Abstract
Morphology of right ventricular outflow tract (RVOT) is potentially related to cardiovascular outcomes. However, this relationship still remains to be verified with direct evidence. We retrospectively reviewed cases from the autopsy specimen library in the Center of Forensic Medicine in Sun Yat-sen University from 2017 to 2023. Six RVOT morphological characteristics were measured and their association with cardiovascular diseases (CVDs), sudden cardiac death (SCD) and age at death was evaluated. Relationship between myocardial fibrosis in RVOT and CVDs was investigated. RVOT characteristics were recruited by machine learning algorithms for diagnosing CVDs. A total of 2370 cases were finally recruited. Perimeter of sub-valve plane (pSBV) in RVOT was positively associated with risk of CVDs and SCD (OR: 1.21, 95%CI: 1.07–1.37, p = 0.003; OR: 1.33, 95%CI: 1.16–1.52, p < 0.001). Compared with thickness of septum (tS) < 3.0 mm, tS ≥ 3.0 mm was associated with premature death in disease-dominant death (β=-0.16, 95%CI: -0.20 to -0.11, p < 0.001) and SCD (β=-0.15, 95%CI: -0.21 to -0.10, p < 0.001). Degree of myocardial fibrosis in the posterior septum was increased in coronary atherosclerosis (6.86%±2.48% vs. 4.91%±2.14%, p = 0.011) and cardiomyopathies (8.11%±3.24% vs. 4.88%±3.11%, p = 0.005). A logistic regression model, recruiting age, left and right ventricular wall thickness, pSBV, circumference of pulmonary annulus and aortic annulus, was elected as an optimal diagnostic model of CVDs, yielding AUC of 0.734 (95%CI: 0.705–0.763), 0.781 (0.740–0.821) and 0.763 (0.725-0.800) in training, validation and test sets. Increased pSBV significantly correlates with higher risk of CVDs and SCD. And tS ≥ 3.0 mm is an independent risk factor of premature death regardless of diseases.
Similar content being viewed by others
Introduction
Right ventricular outflow tract (RVOT), also called infundibulum or conus arteriosus, begins at the distal continuation of the supraventricular crest and extends superiorly and posteriorly toward the pulmonary valve1,2. Based on clinical and electrophysiological studies, RVOT has been recognized as a common origin of ventricular arrhythmias, such as idiopathic ventricular arrhythmia, arrhythmogenic right ventricular cardiomyopathy (ARVC) and Brugada syndrome (Brs)3,4,5,6. Recent clinical studies reported several morphological and functional changes of RVOT in Brs and ARVC patients, including the RVOT volume increase, wall motion abnormalities and impaired endocardial radial strain7,8. A recent case report showed that a patient with thinning free wall and advanced fibrofatty degeneration of RVOT presented with overlapping disease states of Brs and ARVC9. These observations suggested that the primary or secondary changes of RVOT morphology probably have pathophysiological significance.
There were several studies about measuring morphological indexes of RVOT by imaging methods, including echocardiography, CT and cardiac magnetic resonance feature tracking8,10,11. However, comprehensive and direct observations demonstrating the association of RVOT morphological phenotype with cardiovascular diseases (CVDs) and sudden death remain lacked.
To shed light on these issues, we conducted a two-stage autopsy-based retrospective study to systematically examine the morphological characteristics of RVOT and to determine whether the morphological phenotype of RVOT is associated with risk of CVDs and individuals’ survival.
Results
The study design was displayed in Fig. 1.
Flowchart of the study design and cases selection. 2370 cases were reviewed and analyzed. CAS = coronary atherosclerosis; CM = cardiomyopathies; CVD = cardiovascular disease; SCD = sudden cardiac death; pSBV = perimeter of sub-valve; pSPV = perimeter of supra-valve; pMV = perimeter of mid-valve; iSCPV = interval between supraventricular crest-pulmonary valve; tF = thickness of free wall; tS = thickness of septum.
Baseline characteristics
A total of 2,370 cases were reviewed (1,759 men, mean age at death 46.4 ± 15.1y), 1,746 of which (73.7%) were classified as diseases-dominant death and 613 (25.9%) were violence-dominant death (Table 1). Six morphological characteristics of RVOT in the stage-one study were listed in Supplemental Table 1.
Morphological characteristics of RVOT were associated with cardiovascular diseases and sudden cardiac death
Association between RVOT morphology and cardiovascular diseases
Among the 1,305 cases in the stage-one study, 755 cases were diagnosed with CVDs. Logistic regression analysis was performed to analyze the association of CVDs with six morphological characteristics. We found that perimeter of sub-valve (pSBV) was positively related to CVDs risk (OR:1.89, 95%CI:1.65–2.17, p < 0.001) (Table 2). A multivariate model was created by including covariates of age at death, sex, heart weight and abdominal subcutaneous fat thickness. Significant association remained between pSBV and CVDs risk (OR:1.22, 95%CI:1.03–1.44, p = 0.022) (Table 2). No significant differences were found between the other morphological characteristics and CVDs risk in the multivariate models (Supplemental Table 2). In the stage-two study, there were 2370 cases and 1446 with CVDs. The positive association between pSBV and risk of CVDs was validated (OR:1.21, 95%CI:1.07–1.37, p = 0.003) (Table 2). Further, when converting pSBV to a dichotomous variable based on the median value (7.7 cm) in the 2370 cases, cases with pSBV ≥ 7.7 cm had a higher risk of CVDs than those with pSBV < 7.7 cm (OR:1.26, 95%CI:1.03–1.55, p = 0.024) (Supplemental Table 3).
Association between RVOT morphology and coronary atherosclerosis
It is unknown whether there is a potential link between RVOT morphology and coronary atherosclerosis (CAS). To shed light on this issue, the association between pSBV and CAS risk was analyzed in 829 CAS cases with no other CVDs and 924 control cases in stage two. By adjusting for age at death, sex, heart weight and abdominal subcutaneous fat thickness, it was found that the risk of CAS in pSBV ≥ 7.6 cm (median) was higher than that in pSBV < 7.6 cm (OR:1.33, 95%CI:1.04–1.68, p = 0.021) (Supplemental Table 4).
Association between RVOT morphology and cardiomyopathies
Besides CAS, the association between pSBV and cardiomyopathies (CM) risk was also analyzed in 76 CM cases with no other CVDs and 924 control cases in stage two. By adjusting for age at death, sex, heart weight and abdominal subcutaneous fat thickness, it was found that the risk of CM in pSBV ≥ 7.5 cm (median) was higher than that in pSBV < 7.5 cm (OR:2.66, 95%CI:1.32–5.36, p = 0.006) (Supplemental Table 5).
Association between RVOT morphology and sudden cardiac death
In the stage-one study, there were 924 cases of disease-dominant death and 525 of sudden cardiac death (SCD). We found that pSBV was positively related to SCD risk (OR:1.56, 95%CI:1.34–1.83, p < 0.001) (Table 3). By adjusting for age at death, sex, heart weight, CAS and CM, there was a borderline significance of the association between pSBV and SCD risk (OR:1.20, 95%CI:1.00-1.44, p = 0.055). (Table 3). In the stage-two study, there were 1,746 cases of disease-dominant death and 946 of SCD. For validation, it was found that pSBV was strongly associated with risk of SCD in the multivariate model (OR:1.33, 95%CI:1.16–1.52, p < 0.001) (Table 3). In addition, the risk of SCD in pSBV ≥ 7.8 cm (median) was higher than that in pSBV < 7.8 cm (OR:1.45, 95%CI:1.17–1.80, p = 0.001) (Supplemental Table 6). Besides, in the stage-one study we found that the risk of SCD in cases with thickness of septum (tS) ≥ 3.0 mm (Q3) was higher than those with tS < 3.0 mm (OR:1.91, 95%CI:1.45–2.52, p < 0.001) (Supplemental Table 7). By adjusting for age at death, sex, heart weight, CAS and CM, significant association remained in the multivariate model (OR:1.45, 95%CI:1.07–1.97, p = 0.016) (Supplemental Table 7). In the stage-two study, the risk of SCD in cases with tS ≥ 3.0 mm was still higher than those with tS < 3.0 mm in the multivariate model (OR:1.27, 95%CI:1.02–1.57, p = 0.033) (Supplemental Table 7). Perimeter of mid-valve (pMV) was positively related to the risk of SCD in stage one (OR:1.28, 95%CI:1.03–1.59, p = 0.028), while no significant association was found between other characteristics, e.g., perimeter of supra-valve (pSPV), interval between supraventricular crest and pulmonary valves (iSCPV), thickness of free wall (tF), and SCD risk (Supplemental Table 8).
Increased tS was associated with younger age at death
Besides, we explored the association of RVOT morphological phenotype with individuals’ survival, and tS was found to be associated with age at death in disease-dominant death and SCD groups.
The disease-dominant death group (n = 1,746, 1,282 men, mean age at death 47.4 ± 13.9y) was divided by quartiles of tS (2.0 mm, 3.0 mm), and the mean age at death in cases with tS ≥ 3.0 mm was found to be significantly younger than that in the 2.0 mm ≤ tS < 3.0 mm group (45.4 ± 14.1y vs. 48.6 ± 14.3y, p < 0.001). The mean age at death did not differ between tS < 2.0 mm and 2.0 mm ≤ tS < 3.0 mm (47.4 ± 15.3y vs. 48.6 ± 14.3y, p = 0.591) (Supplemental Table 9). To observe whether the tS affected individuals’ survival, linear regression analysis was performed to further analyze the association of age at death with tS. After adjusting for sex, heart weight, abdominal subcutaneous fat thickness and CVDs, we found that the age at death in cases with tS ≥ 3.0 mm was 4.6y younger than those with tS < 3.0 mm (β=-0.16, 95%CI: -0.20 to -0.11, p < 0.001) (Table 4).
In the SCD group (n = 946, 775 men, mean age at death 47.8 ± 14.3y), the mean age at death in cases with tS ≥ 3.0 mm was still significantly younger than those with 2.0 mm ≤ tS < 3.0 mm (45.1 ± 13.6y vs. 50.1 ± 14.4y, p < 0.001) (Supplemental Table 9). After adjusting for sex, heart weight, abdominal subcutaneous fat thickness and CAS, compared with tS < 3.0 mm, the age at death in cases with tS ≥ 3.0 mm was 4.4y younger in SCD group (β=-0.15, 95%CI: -0.21 to -0.10, p < 0.001) (Supplemental Table 10).
Myocardial fibrosis of RVOT was associated with coronary atherosclerosis and cardiomyopathies
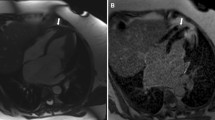
We speculate fibrosis is one of the histological changes corresponding to the gross morphological characteristics of RVOT. So, we further analyzed myocardial fibrosis in RVOT in different CVDs cases. Fifteen pairs of CAS and non-CAS cases (11 pairs of men, 4 pairs of women) with matched age at death, sex and heart weight were selected for this analysis. The heart sections were stained with Masson’s Trichrome, and the results were shown in Fig. 2A-C. The total percentage area of fibrosis in RVOT posterior septum was significantly higher in the CAS group than in the non-CAS group (% Fibrosis: CAS, 6.86%±2.48%; non-CAS, 4.91%±2.14%; p = 0.011, n = 15–15). Other sections of the heart did not show significant differences for the total percentage area of fibrosis between CAS and non-CAS groups (% Fibrosis: anterior septum, 5.66%±3.73% vs. 5.19%±2.64%, p = 0.727; middle septum, 6.42%±2.09% vs. 5.89%±2.97%, p = 0.359; anterior free wall, 3.53%±1.48% vs. 3.84%±2.03%, p = 0.594; anterior wall of left ventricle, 2.12%±0.81% vs. 1.66%±0.70%, p = 0.057; n = 15–15).
Comparison of myocardial fibrosis. Trichrome staining highlights collagen content (blue staining) in the posterior septum myocardium of RVOT in two groups. (A, B) Representative images of the posterior septum sections from CAS and non-CAS subjects, respectively. (C) Quantitative analysis of myocardial collagen volume fraction. N = 15 (CAS) and 15 (non-CAS). (D, E) Representative images of the posterior septum sections from CM and non-CM subjects, respectively. (F) Quantitative analysis of myocardial collagen volume fraction. N = 11 (CM) and 11 (non-CM). Scale bar: 50 μm. CAS = coronary atherosclerosis; CM = cardiomyopathies.
Eleven pairs of CM and non-CM cases (6 pairs of men, 5 pairs of women) with matched age at death, sex and heart weight were selected for analysis of myocardial fibrosis in RVOT. The total percentage area of fibrosis in RVOT posterior septum was also significantly higher in the CM group than in the non-CM group (% Fibrosis: CM, 8.11%±3.24%; non-CM, 4.88%±3.11%; p = 0.005, n = 11–11) (Fig. 2D-F). While other sections did not show significant differences for the total percentage area of fibrosis between CM and non-CM groups (% Fibrosis: anterior septum, 7.82%±7.66% vs. 5.70%±4.42%, p = 0.464; middle septum, 9.10%±4.25% vs. 7.59%±5.09%, p = 0.316; anterior free wall, 3.95%±1.27% vs. 2.84%±1.91%, p = 0.143; anterior wall of left ventricle, 2.47%±1.44% vs. 2.65%±2.47%, p = 0.838; n = 11–11).
Machine learning models for diagnosing cardiovascular diseases
Dataset preparation
To investigate the value of the morphological characteristics of RVOT for diagnosing CVDs, 1,716 cases collected in 2017–2021 were selected as internal dataset and 654 cases collected in 2022–2023 were used as a test set for constructing diagnostic models. In the two sets of cases, the CVDs prevalence was 60.1% and 63.5%, respectively. The internal dataset was further divided to a training set (1,204 cases) and a validation set (512 cases). The missingness rates were 2.1%, 2.0% and 0.3% in the training, validation, and test sets, respectively (Supplemental Fig. 1). The candidate predictors in the imputed training, validation, and test sets were described in Supplemental Table 11.
Feature selection
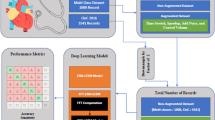
A total of 12 variables, including 4 general variables and 6 anatomical variables of heart, and 2 morphologic variables of RVOT (Supplemental Table 11), were selected as the candidate variables for the diagnosis of CVDs and included in the least absolute shrinkage and selection operator (LASSO) regression for feature selection (Fig. 3A-B). Six variables were finally selected, including age, left and right ventricular wall thickness, circumference of pulmonary annulus, circumference of aortic annulus and pSBV.
Development and evaluation of machine learning models. (A) Plot of mean squared error versus log (λ). Dotted vertical lines were drawn at the optimal values by utilizing the minimum criteria (left line) and the 1 standard error criterion (right line, selected as optimal the penalty parameter in the analysis). (B) LASSO coefficient profiles. Curves with different color represents the coefficient profile of one specific feature against the log (λ) sequence. (C-E) Receiver operating characteristic curves of machine learning models in the training, validation, and test set, respectively. (F-H) Calibration curve of logistic regression model in the training, validation, and test set, respectively. (I-K) Decision curve of logistic regression model in the training, validation, and test set, respectively. (L) Nomogram based on the logistic regression model. LVWT = left ventricular wall thickness; RVWT = right ventricular wall thickness; cPA = circumference of pulmonary annulus; cAA = circumference of aortic annulus; pSBV = perimeter of sub-valve; CAS = coronary atherosclerosis; CVD = cardiovascular disease.
Model development and evaluation
Eight machine learning algorithms were applied to construct the diagnostic model. The AUC of these models ranged from 0.561 to 0.734, 0.570 to 0.786, and 0.592 to 0.763 in the training, validation, and test sets, respectively (Fig. 3C-E). In the training set, all models except for k-nearest neighbor achieved an AUC above 0.7 (Fig. 3C). In the validation set, logistic regression (LR), Gaussian Naïve Bayes, adaptive boosting trees, extreme gradient boosting trees, support vector machine, single-hidden-layer neural network performed well with an AUC higher than 0.75 (Fig. 3D). In the test set, well performance (AUC > 0.75) remained in 5 models, including LR, adaptive boosting trees, extreme gradient boosting trees, support vector machine, single-hidden-layer neural network (Fig. 3E). Other statistical indicators in three datasets were shown in Supplemental Table 12. Considering the diagnostic performance, robustness, simplicity and interpretability, the LR model was found to be an optimal diagnostic model, yielding AUC of 0.734 (95%CI, 0.705–0.763), 0.781 (0.740–0.821) and 0.763 (0.725-0.800) in the three sets, respectively (Fig. 3C-E). When the cutoff probability was set at 0.568, the accuracy was 0.681 (0.654–0.707), 0.715 (0.674–0.754) and 0.711 (0.675–0.745) in training, validation and test sets, respectively. The calibration curves subsequently demonstrated satisfactory calibration capacity of the LR model in the three sets (Fig. 3F-H). The calibration slope was 1.00 (0.85–1.15), 1.23 (0.98–1.49) and 1.24 (1.00 to 1.49) in training, validation, and test set, respectively, while the calibration-in-the-large was 0.00 (-0.13-1.13), 0.18 (-0.02-0.37) and 0.16 (-0.01-0.33) in the three datasets. Decision curve analyses also indicated certain application value of the LR model (Fig. 3I-K). Furthermore, a nomogram based on the coefficient in LR was developed to visualize the diagnostic model (Fig. 3L). A patient was given a point for each included variable and the sum of points indicated the probability of suffering from CVDs. The higher the total points, the more likely the individual had CVDs.
Then we explored the value of the established nomogram in diagnosing CAS based on the same coefficients, the AUCs of nomogram for CAS were 0.727 (95%CI, 0.699–0.755), 0.768 (0.727–0.809) and 0.733 (0.695–0.771) in training, validation and test sets, respectively (Supplemental Fig. 2A-C). The calibration was acceptable after adjusting the intercept of LR model in the training set (Supplemental Fig. 2D-F). Decision curve analyses also revealed satisfactory application value of the nomogram in diagnosing CAS (Supplemental Fig. 2G-I).
Discussion
In this study, we dissected human hearts to access morphological characteristics of RVOT and investigated the association between morphological phenotype of RVOT and CVDs, SCD, and individuals’ survival. The results showed that pSBV and tS were significantly associated with the risk of CVDs and SCD. Of crucial importance, we found that larger tS was significantly associated with premature death in disease-dominant death and SCD cases. Moreover, our study showed that increased myocardial fibrosis in RVOT posterior septum was associated with CAS and CM.
There is a paucity of study on the relevance of morphologic changes of RVOT to cardiovascular outcomes. Previous studies mostly focused on electrophysiological and molecular phenotypes of RVOT in idiopathic ventricular arrhythmia, ARVC and Brs patients12,13,14,15. As for morphologic data, the data were collected by imaging techniques such as echocardiography, cardiac magnetic resonance and CT, which measured RVOT diameters, radial and longitudinal strain and strain-rate parameters8,11,16. Although these imaging modalities can describe RVOT efficiently from a multi-perspective view, there is still a lack of unified diagnostic criteria. Compared to radiological imaging, dissected hearts could provide us a more intuitive approach to examine morphologic characteristics of RVOT. In our previous study17, we found that the average circumferences of cardiac valves, including pulmonary valve, tended to be increased in sudden unexpected death syndrome compared with control. To further explore morphology of RVOT based on dissected hearts from autopsies, this study described the perimeters of three planes of pulmonary valve, interval between supraventricular crest and pulmonary valve, myocardial thickness of septum and free wall.
Previous studies showed that heart weight was an independent risk factor for SCD18,19, which is consistent with our results on CVDs and SCD. After adjusting for heart weight, our results demonstrated that morphological characteristics of RVOT were still independent risk factors for CVDs and SCD.
First, we found that larger pSBV was associated with a higher risk of CVDs and SCD. This result was consistent with the data on ARVC phenotype based on the 2010 revised Task Force Criteria, in which RVOT dilation was defined by transthoracic echocardiography from parasternal long-axis view and parasternal short-axis view16,20. Additionally, using CMR imaging, both Brs and ARVC patients exhibited larger RVOT volumes compared with controls7. Larger pSBV implies RVOT enlargement, which could lead to impaired systolic function, right ventricular hemodynamic changes, and progression to CVDs and death. In contrast to pSBV, the perimeter of supra-valve plane was not associated with CVDs and SCD, which could be explained by anatomical complexity of RVOT-pulmonary artery transition zone. Previous study showed that the variation over the cardiac cycle of the RVOT/pulmonary artery section shapes was significantly higher in sub-valve than supra-valve21. Thus, the morphology of sub-valve plane tends to be influenced because of higher compliance. Further studies looking into the correlation between autopsy findings and imaging findings are needed, so as to find measurable abnormalities in patients before an event or death occurs. For clinical validation and application, we’ve developed an imaging method to examine pSBV in a living person. Since there is a linear relationship between perimeter and diameter, by cardiac ultrasound end-diastolic RVOT diameter was measured at the plane below pulmonary valves from parasternal short axis section (Supplemental Fig. 3).
We also found that larger tS was associated with a higher risk of SCD. Recently studies showed that the morphology and function of right ventricle were closely related to cardiovascular death22. In Gray’s study7, both Brs and ARVC patients showed more RVOT wall motion abnormalities than controls. And it was found that in patients with structural heart disease focal ventricular tachycardia commonly originated from the right ventricle, especially RVOT septum23. Combined with our finding, RVOT septal enlargement in CVDs could be associated with occurrence of ventricular arrhythmias originated from RVOT, which may lead to sudden death.
Besides, larger tS was associated with premature death. In disease-dominant death and SCD cases, we estimated that the age at death in cases with tS ≥ 3.0 mm was 4.6y and 4.4y earlier than those with tS < 3.0 mm, respectively. In a study on hypertrophic cardiomyopathy, it was found that patients with abnormal right ventricle (higher right ventricle systolic pressure, larger right ventricle, etc.) had higher all-cause mortality compared with those with normal size and function24. Combined with our results, we hypothesize that septal enlargement might be an anatomic phenotype potentially exerting additive influence on disease-dominant death and SCD, accelerating early mortality.
Based on the statistical results, we tried to construct machine learning models for diagnosis of CVDs and CAS. Despite moderate performance, the elected LR model together with nomogram still has a considerable potential in the field of CVD screening owing to the noninvasiveness and easiness to measure the morphological characteristics by echocardiography. Nevertheless, large-scale real-world translational studies are warranted before its clinical application and it is expected to be advantageous to construct a multi-element screening model recruiting additional variables, especially those demographic features verified to be risk factors of CVDs.
The underlying mechanism of abnormal RVOT Phenotype is likely multifactorial. Previous study showed that in patients with pulmonary hypertension, secondary adaptation to an increased right ventricle afterload was likely a contributor to right ventricle size and function25, which could also change RVOT morphology. Intrinsic abnormalities of RVOT myocardium must also be considered in the etiology of or evolution towards an abnormal RVOT phenotype. The histological structure of RVOT is interspersed with heterogeneous distribution of fibrous and fat tissue, and myocardial fibers are arranged in multiple layers with different directions, especially the septal myocardium below the pulmonary valve, which could render RVOT more vulnerable to myofiber disarray6.26. Our study showed that myocardial fibrosis of posterior septum was increased in CAS and CM groups, which may be evidence of CVDs affecting myocardial structure of RVOT. It was found that Brs was associated with epicardial surface and interstitial fibrosis and reduced gap junction expression in RVOT15,27. Another study showed that in Brs, there were cytoplasmic degeneration of myocytes, mitochondrial swelling, and vacuolization in the lesion of RVOT28. These histological and molecular changes might be related to morphologic changes of RVOT, and further investigation focusing on this relationship is of particular interest.
This is a retrospective study conducted in a single center and is subject to inherent limitations to its design. First, this study only involved limited Chinese decedents with a men-predominance, which limited us to assess RVOT in a refining healthy condition. Second, because of incomplete clinical records, we could not rule out other influence of confounding factors (e.g. diabetes and hyperlipidemia) on CVDs, CAS or CM, or interpret the clinic-pathological correlation of RVOT morphology. Third, among all CVDs cases, we only evaluated the association of RVOT morphology (pSBV) with CAS and CM risk, and the association between RVOT morphology and other CVDs remained to be analyzed. Fourth, though we’ve come out an imaging method to measure RVOT diameter as an appropriate surrogate to pSBV, the measurement cutoffs used in this study have not been validated in living persons. And we’ve not yet found a suitable imaging modality to detect tS in a living person because the size is so small. Thus, we’ve not delved into correlation between autopsy findings and imaging findings. Finally, we only measured a limited list of morphological aspects. Well-designed investigations in both pathology and clinic that morphologically evaluate the impact of RVOT could be expected to establish a more precise description of RVOT morphology and applied for clinical practice, so as to find measurable abnormalities in patients before a cardiac event or death occurs.
In summary, our study shows that morphological characteristics of RVOT are associated with cardiovascular outcomes. Large perimeter of sub-valve plane is a potential predictor for CVDs and SCD. Large myocardial thickness of septum is also a potential predictor for SCD. Additionally, tS ≥ 3.0 mm is associated with premature death regardless of diseases. Increased myocardial fibrosis in the posterior septum may serve as an independent risk factor of CAS and CM. After clinical validation, these findings could be of great clinical significance in helping to risk stratify patients who may have a cardiac event.
Methods
Study population
Hearts were consecutively reviewed from the autopsy specimen library in the Center of Forensic Medicine in Sun Yat-sen University from January 2017 to December 2023. Exclusion criteria included: (1) under 16 years old, (2) with a medical history of pulmonary valve surgeries or heart transplants, (3) diagnosed with pulmonary hypertension or other diseases that could cause pulmonary hypertension, or presenting pathological changes of pulmonary hypertension (mainly pulmonary vascular fibrosis), and (4) without postmortem reports. The criteria of patients referred for autopsy was unknown or disputed cause of death, with no selection bias. All corpses underwent systematic forensic autopsy, histopathological examinations and routine toxicological tests. Epidemical information, pathological diagnosis, and causes of death were abstracted from postmortem reports.
According to the cause of death, cases were classified as disease- or violence-dominant death. Disease–dominant death was defined as death caused by the natural progression of diseases. And violence-dominant death was those caused by physical, chemical, biological and other external forces. From disease–dominant death cases, we selected SCD cases for further study, which was defined as an unexpected natural death from heart attack that occurred within a short time period, generally within 24 h from the onset of symptoms. Additionally, we selected patients who suffered from CVDs from total cases, including CAS, CM, cardiac inflammation (including myocarditis, endocarditis, epicarditis, pericarditis and valvulitis), pulmonary embolism, aortic dissection, cardiac conduction diseases and congenital heart diseases. The diagnosis of these diseases was based on the postmortem pathological (both macro- and microscopic) examination as well as the clinical records if available. In detail, CAS was diagnosed as the presence of atherosclerotic plaques in coronary arteries microscopically. There were primary and secondary CM, and the diagnostic standard for primary CM was described as follow: hypertrophic cardiomyopathy was diagnosed as maximum left ventricular thickness ≥ 15 mm at any site in the chamber; dilated cardiomyopathy was diagnosed as heart weight ≥ 500 g in male or 450 g in female and left ventricular or biventricular dilatation that couldn’t be explained by abnormal loading conditions or coronary artery disease, with uneven cardiomyocyte hypertrophy and myocardial fibrosis; ARVC was diagnosed as residual myocytes < 50% with fibrous replacement of the RV free wall myocardium, with or without fatty replacement. Myocarditis was diagnosed as inflammatory infiltration of myocardium with inflammatory cell density ≥ 14 cells/mm2.
Gross examination and tissue processing
The entire RVOT was shown in Fig. 4A. Schievano et al.21 measured perimeters, maximum and minimum diameters at ten section planes along the RVOT/pulmonary artery during the cardiac cycle by CT, including sub-valve, supra-valve, mid-valve, etc. In patients with idiopathic RVOT premature ventricular contractions, the most successful ablation targets were located in the septum, especially regions below the pulmonary valve29. Based on these clinical studies, we developed pathological measurements of RVOT which were applied in forensic practice. In our stage-one study, six morphological characteristics of RVOT were measured in 1,305 hearts collected in between 2017 and 2020, including pSBV, pSPV and pMV, iSCPV, tS and tF (Fig. 4B) (details in Supplemental Method 1). In our stage-two study, additional 1,065 hearts were collected between 2021 and 2023 and combined with those in stage one for further studying pSBV and tS. For histological analysis, three parallel longitudinal sections were taken from the septum of RVOT with partial aortic root and left ventricular outflow tract. In addition, we took one longitudinal section each from the anterior free wall of RVOT and the anterior wall of left ventricle (Fig. 4C-D).
Gross examinations and tissue processing of RVOT. (A) Representative picture of the entire RVOT. The RVOT is divided into two parts, which are free wall (white dotted area) and septum (red dotted area). (B) Measurements of six morphological characteristics of RVOT. pSBV (blue dash line), pSPV (red dash line), pMV (orange dash line), iSCPV (green dash line), tF (yellow dash line), tS (wathet dash line). (C) Locations of tissue sampling, including RVOT septum, RV wall and LV wall. The red frame part is the sampling site. (D) Five tissue sections of human heart during examination. From front to rear, the septum is divided into anterior, middle and posterior sections. Anterior free wall of RVOT and anterior wall of left ventricle are also included. APV = anterior pulmonary valve; RPV = right pulmonary valve; LPV = left pulmonary valve; pSBV = perimeter of sub-valve; pSPV = perimeter of supra-valve; pMV = perimeter of mid-valve; iSCPV = interval between supraventricular crest-pulmonary valve; tF = thickness of free wall; tS = thickness of septum; RVOT = right ventricular outflow tract; RV = right ventricular; LV = left ventricular.
Morphometric analysis for post-mortem myocardial collagen/fibrosis
All post-mortem RVOT sections were stained with Masson’s Trichrome, and compared with anterior wall tissue of left ventricles. All of the sections were scanned by an automatic digital slide scanning system (AxioScan.Z1), and the area fraction of myocardial collagen was analyzed using ImageJ (National Institutes of Health).
Development and validation of machine learning models
The LASSO method was applied for the feature selection among 12 variables that consist of the anatomical features of heart, morphologic features of RVOT, and general information, including age, sex, body height, and abdominal subcutaneous fat thickness. Eight machine learning algorithms were used to construct the diagnostic models, including random forest, extreme gradient boosting trees, adaptive boosting trees, LR, support vector machine with radial basis function kernel, Gaussian Naïve Bayes, k-nearest neighbor, and single-hidden-layer neural network. Cases collected from January 2017 to December 2021 were enrolled as the internal dataset, which was then split into training and validation set at a ratio of 7:3. The models were developed in the training set with 5-fold validation and evaluated in the validation set. A test set that consisted of cases in 2022 and 2023 was utilized for the final assessment of these models. The models were systematically appraised from the aspects of discrimination, calibration and clinical usefulness (details in Supplemental Method 2).
Statistical analysis
Continuous variables were shown as the mean ± SD or median (quartiles [Q1, Q3]), and were analyzed using the unpaired Student’s t-test or Mann-Whitney test after the tests of normality. Categorical variables were summarized as the count (percentage) and analyzed using chi-square tests. Paired samples were analyzed by paired Student’s t-tests or Wilcoxon signed-rank tests. The differences between groups were assessed by analysis of variance (ANOVA) or Kruskal-Wallis H test. Univariate and multivariate logistic regression analyses were used to explore risk factors for diseases and causes of death. Linear regression analyses were performed to explore the association of variables with age at death. Two-tailed p-value of < 0.05 was defined to be statistically significant. All statistical analyses were performed on IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA) and R 4.0.5 software.
Statement
The bereaved provided consent to use of cadaveric tissues and medical records for research purposes. The Medical Ethics Committee of Sun Yat-sen university approved this study (Approval No. 2018-006) and waived the requirement for written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wang, J. M. H. et al. An anatomical review of the right ventricle. Transl Res. Anat. 17, 100049 (2019).
Sanz, J. et al. Function, and dysfunction of the right ventricle: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 1463–1482 (2019).
Maury, P. Why is the right ventricular outflow tract so arrhythmogenic? (… or is it really?…). Heart. 97, 1631–1633 (2011).
Kim, R. J. et al. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J. Am. Coll. Cardiol. 49, 2035–2043 (2007).
Gandjbakhch, E., Redheuil, A., Pousset, F., Charron, P. & Frank, R. Clinical diagnosis, imaging, and Genetics of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: JACC State-of-the-art review. J. Am. Coll. Cardiol. 72, 784–804 (2018).
Blok, M. & Boukens, B. J. Mechanisms of Arrhythmias in the Brugada Syndrome. Int. J. Mol. Sci. 21, 7051 (2020).
Gray, B. et al. Relations between right ventricular morphology and clinical, electrical and genetic parameters in Brugada Syndrome. PLoS One. 13, e0195594 (2018).
Heermann, P. et al. Biventricular myocardial strain analysis using cardiac magnetic resonance feature tracking (CMR-FT) in patients with distinct types of right ventricular diseases comparing arrhythmogenic right ventricular cardiomyopathy (ARVC), right ventricular outflow-tract tachycardia (RVOT-VT), and Brugada syndrome (BrS). Clin. Res. Cardiol. 108, 1147–1162 (2019).
Murase, Y. et al. Histopathological characteristics of the arrhythmogenic right ventricular cardiomyopathy presenting the electrocardiographic characteristics with Brugada syndrome. J. Cardiovasc. Electrophysiol. 34, 2006–2009 (2023).
Saberniak, J. et al. Comparison of patients with early-phase arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract ventricular tachycardia. Eur. Heart J-Card Img. 18, 62–69 (2017).
Marginean, A. et al. Performance of the right ventricular outflow tract/aortic diameter as a novel predictor of risk in patients with acute pulmonary embolism. J. Thromb. Thrombolysis. 50, 165–173 (2020).
Lerman, B. B. Mechanism, diagnosis, and treatment of outflow tract tachycardia. Nat. Rev. Cardiol. 12, 597–608 (2015).
Novak, J. et al. Electrocardiographic differentiation of idiopathic right ventricular outflow tract ectopy from early arrhythmogenic right ventricular cardiomyopathy. Europace. 19, 622–628 (2017).
Pieroni, M. et al. Electroanatomic and Pathologic Right Ventricular Outflow Tract Abnormalities in patients with Brugada Syndrome. J. Am. Coll. Cardiol. 72, 2747–2757 (2018).
Behr, E. R., Ben-Haim, Y., Ackerman, M. J., Krahn, A. D. & Wilde, A. A. M. Brugada syndrome and reduced right ventricular outflow tract conduction reserve: a final common pathway? Eur. Heart J. 42, 1073–1081 (2021).
Gotschy, A. et al. Right ventricular outflow tract dimensions in arrhythmogenic right ventricular cardiomyopathy/dysplasia-a multicentre study comparing echocardiography and cardiovascular magnetic resonance. Eur. Heart J-Card Img. 19, 516–523 (2018).
Zhang, L. et al. Does Sudden Unexplained Nocturnal Death Syndrome remain the autopsy-negative disorder: a gross, microscopic, and molecular autopsy investigation in Southern China. Mayo Clin. Proc. 91, 1503–1514 (2016).
Garland, J. et al. Heart Weight is an independent factor Associated with, but is a poor predictor for, Sudden Cardiac Death. Am. J. Foren Med. Path. 43, 18–22 (2022).
Schoppen, Z. J. et al. Prevalence of abnormal Heart Weight after Sudden Death in people younger than 40 years of age. J. Am. Heart Assoc. 9, e015699 (2020).
Marcus, F. I. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy /dysplasia: proposed modification of the Task Force Criteria. Eur. Heart J. 31, 806–814 (2010).
Schievano, S. et al. Four-dimensional computed tomography: a method of assessing right ventricular outflow tract and pulmonary artery deformations throughout the cardiac cycle. Eur. Radiol. 21, 36–45 (2011).
Kawut, S. M. et al. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the multi-ethnic study of atherosclerosis (MESA)--right ventricle study. Circulation. 126, 1681–1688 (2012).
Anderson, R. D. et al. Focal ventricular tachycardias in Structural Heart Disease: prevalence, characteristics, and clinical outcomes after catheter ablation. JACC Clin. Electrophysiol. 6, 56–69 (2020).
Wen, S. et al. Right ventricular enlargement and dysfunction are Associated with increased all-cause mortality in hypertrophic cardiomyopathy. Mayo Clin. Proc. 97, 1123–1133 (2022).
Fischer, L. et al. Right heart size and function significantly correlate in patients with pulmonary arterial hypertension - a cross-sectional study. Respir Res. 19, 216 (2018).
Lu, Y. Y., Chen, Y. C., Lin, Y. K., Chen, S. A. & Chen, Y. J. Electrical and structural insights into right ventricular outflow Tract Arrhythmogenesis. Int. J. Mol. Sci. 23, 11795 (2023).
Nademanee, K. et al. Connexin-43, and conduction abnormalities in the Brugada Syndrome. J. Am. Coll. Cardiol. 66, 1976–1986 (2015).
Tauber, P. E. et al. Correlation between functional and ultrastructural substrate in Brugada syndrome. HeartRhythm Case Rep. 2, 211–216 (2016).
Zhang, Z., Hou, X., Qian, Z., Guo, J. & Zou, J. Target characteristics and voltage mapping of the Matrix in Idiopathic premature ventricular contractions originating from the right ventricular outflow tract. Biomed. Res. Int. 2021, 1–6 (2021).
Acknowledgements
This study was conducted using resources from specimen library of Sun Yat-sen University. We are greatly indebted to staff from the department of forensic pathology for their efforts to collect these cases. And we thank professor Qing Wang from College of Life Science & Technology, Huazhong University of Science and Technology for his valuable suggestions on revision of this manuscript.
Author information
Authors and Affiliations
Contributions
We would like to declare that all co-authors had made substantial efforts on this manuscript: E. H., D. M. and C. L. designed this project and approved the final manuscript; D. M., C. L., D. Z., K. J., Y. H., Y. F., Y. B., Q. L., Xy. L., Q.Y. and Y. M. formed the examination group; B. L., H. Y., S. Z., S. T., S. L., Xs. L. and L. Q. participated in autopsies and cases collection; D. Z. and K. J. collected and interpreted the data; D. M. collected specimens and analyzed for myocardial fibrosis; C. L. developed and validated the machine learning model; D. M. validated statistical analysis; D. M. and C. L. wrote the original manuscript, which was revised by D. Z., K. J. and E. H. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Financial support
This study was supported by the National Natural Science Foundation of China (Grant No. 81920108021), the National Key R&D Program of China (Grant No. 2023YFC3303902 & 2023YFA1800901) and the Guangzhou Science and Technology Project (Grant No. 202201011792).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mao, D., Li, C., Zheng, D. et al. Morphological phenotype of right ventricular outflow tract is associated with cardiovascular outcomes and premature death. Sci Rep 14, 25128 (2024). https://doi.org/10.1038/s41598-024-77023-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77023-3