Abstract
Phytochrome interacting factors (PIFs) serve as crucial regulators in the light signal transduction pathway and also mediate light signals to regulate secondary metabolite synthesis in plants. However, the regulator role of PIFs in secondary metabolites often varies among different plants. Isorhynchophylline (IRN), an iconic secondary metabolite of Uncaria rhynchophylla, holds significant medicinal value. Low light induces the synthesis of IRN in previous research, but PIFs in U. rhynchophylla have not been studied to date. Building on this, we identified a PIF protein, UrPIF3, which possesses the typical conserved domains of the PIFs and is localized in the nucleus. Moreover, the expression level of UrPIF3 is consistently positively correlated with the expression of two key enzyme genes (UrSGD and UrSTR) in the IRN biosynthesis pathway, regardless of whether under low light or restoring light conditions. Yeast one-hybrid and dual-luciferase assays further demonstrated that UrPIF3 can directly upregulate UrSGD. Conversely, silencing UrPIF3 inhibits IRN synthesis, and significantly reduces the expression levels of UrSGD and UrSTR. In summary, our results suggest that under low light conditions, UrPIF3 can directly upregulate UrSGD and indirectly upregulate UrSTR, thereby promoting the synthesis of IRN.
Similar content being viewed by others
Introduction
Light, as a crucial environmental signal, regulates not only plant growth and development but also the synthesis of secondary metabolites such as anthocyanins, artemisinin, and vindoline1,2,3,4. Phytochrome interacting factors (PIFs) are well-known bHLH transcription factors that interact with phytochrome proteins, acting as key nodes in light signal transduction5,6. In Catharanthus roseus, CrPIF1 invovled in tabersonine accumulation under red light conditions. Moreove, PIF4 and PIF5 as a negative regulators of anthocyanin accumulation induced by red light. This implies that PIFs play a important role in the regulation of secondary metabolite synthesis triggered by light signaling. In Arabidopsis thaliana, there are seven PIF members (AtPIF1, AtPIF3-8), which possess three important structural features: the APB domain for binding to phytochrome B, the APA domain for binding to phytochrome A (present only in PIF1 and PIF3), and the bHLH domain for binding to G-box motif (5’-CACGTG-3’) or E-box motif (5’-CANNTG-3’)7. These structures are fundamental for PIFs to mediate light signals and exert regulatory effects. For instance, under red light, phytochrome in its inactive form (Pr) converts to its active form (Pfr). Pfr then enters the nucleus and interacts with PIFs, leading to PIFs degradation and inhibiting PIFs binding to target gene promoters8.
Phytochromes are crucial partners in PIF-mediated light signal regulation. It has been confirmed that phytochromes can bind to PIFs in plants such as A. thaliana, Oryza sativa, Zea mays, Physcomitrella, and Marchantia6,9,10,11,12. This suggests that the phytochrome-PIF signaling module is conserved in the PIF-mediated light signal transduction process. For the PIF-secondary metabolism signaling module, significant progress has also been revealed. In Artemisia annua, overexpression of PIF3 significantly enhances the expression levels of artemisinin synthase genes (ADS, CYP71AV1, DBR2, and LDH1), thereby significantly increasing the artemisinin content13. In Catharanthus roseus, PIF3 does not affect the expression of genes in the vindoline pathway, but PIF1 is a negative regulator of vindoline biosynthesis. PIF1 represses CrGATA1 (a positive regulator of vindoline synthesis) by binding to the G-box, and represses DAT(an enzyme gene in vindoline synthesis) by binding to the PBE-box3. The PIF-secondary metabolism synthesis signaling module shows significant diversity and complexity among different plants. To date, the PIFs in Uncaria rhynchophylla have not been studied, particularly in relation to the PIF-secondary metabolism synthesis signaling module.
Uncaria rhynchophylla, a member of the Rubiaceae family, is a significant plant known for its medicinal properties. Research indicates that rhynchophylline (RIN) and isorhynchophylline (IRN) are the primary and most important alkaloids in U. rhynchophylla, with antihypertensive, sedative, anti-inflammatory, and anti-addictive effects14,15. In recent years, these two alkaloids have shown great potential in treating and preventing central nervous system diseases, such as Alzheimer’s disease16,17. Therefore, studying the regulatory mechanisms of their synthesis is crucial. Our previous research has demonstrated that light signal is a key environmental factor for IRN synthesis. The low light (around 60 ~ 300 µmol m−2 s−1) promotes IRN production compared to the control light around 400 ~ 1500 µmol m−2 s−118. Given that PIF is a critical node in light signal transduction, we hypothesize that it may be involved in mediating low light signaling to regulate IRN synthesis. Thus, we screened a potential PIF (UrPIF3) from previous transcriptome databases. Based on the structural characteristics, localization, expression under low light and restoring normal light conditions, binding with target genes, and its impact on IRN synthesis of the UrPIF3, we reveal that UrPIF3 can mediate the regulation of IRN synthesis under low light.
Results
PIF3 responds to low light and has a strong positive correlation with IRN synthesis genes in U. Rhynchophylla
PIFs act as negative regulators of light signals, with low light increasing their expression levels19,20,21. For this reason, we investigated the response of PIFs to low light signals in U. rhynchophylla. From previous transcriptome databases18, we identified three low-light responsive PIF members (UrPIF3, UrPIF5-1, and UrPIF5-2) and analyzed their expression under low light and restored normal light conditions. We found that the expression level of UrPIF3 gradually increased under low light (from 0 h to 24 h) but rapidly decreased after restoring normal light (from − 1 h to -24 h). However, under low light, the expression level of UrPIF5-1 initially increased and then decreased, while the expression level of UrPIF5-2 fluctuated. Upon returning to normal light, both UrPIF5-1 and UrPIF5-2 expression initially increased and then decreased (Fig. 1). Additionally, we screened six enzyme genes in the IRN synthesis pathway that respond to low light from previous transcriptome databases. We further analyzed their expression under low light and restored normal light conditions. The expression levels of six enzyme genes(UrLAMT, Ur7DLGT, UrSLS, UrTDC, UrSTR, and UrSGD)were induced by low light conditions. At the 24 h time, only UrTDC, UrSTR, and UrSGD showed the most significant increase in expression (more than twice the level at 0 h). Upon restoring normal light, the expression levels of Ur7DLGT and UrSLS exhibited fluctuations, while the expression levels of UrLAMT, UrTDC, UrSTR, and UrSGD rapidly decreased. (Figure S1). We also analyzed the correlation between the expression levels of these PIFs and the six enzyme genes. We notice that, regardless of whether the treatment was low light or restored normal light, the expression levels of UrPIF5-1 and UrSGD showed a significant positive correlation, as did UrPIF3 with both UrSTR and UrSGD (Tables 1 and 2).
The molecular characteristics of PIF3 protein in U. Rhynchophylla
Inspired by the expression level of PIF3 under low light and normal light recovery conditions, and its positive correlation with the expression of UrSTR and UrSGD, we hypothesize that UrPIF3 plays a significant role in regulating IRN synthesis. We further cloned the coding sequence of UrPIF3 and analyzed its molecular characteristics. In A. thaliana, PIF3 is a bHLH family member with three main structural features: a conserved bHLH domain for DNA binding, an APB domain for binding to phytochrome B, and an APA domain for binding to phytochrome A22. In this study, our results showed that UrPIF3 protein contains a typical bHLH conserved structure with 32 conserved amino acid residues. Notably, residues at positions 503 and 507 differ from those of all PIF members in A. thaliana (Fig. 2). In UrPIF3 protein, we also found a conserved structure similar to APA domain (conserved sites, -NF–F-R–)22, with conserved sites-NFSHF-RPA (Fig. 2).
Generally, transcription factors are located in the nucleus, where they regulate the expression of target genes. PIF3 is also located in nucleus in A. annua and A. thaliana13,23. According to previously reported methods, we analyzed the localization of UrPIF3 using heterologous expression in tobacco13. After staining tobacco leaf cells with DAPI, blue fluorescence was observed in the nuclei, which aligns with earlier findings24. When GFP was transiently expressed in tobacco leaf cells, green fluorescence was dispersed throughout the cell. However, when UrPIF3-GFP was transiently expressed, the green fluorescence perfectly overlapped with the blue nuclear fluorescence (Fig. 3). These results indicate that the UrPIF3 protein is located in nucleus like other PIF3 proteins.
UrPIF3 protein can directly activate the expression of UrSGD
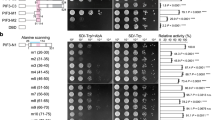
Based on the molecular characteristics of UrPIF3 protein and its significant positive correlation with the expression of UrSTR and UrSGD, we hypothesized that UrPIF3 protein can enter the nucleus and regulate their expression. We further cloned the promoter of UrSTR and UrSGD, and then analyzed the interaction between UrPIF3 protein and these two genes. Our results showed that the UrSTR and UrSGDpromoters contain several motifs, such as the low-temperature responsive element LTR, gibberellin responsive element GARE, and methyl jasmonate responsive element CGTCA-motif. Both promoters also contain the light-responsive element G-box (Figure S2). Previous studies suggest that PIF3 can bind to G-box element in the target gene promoter to regulate gene expression25,26. Thus, UrPIF3 likely binds to the UrSTR and UrSGD promoters to regulate their expression. To test this, we conducted yeast one-hybrid assays. We found that yeast co-transformed with UrPIF3 and UrSGD promoter grew normally on SD-Trp/-Leu/-His medium with various concentrations of 3-AT, while yeast co-transformed with UrPIF3 and UrSTR promoter did not grow. This indicates that UrPIF3 can directly bind to the UrSGD promoter but not to the UrSTR promoter (Fig. 4A). Dual-luciferase assays further confirmed that UrPIF3 can directly bind to the UrSGD promoter and activate its expression (Fig. 4B).
Silencing UrPIF3 inhibits the expression of UrSTR and UrSGD, reducing IRN synthesis
IRN and its isomer RIN are the main active ingredients in U. rhynchophylla, capable of interconversion under the catalysis of isomerase27. For IRN biosynthesis, UrSTR and UrSGDare crucial enzyme genes, and their expression levels directly impact IRN synthesis27,28. In this text, UrPIF3 is significantly positively correlated with the expression of these two enzyme genes and can directly activate UrSGD expression. Thus, we hypothesize that UrPIF3 can influence IRN production. To test this hypothesis, we silenced UrPIF3using previous VIGS technology29. In plant, the phytoene desaturase (PDS) gene is one of the key enzymes in the carotenoid biosynthesis pathway. Once the PDSis successfully silenced, young leaves exhibit photobleaching symptoms30, so we used the PDS as a control marker. In our results, compared to the control plants (Fig. 5A I), the marker plants (vigs-pds) exhibited photobleaching on the 25th day after UrPDS gene silencing (Fig. 5A II). This indicates that the silencing effect had taken place by this time. For experimental plants (vigs-pif3), no abnormal phenotype was observed in the leaves (Fig. 5A III). However, both transcriptional and physiological levels have changed. Compared with the control, the gene expressional level of both UrPDS and UrPIF3 decreased by 78–90% after gene silencing (Fig. 5BC), and the production of IRN/RIN has significantly decreased in vigs-pif3 plants. Interestingly, the expressional level of both UrSTR and UrSGD decreased in vigs-pif3 plants. This suggested that UrPIF3 can influence the expression of UrSTR and UrSGD (Fig. 5 DE).
The effect of UrPIF3 silencing on IRN/RIN biosynthesis in U.rhynchophylla.A The phenotype of U.rhynchophylla. I: CK, II: Vigs-pds, III: Vigs-pif3; B The expression level of UrPDS in Vigs-pds; C The expression level of UrPIF3 in Vigs-pif3; D The expression level of UrSTR1 and UrSGD1 in Vigs-pif3; E The content of IRN/RIN in Vigs-pif3.
Discussion
Light is an indispensable environmental signal for plant growth, development, and metabolism. PIFs play an important role in light signal transduction, making it essential to elucidate their functions in plants. In A. thaliana, PIF3 was the first identified PIF member31, followed by six other members (PIF1, PIF4, PIF5, PIF6, PIF7, and PIF8)5. All of these PIF members possess two key structural features: a typical bHLH domain for DNA binding and an active phytochrome B binding (APB) domain32. In our results, we identified a PIF member UrPIF3 (GeneBank number: OM982855) in U. rhynchophylla. Molecular characterization revealed that the UrPIF3 protein has the typical structural features of PIFs, including the bHLH and APB domains. Subcellular localization studies showed that the UrPIF3 protein is located in the nucleus. These are consistent with previous PIFs localization reports13,33. All these findings confirm that UrPIF3 is a genuine member of the PIF family.
In A. thaliana, several PIFs, including PIF1, PIF3, PIF4, PIF5, and PIF8, can activate the expression of their target genes by binding to the G-box motif in promoters of these genes22,34,35,36. In our study, the UrSGD promoter contains three G-box, also UrPIF3 protein can directly activate UrSGD expression, as demonstrated by yeast one-hybrid and dual-luciferase assays. These suggest UrPIF3 protein may activate UrSGD by binding to the G-box motif in the UrSGDpromoter. Notably, the bHLH domain is the structural foundation for the binding of bHLH family TFs (transcription factors) to the G-box motif26,37. In the bHLH domain of the UrPIF3 protein, the amino acid residues at positions 503 and 507 differ from those in all PIF members in A. thalian (Fig. 2). This implies that the UrPIF3 protein might have a distinctive capability for binding to the G-box motif in U. rhynchophylla. Additionally, in A. thaliana, AtPIF3 and AtPIF1, also have the APA domain, which is essential for binding phytochrome A22. Our tests show that the UrPIF3 protein has an APA domain similar to that of the AtPIF3 protein, with highly conserved sites within the APA domain (Fig. 2). Therefore, UrPIF3 may also have the ability to bind phytochrome A.
PIF3 is well known for its roles in morphology development, seed germination, flowering, stress tolerance, and anthocyanin biosynthesis in A. thaliana5. Recently, PIF3 has been found to regulate the biosynthesis of artemisinin in A. annua13, and control pollen development in Solanum lycopersicum38. These findings show that PIF3 has different functions in different plants. In U. rhynchophylla, silencing UrPIF3 reduces the production of IRN/RIN and decreases the expression levels of the IRN synthetase genes UrSTR and UrSGD (Fig. 5DE). This indicates that PIF3 has a regulatory function in the biosynthesis of IRN/RIN. Besides, forward validation experiments show that UrPIF3 positively correlates with the expression levels of the enzyme genes UrSTR and UrSGD (Tables 1 and 2), directly activating UrSGD expression (Fig. 4AB). Reverse validation confirms that silencing UrPIF3 significantly decreased UrSTR and UrSGD expression levels (Fig. 5D). These further indicate that UrPIF3 is a positive regulator of IRN biosynthesis.
The regulation of secondary metabolite synthesis by PIFs, especially anthocyanin synthesis, has been extensively studied. For example, PIF3, PIF4, and PIF5 interact directly with the promoters of anthocyanin synthase genes, then positively regulating (PIF3) or negatively regulating (PIF4, PIF5) anthocyanin synthesis36,39. In C. roseus, CrPIF1 directly activates T16H2 and DAT, positively regulating the biosynthesis of vindoline3. These researchers show that PIFs control metabolite synthesis by directly activating enzyme genes involved in the synthesis process. Moreover, in apple, MdPIF7 also be found to weaken the transcriptional activation of MY5 (an anthocyanin regulatory factor) by MdBBX23, thus inhibiting anthocyanin biosynthesis19. This finding indicates that PIFs can also indirectly control metabolite synthesis through controlling other TFs. In U. rhynchophylla, UrPIF3 cannot directly activate UrSTR, but silencing UrPIF3 decreases UrSTR expression. This suggests that UrPIF3’s effect on UrSTR expression may involve other unknown transcription factors.
IRN is a secondary metabolite specific to most plants of the Uncaria, classified under monoterpene indole alkaloids with various pharmacological effects. Its role in combating Alzheimer’s disease has garnered significant attention40. Our previous research has shown that low light promotes the accumulation of IRN in U. rhynchophylla18, suggesting that IRN biosynthesis is regulated by light signals. Since light is crucial for plant growth, understanding the role of PIF in regulating IRN biosynthesis is of considerable importance. To date, PIFs have been found to mediate light-regulated anthocyanin biosynthesis in A. thaliana and M. domestica, as well as the biosynthesis of vindoline in C. roseus3,19,36. For instance, CrPIF1 is a negative transcriptional regulator of vindoline, repressing the expression levels of two enzyme genes involved in vindoline biosynthesis. Under dark conditions, CrPIF1expression levels increase, negatively regulating vindoline biosynthesis by controlling these enzyme genes3. Conversely, UrPIF3 acts as a positive regulator of IRN synthesis, directly activating UrSGD and indirectly regulating UrSTR, as demonstrated in this paper. Low light not only promotes IRN accumulation18 but also upregulates the expression levels of UrPIF3, UrSGD, and UrSTR in this paper. Therefore, we propose a potential molecular model where UrPIF3 mediates the low light signal to regulate IRN biosynthesis. In a low-light environment, the expression level of UrPIF3 increases, which then directly activates UrSGD and indirectly activates UrSTR, leading to the accumulation of RIN and IRN (Fig. 6).
Conclusion
The UrPIF3 protein is a typical member of the PIF family, characterized by having a bHLH domain and an APB domain, and it is located in the nucleus. Also, UrPIF3 protein possesses an APA domain highly similar to that of AtPIF3, which may enable it to bind with phytochrome A. Moreover, UrPIF3 acts as a positive regulator of IRN biosynthesis, directly activating UrSGD and indirectly activating UrSTR to control IRN production. Under low light condition, UrPIF3 expression increases, allowing UrPIF3 to regulate the two key enzyme genes, UrSGD and UrSTR, thereby enhancing the production of IRN/RIN. Overall, our research reveals that PIF3, which plays a crucial role in the light signal transduction pathway, also has an important function in regulating IRN biosynthesis in U. rhynchophylla.
Methods
Plant growth and treatment conditions
The plants used in this study include Uncaria rhynchophylla、tobacco (♂ Yunyan 2 × ♀ K326). The use of plants in this study complies with international, national and/or institutional guidelines. All seeds used in this study were purchased from Guizhou Peili Nongbenfang Chinese Medicine Co., Ltd. The seeds germinated on nutrient soil at 25 ± 3℃ 16 h light/ 8 h dark photoperiod. After 5 weeks, tobacco seedlings with four young leaves were for dual‑luciferase assay, and U.rhynchophylla seedlings with four young leaves were for Virus-Induced Gene Silencing and the shading experiment. The light intensities of control group and treatment group were about 400 and 100 µmol m−2s−1 in experiment of quantitative reverse transcription PCR.
Quantitative reverse transcription PCR analysis (qRT-PCR)
Plant materials from the treatment and control groups were collected in liquid nitrogen, then extract total RNA by using RNA isolation kit (Omega Bio-Tek, Beijing, China). The qualified RNA was used to synthesize the first stand of cDNA (GenStar, Beijing, China). GAPDH and α-tub as internal control. PCR amplification was used Bio-Rad CFX system (Bio-Rad, USA). The gene relative expression was calculated by using the 2 − ΔΔ CT method41. All primers used for qRT-PCR are shown in Table S2.
UrPIF3 isolation and multiple sequence alignment of UrPIF3 protein
The full cDNA of UrPIF3 was cloned from previous transcriptome data (Access number: PRJNA691069) by PCR, and full-length cDNA was uploaded to the NCBI database (GeneBank: OM982855). Multiple sequence alignment of PIFs used DNAMAN, and the sequence of PIFs in A. thaliana was obtained from TAIR (Table S1).
Subcellular localization of UrPIF3
The full cDNA of UrPIF3 was inserted into the pCAMBIA1300-GFP by using Kpn I and Xba I, and then the recombinant plasmid pCAMBIA1300-UrPIF3-GFP was transformed into Agrobacterium tumefaciens GV3101 as test strain. Nuclei were specifically stained using 4’,6-diamidino-2-phenylindole (DAPI). The GV3101 strain with recombinant plasmid was used to infect tobacco leaves, after 72 h, GFP fluorescence signals were detected by laser scanning confocal microscope (FV10-ASWOLYMPUS, Japan). The primers used for Subcellular localization are shown in Table S2.
Promoter isolation and analysis
The promoter of UrSTR1 and UrSGD1was isolated from genomic by PCR (BioProject number: PRJCA026507), and the analysis of promoter sequences using PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Yeast‑one hybrid (Y1H) and dual‑luciferase assay
The promoter of UrSTR1and UrSGD1 were inserted into vector pHIS2 by using EcoR I and Mlu I respectively, and the full cDNA of UrPIF3 was inserted into vector pGADT7 by using EcoR I and BamH I. The recombinant plasmid pro-UrSTR/UrSGD-pHIS2 and pGADT7-UrPIF3were co-transformed into Y187 as test strain. The negative controls and positive controls refer to previous reports42.
The promoter of UrSTR1and UrSGD1 were inserted into vector pGreen II-0800-LUC by using Kpn I and BamH I respectively, and the full cDNA of UrPIF3 was inserted into vector pHB by using Sac I and BamH I. The recombinant plasmid pGreen II-0800-proUrSTR1/UrSGD-LUC as a reporter, and the recombinant plasmid pHB-UrPIF3as an effector. The reporter plasmid was transformed into GV3101 pSoup, and the effector plasmid was transformed into GV3101. Mix GV3101 pSoup and GV3101 in a 1:1 ratio, then the mixture was used to infect tobacco leaves. After 72 h, the LUC activity of leaves was measured by the LUC detection kit (Promega, Madison, WI, USA)43. The primers used for Y1H and dual‑luciferase assay are shown in Table S2.
Virus-induced gene silencing
The target sequence (about 400 bp) of gene silencing was amplified using specific primers (Table S2), then the target sequence was fused into the vector TRV2 to form recombinant plasmids. The recombinant plasmid and TRV1 plasmid were transformed into Agrobacterium tumefaciens EHA105 by the heat shock method. TRV1-EHA105 and TRV2-EHA105 were mixed in equal proportion, and then the mixture was used to infect U. rhynchophyllaseedlings. These seedlings were cultured in the plant growth chamber for two days at 25 ± 2 °C in the dark, and then they were grown following a normal photoperiod (16 h light/8 h dark). After successfully silencing the gene in these seedlings, the contents of RIN/IRN and the gene expression level were detected29.
IRN/RIN measurement
The quantitative analysis of IRN/RIN was measured by the HPLC system (Waters Alliance 2695). Chromatographic separation was used Agilent ZORBAX SB-C18 column, the mobile phase was methanol and 0.1% ammonia (70:30, V/V), and the detective wavelength was 245 nm. The detailed steps reference to previous research27.
Statistical analysis
Statistical analysis of data was calculated by SPSS (22.0). The significance of difference was analyzed by Kendall’s test, and the correlation analysis of expression patterns was analyzed by Spearman’s correlation coefficient.
Data availability
Sequence data that support the findings of this study have been deposited in the NCBI with the primary accession code OM982855.1. All the data produced or examined during this study are presented within this article. The full datasets generated or analysed during the current study are available in the NCBI repository, the accession numbers: OM982855.1 (https://www.ncbi.nlm.nih.gov/nuccore/OM982855.1/).
References
Sun, C. et al. A Transcriptional Network promotes anthocyanin biosynthesis in Tomato Flesh. Mol. Plant. 13, 42–58 (2020).
Zhang, D. et al. Red and Blue Light promote the Accumulation of Artemisinin in Artemisia Annua L. Molecules. 23, 1329 (2018).
Liu, Y., Patra, B., Pattanaik, S., Wang, Y. & Yuan, L. GATA and phytochrome interacting factor transcription factors regulate light-Induced Vindoline Biosynthesis in Catharanthus roseus. Plant. Physiol. 180, 1336–1350 (2019).
Zhang, X. et al. A PIF1/PIF3-HY5-BBX23 transcription factor Cascade affects photomorphogenesis. Plant. Physiol. 174, 2487–2500 (2017).
Sharma, A. et al. Functions of phytochrome-interacting factors (PIFs) in the regulation of plant growth and development: a comprehensive review. Int. J. Biol. Macromol. 244, 125234 (2023).
Pham, V. N., Kathare, P. K. & Huq, E. Phytochromes and phytochrome interacting factors. Plant. Physiol. 176, 1025–1038 (2018).
Lee, N. & Choi, G. Phytochrome-interacting factor from Arabidopsis to liverwort. Curr. Opin. Plant. Biol. 35, 54–60 (2017).
Park, E., Kim, Y., Choi, G., Phytochrome, B. & Requires, P. I. F. Degradation and sequestration to induce light responses across a wide range of light conditions. Plant. Cell. 30, 1277–1292 (2018).
Gao, Y. et al. A maize phytochrome-interacting factors protein ZmPIF1 enhances drought tolerance by inducing stomatal closure and improves grain yield in Oryza sativa. Plant. Biotechnol. J. 16, 1375–1387 (2018).
Kumar, I., Swaminathan, K., Hudson, K. & Hudson, M. E. Evolutionary divergence of phytochrome protein function in Zea mays PIF3 signaling. J. Exp. Bot. 67, 4231–4240 (2016).
Xu, T. & Hiltbrunner, A. PHYTOCHROME INTERACTING FACTORs from Physcomitrella patens are active in Arabidopsis and complement the pif quadruple mutant. Plant. Signal. Behav. 12, e1388975 (2017).
Inoue, K., Nishihama, R. & Kohchi, T. Phytochrome and Light Signaling in Marchantia. Methods Mol. Biol. 2026, 215–223 (2019).
Zhang, Q. Z. W. N. et al. Overexpression of AaPIF3 promotes artemisinin production in Artemisia annua. Ind. Crops Prod. 138, 11476 (2019).
Li, H. Q. et al. Isorhynchophylline ameliorates cognitive impairment via modulating amyloid pathology, tau hyperphosphorylation and neuroinflammation: studies in a transgenic mouse model of Alzheimer’s disease. Brain Behav. Immun. 82, 264–278 (2019).
Liang, J. H. et al. The genus Uncaria: a review on phytochemical metabolites and biological aspects. Fitoterapia. 147, 104772 (2020).
Qin, N. et al. Recent research progress of Uncaria spp. based on alkaloids: phytochemistry, pharmacology and structural chemistry. Eur. J. Med. Chem. 210, 112960–112973 (2021).
Egan, M. F. et al. Randomized Trial of Verubecestat for mild-to-moderate Alzheimer’s Disease. N Engl. J. Med. 378, 1691–1703 (2018).
Wang, X. H. L. X., Qiang, W., Yu, X. S., Zheng, H. J. & Zhang, M. S. Comparative transcriptome analysis revealed the molecular mechanism of the effect of light intensity on the accumulation of rhynchophylline and isorhynchophylline in Uncaria rhynchophylla. Physiol. Mol. Biology Plants. 28, 315–331 (2022).
Liu, Y. et al. Phytochrome interacting factor MdPIF7 modulates anthocyanin biosynthesis and hypocotyl growth in apple. Plant. Physiol. 188, 2342–2363 (2022).
Lorenzo, C. D. et al. Shade delays flowering in Medicago sativa. Plant. J. 99, 7–22 (2019).
Pashkovskiy, P. et al. Influence of light of different spectral compositions on the growth, photosynthesis, and expression of light-dependent genes of scots Pine Seedlings. Cells. 10, 3284 (2021).
Oh, J., Park, E., Song, K., Bae, G. & Choi, G. PHYTOCHROME INTERACTING FACTOR8 inhibits phytochrome A-Mediated far-red light responses in Arabidopsis. Plant. Cell. 32, 186–205 (2020).
Li, Y. et al. Arabidopsis EXECUTER1 interacts with WRKY transcription factors to mediate plastid-to-nucleus singlet oxygen signaling. Plant. Cell. 35, 827–851 (2023).
Chazotte, B. Labeling nuclear DNA using DAPI. Cold Spring Harb Protoc, 2011:pdb prot5556. (2011).
Shi, Q. et al. Modulation of starch synthesis in Arabidopsis via phytochrome B-mediated light signal transduction. J. Integr. Plant. Biol. 66, 973–985 (2024).
Zhang, Y. et al. A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet. 9, e1003244 (2013).
Li, X. W. X., Qiang, W., Zheng, H. J., ShangGuan, L. Y. & Zhang, M. S. Transcriptome revealing the dual regulatory mechanism of ethylene on the rhynchophylline and isorhynchophylline in Uncaria rhynchophylla. J. Plant. Res. 135, 485–500 (2022).
Guo, E. et al. Identification of three key enzymes involved in the biosynthesis of tetracyclic oxindole alkaloids in Uncaria rhynchophylla. Bioorg. Chem. 136, 106545 (2023).
Pan, Q. W. C. et al. CrERF5, an AP2/ERF transcription factor, positively regulates the biosynthesis of Bisindole alkaloids and their precursors in Catharanthus roseus. Front. Plant. Sci. 10, 931–944 (2019).
Qi, X. et al. Establishment of virus-induced gene silencing (VIGS) system in Luffa acutangula using Phytoene desaturase (PDS) and tendril synthesis related gene (TEN). Plant. Methods. 19, 94 (2023).
Ni, M., Tepperman, J. M. & Quail, P. H. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell. 95, 657–667 (1998).
Leivar, P. & Monte, E. PIFs: systems integrators in plant development. Plant. Cell. 26, 56–78 (2014).
Liu, L. Y., Jia, M. Z., Wang, S. N., Han, S. & Jiang, J. Identification and characterization of cotton PHYTOCHROME-INTERACTING FACTORS in temperature-dependent flowering. J. Exp. Bot. 74, 3765–3780 (2023).
Kim, J. et al. PIF1-Interacting transcription factors and their binding sequence elements determine the in vivo Targeting sites of PIF1. Plant. Cell. 28, 1388–1405 (2016).
Martinez-Garcia, J. F., Huq, E. & Quail, P. H. Direct targeting of light signals to a promoter element-bound transcription factor. Science. 288, 859–863 (2000).
Liu, Z. et al. Phytochrome-interacting factors PIF4 and PIF5 negatively regulate anthocyanin biosynthesis under red light in Arabidopsis seedlings. Plant. Sci. 238, 64–72 (2015).
Hao, Y., Zong, X., Ren, P., Qian, Y. & Fu, A. Basic Helix-Loop-Helix (bHLH) transcription factors regulate a wide range of functions in Arabidopsis. Int. J. Mol. Sci. 22, 7125 (2021).
Yang, D. et al. Phytochrome interacting factor 3 regulates pollen mitotic division through auxin signalling and sugar metabolism pathways in tomato. New. Phytol. 234, 560–577 (2022).
Shin, J., Park, E. & Choi, G. PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant. J. 49, 981–994 (2007).
Yang, W., Ip, S. P., Liu, L., Xian, Y. F. & Lin, Z. X. Uncaria rhynchophylla and its major constituents on Central Nervous System: a review on their pharmacological actions. Curr. Vasc Pharmacol. 18, 346–357 (2020).
Li, X., Zhang, M. S., Zhao, L. Q., Ling-Hu, Q. Q. & Xu, G. The study on interacting factors and functions of GASA6 in Jatropha curcas L. BMC Plant. Biol. 23, 99 (2023).
Shi, S., Liu, Y., He, Y., Li, L., Li, D. & Chen, H. R2R3-MYB transcription factor SmMYB75 promotes anthocyanin biosynthesis in eggplant (Solanum melongena L.). Scientia Horticulturae. 282, 110020 (2021).
Liu, G. et al. Alterations of mitochondrial protein assembly and jasmonic acid biosynthesis pathway in Honglian (HL)-type cytoplasmic male sterility rice. J. Biol. Chem. 287, 40051–40060 (2012).
Acknowledgements
This work was supported by the Scientific Research Project of Ordinary Undergraduate Colleges and the Universities of Guizhou Provincial Department of Education (Qian Jiao Ji [2022]No. 145), the Guizhou Provincial Science and Technology Projects (Qian Ke He Ji Chu-ZK[2022] General 502), the Guizhou Minzu University (Youth) Fund Research Project (GZMUZK[2023]QN06), the Guizhou Minzu University Doctoral Research Initiation Project (GZMUZK[2024]QD51), the Guizhou Provincial Science and Technology Projects (Qian Ke He Ji Chu-ZK[2022] General096), and the National Natural Science Foundation of China (No. 32460146).
Author information
Authors and Affiliations
Contributions
Designed and conceived the experiments, Ms.Z. and Xh.W.; Conducted experimentation, Xh.W. and X.L.; Analyzed the data, Xh.W. and X.L.; Wrote the paper, X.L.; Revised the paper, Hq.H. and Yl.W.; Retrieve the data, T.H. and W.Q. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Han, Hq., Wei, Yl. et al. Phytochrome interacting factor 3 mediates low light signaling to regulate isorhynchophylline biosynthesis in Uncaria rhynchophylla. Sci Rep 14, 25032 (2024). https://doi.org/10.1038/s41598-024-76939-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76939-0