Sr, Ti, V, Zr and Zn).
One-way analysis of variance ANOVA or AOV was used to compare the means of variables in more than two groups of function AOV21,22.
Results
Principal component analysis
Out of 19 elements analyzed, a total fourteen elements (Al, As, Ca, Cd, Cr, Cu, Fe, Mn, Ni, Pb, S, V, Zn and Zr) were found to be the most abundant and were detected in all samples above the limit of detection (LOD) (Fig. 2). Eight metallic elements (Sr, Sb, Sc, Se, Mo, Na, Mg and K) were below the LOD and therefore were not considered in the analysis.
The first two dimensions of the PCA carried 59.16% (F1) and 16.17% (F2) of the total inertia (Fig. 2). F1 distinctly discriminated the variables Fe (16.20%), Al (15.93%), V (13.18%), Cu (12.59%) and Cr (12.28%) whereas and F2 discriminated As (35.48%), Mn (18.79%) and Ca (13.05%), Zn (13.98%) and S (12.31%).
Circle of correlation of Principal Component Analyses (PCA) showing variables F1 (59.16%) and F2 (16.17%) represents the relationship in organisms (Dictyota dictyota; Thalassia stipulacea; Halophila filiforme and Echninoderm (Lytechinus variegatus)) in the three sites (BT, CA and CE) between all the metallic elements (Al, As, B, Ba, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Pb, S, Sb, Sc, Se, Sr, Ti, V, Zr and Zn).
Together, the first two axes of the PCA discriminated samples of Dictyota spp., were characterized by high concentrations in Fe, Al, V, Cu and Cr, and samples of L. variegatus were characterized by As, Mn, Zn, Ca and S from T. testidinum and H. stipulacea. The two phanerogam species showed a large overlap, indicated similar concentrations of As, Zn and S (Fig. 3).
The PCA did not discriminate samples based on the years (2021 and 2022) or the distance from accumulated Sargassum found in barriers (CE and CA) and the seashore (BT).
Principal Component Analysis (PCA) for the four species: Dictyota dictyota; Thalassia stipulacea; Halophila filiforme and Echninoderm (Lytechinus variegatus) in the three sites (BT, CA and CE) between all the metallic elements (Al, As, B, Ba, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Pb, S, Sb, Sc, Se, Sr, Ti, V, Zr and Zn). Color represents different years in 2021 (red ellipse) and 2022 (blue ellipse) and shape represents distance from Sargassum accumulation near (circle) and far (triangle).
Concentrations of metals and metalloids
The MTE concentrations were higher in stations CE and BC as compared to BT (Supplementary Table 1), for all samples analyzed (Fig. 4).
Concentrations of metallic trace elements (Al, As, Ca, Cr, Cu, Fe, Mn, S, V and Zn) in ppm in: Dictyota dictyota; Thalassia stipulacea; Halophila filiforme and Lytchinus variegatus during 2021 (circle) and 2022 (triangle), near (green) and far (purple) from Sargassum accumulation in the three sites (BT, CA and CE).
Among the four studied organisms, the concentration of the metalloid As were higher in Dictyota. spp. presenting arsenic concentration significantly higher than in T. testidinum, H. stipulacea and L. variegatus. (Supplementary Table 2).
The ANOVA tests did not reveal any significant impact of the proximity to accumulated Sargassum on the MTE composition of the studied organisms (Fig. 5).
Discussion
The aim of the present study is to evaluate the potential transfer of MTE from Sargassum sp. to adjacent marine life. Three species were sampled at varying distances (0 and > 200 m) from the accumulation of Sargassum found in two artificial barriers and in a natural bay. These species were chosen as they were the only ones present at all sites.
Out of the 19 metallic trace elements analyzed, three elements (Al, Fe and As) stand out in the PCA analyses, showing higher contributions. The proximity to accumulated Sargassum did not lead to an increased concentration of MTE in phanerogams (T. testidinum and H. stipulacea) and sea urchin (L. variegatus). Similarly, short-term experiments did not reveal any changes in MTE composition of Dictyota spp. when placed near Sargassum accumulations during four days.
This lack of effect was observed irrespective of the location of Sargassum accumulation, whether it was close to seashore in a natural bay (BT) or in an artificial barrier (CE and CA). Samples with minimal influence from Sargassum were collected at a distance exceeding 200 m from the accumulations. These experiments are based on the assumption that the effects on MTE concentrations in other species would be more pronounced with closer the proximity to Sargassum accumulations. However, there may be a bias if this spatial scale is not appropriate and if effects occur on a larger scale, impacting control areas as well. During the field sampling, the water appeared brown in color due to leachates near the Sargassum accumulations, whereas this turbidity was not observed 10 m away from the Sargassum, suggesting a small-scale effect. The eutrophication associated with Sargassum accumulation stresses the environment at ecosystem scale and reduces biodiversity in coastal habitats9. In the present study, the diversity of benthic species available to evaluate metallic contamination was always higher away from Sargassum. Visual observations of water turbidity and diversity of benthic fauna reveals an impact more reduced 200 m away from Sargassum accumulation suggesting an adapted sampling scale.
The algae Dictyota present the ability to absorb rapidly metals elements like Cr (Chromium) 15 min after exposition (Nandhagopal et al. 2018) and experiments of the present study were conducted during 4 days based on those results. Integration of other metallic compounds can take longer and this potential bias due to experiment duration must be kept in mind.
Spatial transect studies13,14 as well as temporal surveys16 both revealed a constant higher concentration of As in pelagic Sargassum than in most coastal environment organisms. Once arrived at coast, Sargassum rapidly released As16 and the present study is based on the assumption that proximity with accumulated Sargassum increase contamination risks. Sargassum were not sampled during this study as their turn over time is not known in barrier and their metallic concentration would be difficult to link with concentration released in environment.
Brown algae presents high amount of alginate in their cell walls, which gives them a strong affinity with MTEs12,23,24. As a result, brown algae are known to be more efficient than other algae and organisms in absorbing and accumulating metals and metalloids like arsenic25. This ability leads to a consistent and elevated presence of As in Sargassum over large spatial scales13,14,26 and over time16,17. The genus Dictyota has also been found to absorb substantial amounts of metals in the Mediterranean Sea27 and organochlorine molecules such as chlordecone in French West Indies28.
Among the metallic trace elements studied, arsenic stands out in the PCA analysis with a higher contribution to the first principal component compared to others elements. Given the consistent presence of As element in Sargassum13,14,15 this study primarily focuses on the As element. The concentrations of As measured during our campaigns falls within in the range of values regularly measured in macroalgae Chlorophytes (from 0.1 to 23 ppm), Rhodophytes (from 0.1 to 45 ppm) and Phaeophytes (from 1 to 179 ppm)29.
Due to high level of As found in Dictyota spp., this species could serve as a reliable bioindicator with a high sensitivity, enabling the detection of slight changes in MTE concentrations in the environment.
The bioavailability of metals can be influenced by suspended or sedimented organic matter (OM) influencing the metals bioavailability due to its high ability to chelate metal elements30. Due to terrestrial runoff and local productions, coastal environments and particularly mangroves present significant amount of OM31.
OM constitues an ubiquitous sorbent for Arsenic32,33,34 with the ability to bind both As(V) and As(III) forming OM-As complexes due to various functionals groups such as sulfhydryl and amine35,36. A potential explanation of the absence of As increases in organism located close to Sargassum accumulation would be that the high amount of OM in coastal environments reduce As bioavailability.
Once stranded, Sargassum are releasing phosphorus in surrounding water9. Phosphate ion (PO43−) and (AsO43) are presenting chemical similarities and As is consequently entering in algal cells using P transportation systems37. High amount of P in the environment tend to decrease the amount of As entering in Sargassum38,39. Arrived in coastal waters Sargassum are simultaneously releasing As and P. The amount of As entering in adjacent organisms could be limited through competition with P explaining the reduced effect observed in Dictyota spp and phanerogams.
Even if As is entering in brown algae through P transportation systems, brown algae plant have set up a regulation mechanism in order to reduce the toxicity of As. The major part of the arsenate absorbed by the algae is transformed in arsenite As(III)40,41,42 and then stocked in the brown algae in the form of nontoxic arsenosugars29. Experiments conducted with caged Sargassum suggest a rapid release of As19. However, the speciation of As released by Sargassum is not known and this form could be non-bioavailable explaining the absence of increased As in organisms adjacent to Sargassum accumulations.
Conclusion
Artificial barriers represent a managing solution to limit the stranding of Sargassum and their negative impacts on environment, public health and economy. Sargassum accumulated in barriers can release their heavy metals in coastal environments. To evaluate this risk, two approaches were used in the present study: i) observation of metallic compositions of organisms according to distance from accumulated sargassum (during both periods of intense and limited sargassum stranding) and ii) temporal evolution of metallic contamination of algae Dictyota sp. placed closed to accumulated Sargassum. Despite potential bias of this study associated with spatial and temporal scale used, MTE concentrations of organisms were not changed according to their proximity with Sargassum accumulations. As a result, the use of barriers to manage Sargassum stranding would constitute a limited threat of MTE contamination of marine environment.
Data availability
All the relevant data are provided within the manuscript. The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Guiry, M. D. & Guiry, G. M. World-Wide electronic publication. Natl. Univ. Ireland Algaebase. Retrieved from http://www.algaebase.org (2022).
Parr, A. E. Quantitative observations on the pelagic sargassum vegetation of the western north Atlantic. Bull. Bingham Oceanogr. Collect. 6, 1–94 (1939).
Sandt, V. J. & Stoner, A. W. Ontogenetic shift in habitat by early juvenile queen conch, Strombus gigas: patterns and potential mechanisms. Fish. Bull. 91, 516–525 (1993).
Fidai, Y. A., Dash, J., Tompkins, E. L. & Tonon, T. A systematic review of floating and beach landing records of sargassum beyond the sargasso sea. Environ. Res. Commun. 2, 122001 (2020).
Dawes, C. & Mathieson, A. C. The seaweeds of Florida. Univ. Press. Fla. Gainesv. https://doi.org/10.2989/10220110509485863 (2008).
Amaral-Zettler, L. A. et al. Comparative mitochondrial and chloroplast genomics of a genetically distinct form of Sargassum contributing to recent Golden tides in the Western Atlantic. Ecol. Evol. 7, 516–525 (2017).
Gower, J. F. R. & King, S. A. Distribution of floating Sargassum in the Gulf of Mexico and the Atlantic ocean mapped using MERIS. Int. J. Remote Sens. 32, 1917–1929 (2011).
Witherington, B., Hirama, S. & Hardy, R. Young sea turtles of the pelagic Sargassum-dominated drift community: habitat use, population density, and threats. Mar. Ecol. Prog Ser. 463, 1–22 (2012).
van Tussenbroek, B. I. et al. Severe impacts of brown tides caused by Sargassum spp. on near-shore caribbean seagrass communities. Mar. Pollut Bull. 122, 272–281 (2017).
Resiere, D. et al. Sargassum seaweed on Caribbean islands: an international public health concern. Lancet 392, 2691 (2018).
Langin, K. Seaweed masses assault Caribbean islands. Science (80-) 360, 1157–1158 (2018).
Volesky, B. & Holan, Z. R. Biosorption of heavy metals. Biotechnol. Prog. 11, 235–250 (1995).
Dassié, E. P., Gourves, P. Y., Cipolloni, O., Pascal, P. Y. & Baudrimont, M. First assessment of Atlantic open ocean Sargassum spp. metal and metalloid concentrations. Environ. Sci. Pollut Res. https://doi.org/10.1007/s11356-021-17047-8 (2021).
Cipolloni, O. A. et al. Metals and metalloids concentrations in three genotypes of pelagic Sargassum from the Atlantic Ocean Basin-Scale. Mar. Pollut Bull. 178, (2022).
Devault, D. A. et al. The silent spring of Sargassum. Environ. Sci. Pollut Res. 28, 15580–15583 (2021).
Cipolloni, O. A., Couture, P., Cordonnier, S. & Pascal, P. Y. Temporal fluctuation of metallic and metalloid concentration in three morphotypes of floating holopelagic Sargassum from the Caribbean coast (Guadeloupe, French West Indies). Mar. Pollut Bull. (2024).
Rodríguez-Martínez, R. E., van Tussenbroek, B. & Jordán-Dahlgren, E. Afluencia masiva de sargazo pelágico a la costa del Caribe mexicano (2014–2015). In Florecimientos algales nocivos en México 352–365 (2017).
Devault, D. A., Massat, F., Baylet, A., Dolique, F. & Lopez, P. J. Arsenic and chlordecone contamination and decontamination toxicokinetics in Sargassum Sp. Environ. Sci. Pollut Res. https://doi.org/10.1007/s11356-020-12127-7 (2021).
Cipolloni, O. A. et al. Kinetics of metal and metalloid concentrations in holopelagic Sargassum reaching coastal environments. Environ. Sci. Pollut Res. https://doi.org/10.1007/s11356-023-29782-1 (2023).
Robledo, D., Vázquez-delfín, E. & Freile-pelegrín, Y. Challenges and opportunities in relation to Sargassum events along the Caribbean sea. Front. Mar. Sci. 8, 1–13 (2021).
R Core Team. R: A Language and Environment for Statistical Computing (R Found. Stat. Comput., 2014).
Chambers, J. M., Freeny, A. & Heiberger, R. M. Analysis of Variance; Designed Experiments. Chapter 5 of Statistical Models in S (Wadsworth Brooks/Cole, 1992).
Davis, T. A., Volesky, B. & Vieira, R. H. S. F. Sargassum seaweed as biosorbent for heavy metals. Elsevier Sci. 34, 4270–4278 (1999).
Davis, T., Volesky, B. & Vieira, R. Sargassum seaweed as biosorbent for heavy metals. Water Res. 34, 4270–4278 (2020).
Zingde, M. D., Singbal, S. Y. S., Reddy, C. V. G., Arsenic, copper, zinc & manganese in the marine flora & fauna of coastal & estuarine waters around Goa. Indian J. Mar. Sci. 5, 212–217 (1976).
Rodríguez-Martínez, R. E., Van Tussenbroek, B. I. & Jordán-Dahlgren, E. Afluencia masiva de sargazo pelágico a la costa del Caribe Mexicano 353–365 (Caribe Mex, 2014).
Chakraborty, S., Bhattacharya, T., Singh, G. & Maity, J. P. Benthic macroalgae as biological indicators of heavy metal pollution in the marine environments: a biomonitoring approach for pollution assessment. Ecotoxicol. Environ. Saf. 100, 61–68 (2014).
Contarini, P. E. & Dromard, C. R. Biosorption capacity of genus Dictyota facing organochlorine pesticide pollutions in coastal areas of the Lesser Antilles. Aquat. Bot. 169, 103346 (2021).
Francesconi, K. A. & Edmonds, J. S. Arsenic and marine organisms. Adv. Inorg. Chem. 44, 147–189 (1996).
Doig, L. E. & Liber, K. Nickel partitioning in formulated and natural freshwater sediments. Chemosphere. 62, 968–979 (2006).
Bouillon, S. et al. Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochem. Cycles 22, 1–12 (2008).
Sharma, P., Ofner, J. & Kappler, A. Formation of binary and ternary colloids and dissolved complexes of organic matter, Fe and As. Environ. Sci. Technol. 44, 4479–4485 (2010).
Liu, G. & Cai, Y. Complexation of arsenite with dissolved organic matter: conditional distribution coefficients and apparent stability constants. Chemosphere. 81, 890–896 (2010).
Ehlert, K., Mikutta, C. & Kretzschmar, R. Impact of birnessite on arsenic and iron speciation during microbial reduction of arsenic-bearing ferrihydrite. Environ. Sci. Technol. 48, 11320–11329 (2014).
Hoffmann, M., Mikutta, C. & Kretzschmar, R. Bisulfide reaction with natural organic matter enhances arsenite sorption: insights from X-ray absorption spectroscopy. Environ. Sci. Technol. 46, 11788–11797 (2012).
Buschmann, J. et al. Arsenite and arsenate binding to dissolved humic acids: influence of pH, type of humic acid, and aluminum. Environ. Sci. Technol. 40, 6015–6020 (2006).
Dixon, H. B. F. The biochemical action of arsonic acids especially as phosphate analogues. Adv. Inorg. Chem. 44, 191–227 (1996).
Alleyne, K. S. T., Johnson, D., Neat, F., Oxenford, H. A. & Vallés, H. Seasonal variation in morphotype composition of pelagic Sargassum influx events is linked to oceanic origin. Sci. Rep. 13, 3753 (2023).
Gobert, T. et al. Trace metal content from holopelagic Sargassum spp. sampled in the tropical North Atlantic Ocean: emphasis on spatial variation of arsenic and phosphorus. Chemosphere. 308, 136186 (2022).
Andreae, M. O. & Klumpp, D. Biosynthesis and release of organoarsenic compounds by marine algae. Environ. Sci. Technol. 13, 738–741 (1979).
Sanders, J. G. & Windom, H. L. The uptake and reduction of arsenic species by marine algae. Estuar. Coast. Mar. Sci. 10, 555–567 (1980).
Howard, A. G., Comber, S. D. W., Kifle, D., Antai, E. E. & Purdie, D. A. Arsenic speciation and seasonal changes in nutrient availability and micro-plankton abundance in southampton water, UK. Estuar. Coast. Shelf Sci. 40, 435–450 (1995).
Acknowledgements
We thank Brigitta I.Van-Tussenbroek and Paco Bustamante to proofread the manuscript and the correction. Metal analyses were funded by a Discovery Grant from the Natural Science and Engineering Research Council of Canada to Patrice Couture. We thank the OFB – French Office of Biodiversity to supported financially. We thank Sébastien Cordonnier and the fishermen for their help on the field with sampling. OC was partly supported financially by the institution of Guadeloupe Region.
Author information
Authors and Affiliations
Contributions
O-A.C. conceived the research, collected the samples, identified Sargassum species, carried out sample preparation, acquired the data, performed data analyses, conducted the analytical study and interpretation, and wrote the first draft of the paper. P.C. funded the metal analyses and proofread the manuscript B.S-B. performed data analyses, conducted the analytical study and interpretation. P-Y.P. proofread the manuscript, collected the samples and data analyzes.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cipolloni, OA., Simon-Bouhet, B., Couture, P. et al. Reduced transfer of metals and metalloids from pelagic Sargassum spp. accumulated in artificial floating barrier. Sci Rep 14, 27066 (2024). https://doi.org/10.1038/s41598-024-76899-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76899-5