Abstract
Recent findings suggest that prism adaptation can extend its effects beyond spatial attention, modulating the performance of different cognitive tasks by acting on cerebellar, parietal and temporal-frontal networks. We tested groups of healthy subjects to investigate the effects of rightward vs. leftward prism adaptation vs. neutral lenses exposure in a series of memory tasks, probing either short-term (Digit span, Corsi span) or long-term memory (Supraspan verbal and spatial learning). In the short-term memory tasks, leftward prism adaptation selectively increased verbal span, while rightward prism adaptation increased spatial span. In the long-term memory tasks, leftward prism adaptation selectively increased verbal supraspan, i.e., increased the number of digits in the correct sequence reproduced and reduced the number of repetitions needed to learn the supraspan sequence. On the other hand, rightward prism adaptation selectively increased spatial supraspan, i.e. it increased the number of spatial positions in the correct sequence reproduced and reduced the number of repetitions needed to learn the supraspan sequence. Moreover, rightward, but not leftward, prism adaptation selectively increased supraspan recall after a delay interval, regardless of the stimulus material, i.e., it increased the number of digits or spatial positions recalled after a delay interval. Neutral lenses exposure did not influence any memory task. These findings suggest that prism adaptation can induce both modality/hemispheric-specific and process-specific effects on short-term and long-term explicit memory.
Similar content being viewed by others
Introduction
Prism adaptation (PA) is a form of visuomotor adaptation following visual field displacement induced by prismatic lenses1.
Several findings suggest that PA can influence not only space representation and orienting of attention in different modalities2,3 but also other cognitive functions. In some cases, the modulatory effect of prism adaptation on cognitive functions other than spatial could be explained because of sharing a spatial medium, as in the case of temporal representation in both healthy subjects and neglect patients4,5, visual search in neglect patients6, visually guided actions in neglect patients6, auditory representation in healthy subjects7, constructional disorders in neglect patients8. For other cognitive functions, it has been hypothesised that modality-specific hemispheric modulation of brain areas would prompt changes from the sensorimotor level to high cognitive functions, as in the case of reward-based learning9 or phonological fluency10. As suggested by Michel11,12, this link between low-level sensorimotor plasticity and high-level cognitive functions raises questions about the mechanisms underlying the somewhat unexpected cognitive effects following PA.
Seminal studies in healthy subjects showed that left PA leads to neglect-like shifts in attention, while right PA has only minor effects13,14,15. These findings were recently interpreted as the consequence of an induced instability in the synaptic organization in the posterior parietal cortex contralateral to optical deviation. According to this model, the right PA would not produce any effect as healthy individuals may continue to rely on their right-hemispheric attentional system. Left PA would determine a reshuffling in the right PPC, thus accounting for neglect-like deficits16.
Recent research suggested that, in addition to this modulation of attentional networks and reshuffling of spatial representations in the inferior parietal lobe, visuomotor adaptation elicited by PA could also induce modulation of frontal circuits of the brain hemisphere ipsilateral to prismatic deviation. A study using paired-TMS and recording of motor evoked potentials in healthy subjects17 reported modulation of excitatory brain circuits on the motor cortex ipsilateral to the visual shift induced by prismatic lenses. The left deviation increased the intracortical facilitation of the left motor cortex, and the right deviation increased the intracortical facilitation of the right motor cortex.
The effects of PA on the excitability of the motor cortex are similar in magnitude to those induced by anodal tDCS18.
Additional findings showed that prism deviation increases the power of beta oscillations in the frontal areas of the hemisphere ipsilateral to the optical deviation during motor preparation but not visual attention tasks19.
These results complement those of neuroimaging studies reporting an increase in activation of parietal-frontal areas of the brain hemisphere ipsilateral to prism deviation paralleled to transcallosal inhibition of the homologous regions of the contralateral hemisphere20. This mechanism of action is in line with the model proposed by Clarke et al.16, reporting inhibition of the ventral attentional system of the hemisphere contralateral to prism deviation, which in turn would be consistent with increased activation in the hemisphere ipsilateral to prism deviation21.
These findings imply that PA may induce modality-specific effects on cognitive tasks associated with brain regions of the hemisphere ipsilateral to the side of the visual shift. According to this view, and assuming a spread of activation from the activated motor cortex toward interconnected areas of the same hemisphere, left PA could modulate subjects’ performance on cognitive tasks with a verbal component; on the other hand, right PA could modulate subjects’ performance with a spatial component. This prediction is not in contrast to the results of several studies that showed aftereffects in visuospatial tasks after leftward but not rightward prism adaptation in healthy participants. These effects mainly concerned attentional tasks, where, for example, leftward prisms counteract pseudoneglect14,15,22. Indeed, when testing the effects of prism deviation on other, not strictly visuospatial, cognitive functions, the modality-specific effects of prisms related to hemispheric activation could emerge10.
In the present study, we tested this prediction by investigating the effects of prism adaptation in short- and long-term memory tasks.
Indeed, memory is a cognitive function in which both modality- and process-specific brain activations are observed. For example, short-term verbal memory, as investigated with digit span tasks, is strictly correlated with left hemispheric activation23. In contrast, short-term spatial memory, as studied with Corsi span tasks, strictly correlates with right hemispheric activation24,25. The same modality-specific hemispheric activation applies to long-term episodic memory26. On the other hand, multiple studies show that in long-term memory, the activation of the right hemisphere is greater than that of the left hemisphere during the memory retrieval phase27.
We investigated whether directional effects induced by PA could selectively modulate short-term memory spans and supra-span learning, with specific effects associated with the side of visual field deviation, the stimuli to be memorised (e.g., verbal vs. spatial), and the process challenged by the task (e.g., learning vs. recall after a delay interval).
According to modality-specific effects, we expected that rightward PA modulated spatial spans and supraspans while leftward PA modulated verbal spans and supraspans. According to process-specific impacts, we expected that rightward but not leftward PA modulated the recall of previously learned sequences in both verbal and spatial supra-span tasks.
Results
Participants underwent a neuropsychological assessment to verify that the three groups were homogeneous from a cognitive point of view and that memory modulation was not related to neuropsychological memory deficits at baseline.
There was no significant difference in the performance of the L-PA, R-PA and NL groups on the cognitive baseline tasks: Symbol Digit Modalities Test (SDMT)28 (F1,30 = 0.70, p = 0.453), Modified Five Point Test (MFPT)29 (F1,30 = 0.04, p = 0.750), Stroop test30 (F1,30 = 2.26, p = 0.293), Raven’s Advanced Progressive Matrices (RAPM) (F1,30 = 0.22, p = 0.512), Phonological Word Fluency (FAS)31 (F1,30 = 0.03, p = 0.765), Beck Depression Inventory (BDI)32 (F1,30 = 0.40, p = 0.486), Hamilton Depression Rating Scale (HDRS)33 (F1,30 = 0.44, p = 0.458).
Effects of prism adaptation on short-term memory tasks
The Digit span and Corsi span tests were used to assess verbal and visuospatial short-term memory.
To compare the verbal short-term memory of participants at baseline (pre-PA), a one-way ANOVA with Group (L-PA, R-PA, NL) variable was performed on the number of correct digit sequences reproduced in the Digit span test. The analysis did not show significant differences (F2,45 = 0.94, p = 0.390, ηp2 = 0.04), suggesting that the Digit span task performance was similar for the groups (L-PA = 6.56 ± 0.62; R-PA = 6.43 ± 0,81, NL = 6.25 ± 0.44).
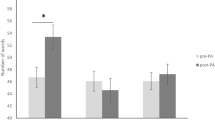
To investigate whether left and rightward PA modulate verbal short-term memory differently, a 2 × 3 repeated measures ANOVA was conducted on the number of correct digits sequences reproduced, with the variables Session (pre-PA, post-PA) as within-subject factor and Group (L-PA, R-PA, NL) as a between subject’s factor. The ANOVA revealed significant effects of Group (F2,45 = 7.35, p = 0.002, ηp2 = 0.24) and Session (F1,45 = 3.52, p = 0.042, ηp2 = 0.07). There was a significant Session x Group interaction (F2,45 = 7.19; p = 0.002, ηp2 = 0.24). L-PA significantly increased the number of digits produced compared to baseline (pre-PA = 6.56 ± 0.63 vs. post-PA = 7.44 ± 0.73; p = 0.002), while R-PA did not (pre-PA = 6.44 ± 0.81 vs. post-PA = 6.25 ±, 0.85; p = 0.948). The number of digits produced following left PA was significantly higher than that following right PA (p = 0.003).
No statistically significant changes in performance were observed in the NL group (pre = 6.25 ± 0.44 vs. post = 6.25 ±, 0.85; p = 1.000). The number of digits produced following left PA was significantly higher than that following post NL (p = 0.002), while the number of digits produced following right PA was not different from that following post NL (p = 1.000).
In sum, the leftward but not the rightward optical deviation increased subjects’ performance in the Digit span task (Fig. 1; see also Table 1 in the Supplementary Materials).
Digit span in baseline (pre-PA) and post-PA sessions. The score indicates the number of correctly reproduced sequence elements in the verbal span task. R-PA rightward prism adaptation, L-PA leftward prism adaptation, NL neutral lenses exposure. Error bars indicate the standard error of the mean. *p < 0.05, **p < 0.001.
To compare the visuospatial short-term memory of participants at baseline (pre-PA), a one-way ANOVA with the Group (L-PA, R-PA, NL) variable was performed on the number of correct spatial positions reproduced in the CORSI span test. The analysis did not show significant differences (F2,45 = 2.28, p = 0.114, ηp2 = 0,09), suggesting that performance on the Corsi span test was similar for the three groups (L-PA = 6.44 ± 0.51; R-PA = 6.38 ± 0.61; NL = 6.00 ± 0.73).
To investigate whether left and rightward PA modulates the Corsi span test differently, a 2 × 3 repeated measures ANOVA was conducted on the number of correct spatial positions reproduced, with the variables Session (pre-PA, post-PA) as within-subject factor and Group (L-PA, R-PA, NL) as a between subject’s factor. The ANOVA showed a non-significant effect of Session (F1,45 = 3.24, p = 0.078, ηp2 = 0.06). There was a significant effect of Group (F2,45 = 4.14, p = 0.022, ηp2 = 0.15) and Session x Group interaction (F2,45 = 9.93; p < 0.001, ηp2 = 0.30). R-PA significantly increased the number of correct spatial positions reproduced compared with baseline (pre-PA = 6.38 ± 0.61 vs. post-PA = 7.00 ± 0.81; p = 0.004), while L-PA did not (pre-PA = 6.44 ± 0.51 vs. post-PA = 6.06 ± 0.77, p = 0.200). The sequence length produced following R-PA was significantly higher than after L-PA (post-L-PA = 6.06 ± 0.77 vs. post-R-PA = 7.00 ± 0.81; p = 0.005).
No statistically significant changes in performance were observed in the NL group (pre = 6.00 ± 0.73 vs. post = 6.25 ± 0.44; p = 0.629). The sequence length reproduced following right PA was significantly higher than that following post NL (p = 0.040), while the sequence length reproduced following left PA was not different from that following post NL (p = 0.973).
In sum, R-PA but not L-PA increased subjects’ performance in the Corsi span task (Fig. 2; see also Table 2 in Supplementary Materials).
Spatial span in baseline (pre-PA) and post-PA sessions. The score indicates the number of correctly reproduced sequence elements in the spatial span task. R-PA rightward prism adaptation, L-PA leftward prism adaptation, NL neutral lenses exposure. Error bars indicate the standard error of the mean. *p < 0.05, **p < 0.001.
Effects of prism adaptation on long-term memory tasks
The Supraspan verbal and the Supraspan spatial learning tests assessed verbal and visuospatial long-term memory.
We investigated whether left and rightward PA modulates the Supraspan verbal learning test differently. We conducted three separate analyses.
The first analysis was conducted to explore the effects of PA on the number of correct digit sequences reproduced. To compare the participant’s performance at baseline (pre-PA), a one-way ANOVA with the Group (L-PA, R-PA, NL) variable was performed on the Supraspan verbal learning test. The analysis did not show significant differences (F2,45 = 0.05, p = 0.947, ηp2 = 0.002), suggesting that the Supraspan task performance was similar for the three groups (L-PA = 19.40 ± 0.78; R-PA = 19.30 ± 0.93; NL = 19.60 ± 1.18).
To investigate whether leftward and rightward PA differently modulate verbal learning, a 2 × 3 repeated measures ANOVA was conducted on the number of correct digits sequences reproduced, with the variables Session (pre-PA, post-PA) as within-subject factor and Group (L-PA, R-PA, NL) as a between subject’s factor. The ANOVA showed a significant effect of Group (F2,45 = 3.23, p = 0.049, ηp2 = 0.01) and Session (F1,45 = 7.36, p = 0.009, ηp2 = 0.14). There was a significant Session x Group interaction (F2,45 = 6.59; p = 0.003, ηp2 = 0.22). L-PA significantly increased the task score compared with baseline (pre-PA = 19.40 ± 0.78 vs. post-PA = 22.80 ± 0.32; p < 0.001), while R-PA did not (pre-PA = 19.30 ± 0.93 vs. post-PA = 19.40 ± 0.86, p = 1.000). The subjects’ performance on the Supraspan verbal learning test following left PA was significantly higher than that following right PA (p < 0.001). The NL -PA group did not exhibit any statistically significant alterations in performance (pre = 19.60 ± 0.18 vs. post = 19.70 ± 0.20, p = 1.000).
The subjects’ performance following left PA was significantly higher than that following post NL (p = 0.003), while the performance following right PA was not different from that following post NL (p = 0.998).
In sum, L-PA but not R-PA increased subjects’ performance in the supraspan verbal learning test (Fig. 3; see also Table 3 in Supplementary Materials).
Supraspan verbal learning in baseline (pre-PA) and post-PA sessions. The score indicates the number of correctly reproduced elements of the supraspan digit sequence. R-PA rightward prism adaptation, L-PA leftward prism adaptation, NL neutral lenses exposure. Error bars indicate the standard error of the mean. *p < 0.05, **p < 0.001.
The second analysis was carried out to investigate the effects of PA on the number of repetitions needed to learn the correct sequence.
To compare the participants’ performance at baseline (pre-PA), a one-way ANOVA with the Group (L-PA, R-PA, NL) variable was performed. The analysis did not show significant differences (F2,45 = 0.752, p = 0.477, ηp2 = 0.03), suggesting that the performance for this variable was similar for the three groups (L-PA = 4.88 ± 0.43; R-PA = 5.70 ± 0.68; NL = 5.00 ± 0.34).
To investigate whether leftward and rightward PA modulate the number of repetitions needed to learn, a 2 × 3 repeated measures ANOVA with the variables Session (pre-PA, post-PA) as within-subject factor and Group (L-PA, R-PA, NL) as a between subject’s factor was conducted. The ANOVA showed significant effects of Group (F2,45 = 3.84, p = 0.029, ηp2 = 0.14) and Session (F1,45 = 13.44, p < 0.001, ηp2 = 0.23). There was a significant Session x Group interaction (F2,45 = 5.53; p = 0.007, ηp2 = 0.19). L-PA significantly decreased the number of repetitions compared to baseline (pre-PA = 4.88 ± 0.43 vs. post-PA = 2.70 ± 0.46; p < 0.001), while R-PA did not show significant effects compared to baseline (pre-PA = 5.70 ± 0.68 vs. post-PA = 5.31 ± 0.66, p = 0.961).
The number of repetitions needed to learn the digit sequence following left PA was significantly lower than that following right PA (p = 0.006) (Fig. 4).
No statistically significant changes in performance were observed in the NL group (pre = 5.00 ± 0.34 vs. post = 4.69 ± 0.25, p = 0.982).
The number of repetitions needed to learn the digit sequence post left PA tended to be lower than that post NL (p = 0.060), while the number of repetitions needed to learn the digit sequence post right PA was not different from that post NL (p = 0.944).
In sum, left but not right PA reduced the number of repetitions needed to learn the verbal supraspan sequence, speeding up learning process (Fig. 4; see also Table 4 in Supplementary Materials).
Supraspan repetitions verbal learning in baseline (pre-PA) and post-PA sessions. The number of repetitions indicates the repetitions needed to learn the correct digit sequence. R-PA rightward prism adaptation, L-PA leftward prism adaptation, NL neutral lenses exposure. Error bars indicate the standard error of the mean. *p < 0.05, **p < 0.001.
The third analysis was conducted to explore the effects of PA on Supraspan verbal learning recall.
To compare the participants’ performance at baseline (pre-PA), a one-way ANOVA with the Group (L-PA, R-PA, NL) variable was performed on the number of correct digits sequences reproduced in the Supraspan verbal learning recall task. The analysis did not show significant differences (F2,45 = 0.75, p = 0.477, ηp2 = 0.03), suggesting that performance was similar for the groups (L-PA = 1.26 ± 0.09; R-PA = 1.27 ± 0.07; NL = 1.10 ± 0.10).
To investigate whether leftward and rightward PA modulate the recall of the previously learned sequence, a 2 × 3 repeated measures ANOVA with the variables Session (pre-PA, post-PA) as within-subject factor and Group (L-PA, R-PA, NL) as a between subject’s factor was conducted on the number of correct digits sequences reproduced. The ANOVA showed a significant effect of Group (F2,45 = 4.50, p = 0.017, ηp2 = 0.1) but not of Session (F1,45 = 2.64, p = 0.111, ηp2 = 0.055). There was a significant effect of Session x Group interaction (F2,45 = 4.12; p = 0.023, ηp2 = 0.15). R-PA significantly increased the number of digits recalled compared to baseline (pre-PA = 1.27 ± 0.07 vs. post-PA = 1.50 ± 0.03, p = 0.029), while L-PA did not (pre-PA = 1.26 ± 0.09 vs. post-PA = 1.29 ± 0.04, p = 0.999). In the NL group, no statistically significant performance changes were detected (pre = 1.10 ± 0.09 vs. post = 1.07 ± 0.08; p = 0.976).
R-PA also significantly increased the number of digits recalled compared to post NL (p < 0.001), while L-PA did not (p = 0.138).
In sum, right but not left PA facilitated recall of previously learned supraspan verbal sequences (Fig. 5; see also Table 5 in Supplementary Materials).
The same three analyses were conducted for the Supraspan spatial learning test.
The first analysis explored the effects of PA on the number of correct spatial positions reproduced.
For the baseline (pre-PA), the ANOVA did not show a significant difference between L-PA, R-PA and NL (F2,45 = 0.30, p = 0.740, ηp2 = 0.01; L-PA = 25.50 ± 0.40; R-PA = 25.10 ± 0.48; NL = 25.20 ± 0.21) indicating that there was not the difference between the groups.
On the 2 × 3 ANOVA, no significant effects of Session (F1,45 = 0.14, p = 0.703, ηp2 = 0.003) and Group (F2,45 = 1.94, p = 0.155, ηp2 = 0.08) were found. There was a significant Session x Group interaction (F2,45 = 10.61; p < 0.001, ηp2 = 0.32). Post hoc tests revealed that R-PA increased the subject’s performance compared to baseline (pre-PA = 25.10 ± 0.48 vs. post-PA = 26.70 ±, 0.39; p < 0.001) while L-PA did not (pre-PA = 25.50 ± 0.40 vs. post-PA = 24.60 ± 0.54, p = 0.278). The subjects’ performance on the Supraspan spatial learning test following right PA was significantly higher than that following left PA (p = 0.021). No statistically significant changes in performance were observed in the NL group (pre = 25.20 ± 0.21 vs. post = 24.70 ± 0.33; p = 0.873).
R-PA increased the subject’s performance compared to post NL (p = 0.003), while L-PA did not (p = 0.867).
In sum, right but not left PA increased performance in the supraspan spatial learning task (Fig. 6; see also Table 6 in Supplementary Materials).
Supraspan spatial learning in baseline (pre-PA) and post-PA sessions. The score indicates the number of correctly reproduced elements of the supraspan spatial sequence. R-PA rightward prism adaptation, L-PA leftward prism adaptation, NL neutral lenses exposure. Error bars indicate the standard error of the mean. *p < 0.05, **p < 0.001.
The second analysis was carried out to investigate the effects of PA on the number of repetitions needed to learn the correct sequence.
To compare the participant’s performance at baseline (pre-PA), a one-way ANOVA with the Group (L-PA, R-PA, NL) variable was performed on the number of correct spatial positions reproduced. The analysis did not show significant differences (F2,45 = 0.17, p = 0.842, ηp2 = 0.008), suggesting that performance for this variable was similar for the three groups (L-PA = 5.32 ± 0.43; R-PA = 5.71 ± 0.55; NL = 5.65 ± 0.48).
To investigate whether leftward and rightward PA modulates the number of repetitions needed to learn, a 2 × 3 repeated measures ANOVA with the variables Session (pre-PA, post-PA) as within-subject factor and Group (L-PA, R-PA, NL) as a between subject’s factor was conducted. The ANOVA showed significant effects of Group (F2,45 = 3.31, p = 0.045, ηp2 = 0.12) and Session (F1,45 = 7.72, p = 0.008, ηp2 = 0.14). There was a significant Session x Group interaction (F2,45 = 10.53; p < 0.001, ηp2 = 0.31). R-PA significantly decreased the number of repetitions compared to baseline (pre-PA = 5.71 ± 0.55 vs. post-PA = 2.94 ± 0.25; p < 0.001), while L-PA did not (pre-PA = 5.32 ± 0.43 vs. post-PA = 5.81 ± 0.46, p = 0.934). No statistically significant changes in performance were observed in the NL group (pre = 5.65 ± 0.48 vs. post = 5.39 ± 0.44; p = 0.996).
The number of repetitions needed to learn a spatial sequence following right PA was significantly lower than that following left PA (p < 0.001). The number of repetitions following right PA was also significantly lower than that post NL (p = 0.003), while the number of repetitions following left PA was not different from that post NL (p = 0.983).
In sum, right but not left PA reduced the number of repetitions needed to learn the spatial supraspan sequence, speeding up learning process (Fig. 7; see also Table 7 in Supplementary Materials).
Supraspan spatial learning in baseline (pre-PA) and post-PA sessions. The number of repetitions indicates the repetitions needed to learn the correct spatial sequence. R-PA rightward prism adaptation, L-PA leftward prism adaptation, NL neutral lenses exposure. Error bars indicate the standard error of the mean. *p < 0.05, **p < 0.001.
The third analysis was conducted to explore the effects of PA on Supraspan spatial learning recall.
To compare the participant’s performance at baseline (pre-PA), a one-way ANOVA with the Group (L-PA, R-PA, NL) variable was performed on the number of correct sequences reproduced in Supraspan spatial recall test. The analysis did not show significant differences (F2,45 = 0.30, p = 0.739, ηp2 = 0.01), suggesting that performance was similar for the groups (L-PA = 1.21 ± 0.11; R-PA = 1.18 ± 0.12; NL = 1.07 ± 0.15).
To investigate whether leftward and rightward PA modulate the recall of the previously learned spatial sequence, a 2 × 3 repeated measures ANOVA with the variables Session (pre-PA, post-PA) as within-subject factor and Group (L-PA, R-PA, NL) as a between-subjects factor was conducted on the number of correct spatial sequences reproduced. The ANOVA did not reveal significant effects of Group (F2,45 = 1.97, p = 0.151, ηp2 = 0.08) and Session (F1,45 = 0.98, p = 0.325, ηp2 = 0.02). There was a significant Session x Group interaction (F2,45 = 5.16; p = 0.010, ηp2 = 0.18).
R-PA significantly increased the number of positions recalled compared to baseline (pre-PA = 1.18 ± 0.12 vs. post-PA = 1.51 ± 0.04, p = 0.030), while L-PA did not (pre-PA = 1.21 ± 0.11 vs. post-PA = 1.11 ± 0.11, p = 0.932). The subjects’ performance on recall of the previously learned spatial sequence following right PA was significantly higher than that following left PA (p = 0.030). No statistically significant changes in performance were observed in the NL group (pre = 1.07 ± 0.15 vs. post = 1.01 ±, 0.14; p = 0.995). The subjects’ performance on recall following right PA was significantly higher than that post NL (p = 0.030), while L-PA did not affect recall compared to post NL (p = 0.992).
In sum, right but not left PA facilitated recall of previously learned supraspan spatial sequences (Fig. 8; see also Table 8 in Supplementary Materials).
Prism adaptation
Aftereffect. The ANOVA revealed significant effects of Group (F2,45 = 75.30; p < 0.001, ηp2 = 0.77), Session (F2,45 = 5.91; p = 0.019, ηp2 = 0.11) and Session x Group interaction (F2,45 = 75.94; p < 0.001, ηp2 = 0.77).
The presence of aftereffects was confirmed by a significant difference between pre-exposure and post- exposure in L-PA (pre-PA = 0.02 ± 0.13 vs. post-PA = 2.10 ± 0.22 degrees; p < 0.001) and R-PA (pre-PA = -0.06 ± 0.16 vs. post-PA = -2.94 ± 0.21 degrees; p < 0.001) groups. No statistically significant changes in performance were observed in the NL group (pre = 0.15 ± 0.26 vs. post = -0.25 ± 0.18; p = 0.722) (Fig. 9).
Prisms aftereffect. The values indicate mean pointing displacement in the four experimental conditions across groups. R-PA rightward prism adaptation, L-PA leftward prism adaptation, NL neutral lenses exposure. Negative values indicate leftward pointing displacement; positive values indicate rightward pointing displacement. Error bars = standard error of the mean; *p < 0.05, **p < 0.001.
Discussion
The main results of the present study show that PA can modulate both short- and long-term memory, as investigated with Digit and Corsi span and Supraspan Verbal and Spatial learning tasks, and that its effects are modality, process and directional specific.
As to modality-specific effects on memory spans, results show that leftward PA selectively improves verbal but not spatial short-term memory span as well as verbal but not spatial supraspan learning; on the other hand, rightward PA selectively improves spatial but not verbal short-term memory span as well as spatial but not verbal supraspan learning.
The effects of PA on supraspan learning are observed in an increased number of reproduced sequences and a reduced number of trials needed to learn a supraspan sequence.
Regarding process-specific effects, rightward but not leftward PA improves the recall, after a delay interval, of learned supra-span sequences regardless of their modality. The effect is observed for verbal (i.e. digits) and spatial (i.e. cubes) material.
It is unlikely that the observed results can be accounted for unspecific effects. Indeed, prism directional-specific effects argue against the presence of practice effects.
Moreover, one could argue that in post-PA sessions persistence of adaptation to prisms could have influenced performance in the Corsi block test. In the present study, we did not test for such an effect. On the other hand, we think it is unlikely that persistence of adaptation could be determinant in inducing facilitation of Corsi block test following right but not left PA.
The present results could be considered at odds with previous findings of PA, which showed that cognitive aftereffects mainly occur following a rightward optical deviation in neglect patients34 and after a leftward prism adaptation in healthy subjects11. However, as also anticipated in the Introduction, previous findings mainly refer to line bisection tasks, that left PA counteracts while they cannot be significantly modulated by right PA35.
Outside of strictly spatial tasks, PA could operate as suggested by studies documenting an increase in excitability of frontal areas ipsilateral to the deviation side17,18. According to this previous evidence, the present results may reflect an increased excitability of left and right hemispheric brain regions associated with processing verbal and spatial material, probably due to reduced threshold of brain activation or recruitment of glutamatergic interneurons17. This interpretation would be consistent with a modality-dependent view of short- and long-term verbal and spatial memory as investigated with digit and Corsi spans and with digit and Corsi supraspan, with a hemispheric asymmetry (i.e. left vs. right hemispheric activation) related to the modality of the processed material (i.e. verbal vs. visuospatial material to be memorised/learned). Furthermore, these findings are in accord with previous literature, suggesting that a domain-specific process would operate for item retention not only in short-term memory spans but also in verbal and spatial supraspan learning tasks36.
The present findings align with the results of a recent trial using a device combining PA and digital cognitive training in left and right brain-damaged patients undergoing rehabilitation. In fact, left PA and right PA improved Digit and Corsi memory spans when applied, respectively, in patients with left and right brain strokes37. On the other hand, the limited number of left-brain damaged patients in that study does not allow us to make definitive conclusions as to the presence of modality specific aftereffects related to prism deviation in stroke.
As to the brain regions involved in the observed effects, although the present study was not aimed at investigating the neural correlates of PA concerning verbal and spatial memory, one can make some speculations based on previous literature on PA. In particular, one can hypothesise a hemispheric specific influence of PA on temporal and parietal circuits, i.e. on areas that PA activates21,38 and that are also necessary for memory spans as suggested by lesion studies23. In addition, one cannot exclude a critical contribution of cerebellar circuits in the observed effects of PA on memory spans and supraspan learning. The cerebellum is known to be activated during error reduction and realignment phases following prism adaptation39,40,41. On the other hand, the cerebellum has also been correlated to explicit, sequential learning, especially of motor sequences42,43. Although the motor aspect of sequence reproduction is explicitly present only in the spatial span tasks, verbal reproduction of a processed auditory sequence of digits, such as in the digit span tasks, can also be assimilated to motor sequence reproduction, even if with different effectors involved (vocal rather than manual).
Recent literature suggested that cognitive aftereffects of PA would be mainly mediated by the activation of prefrontal circuits21,44. Behavioural confirmation of this hypothesis comes from studies proving modulation of executive functions, such as phonological fluency, following PA10. Furthermore, frontal components operate on both span (although mainly in backward versions) and supraspan tasks45,46. In the case of supraspan tasks, the dorsolateral prefrontal cortex would provide a critical neural substrate, enabling learning through exposure to memory sets that exceed short-term memory capacity. In particular, the effect of rightward PA on both digit and spatial supraspan retrieval could be ascribed to the modulation of right hemispheric prefrontal areas according to the HERA model (Hemispheric Encoding Retrieval Asymmetry), which assumes a prevalent activation of of left hemispheric frontal circuits for memory encoding and of right hemispheric ones for memory retrieval47.
To the best of our knowledge, this is the first study investigating the effects of PA on human explicit memory. Modulating memory tasks by PA fits the idea that cognition maps on brain circuits subserving sensorimotor interactions. This theoretical field can conceptualize the prism aftereffects beyond sensorimotor adaptation and extending to higher cognitive functions. These findings could contribute to further elucidating the underlying mechanism of PA, devising new therapies for cortical dysfunctions based on PA, in addition to the well-documented effects on unilateral spatial neglect following right hemispheric damage.
This study presents some limits, that could be specifically addressed in future studies.
The effects of PA combined with other techniques (e.g. neuroimaging) could better address the neural counterparts of the memory modulation observed.
The aftereffect following prism adaptation was not measured at the end of the experiment, i.e. following the last memory task, and the pre-test evaluation of memory tasks was not made on the same day of the post test one.
We limited investigations to memory spans and supraspan learning. Future studies could usefully address the effects of PA on other working memory and long-term memory tasks.
Methods
Participants
Forty-eight healthy subjects (16 males, 32 females; mean age: 26.65 ± 3.2 years) volunteered to participate in the experiments. All participants were University students, native Italian speakers, right-handed, with normal or corrected-to-normal vision, and none reported neurological or psychiatric disease history. Participants were assigned to a leftward Prism Adaptation group (L-PA; n = 16; mean age = 25.82 ± 2.88 years), a rightward Prism Adaptation group (R-PA; n = 16; mean age = 26.70 ± 1.92 years) and a neutral lenses exposure group (NL; n = 16; mean age = 26.30 ± 1.84 years).
Our stopping rule was determined based on the sample size recommended by the a priori power analysis conducted using G*Power 3.1.9.448. We specified a minimum to medium effect size (f = 0.25) and an alpha level of 0.05, while considering correlations among repeated measures (r = 0.5). The analysis indicated that a total sample of 48 participants, divided into three equal-sized groups of n = 16, was necessary to achieve a power of 0.85.
All subjects gave written informed consent for participation in the study. All methods were approved by the ethical committee of the University of Palermo (approval n. 25/2020). The experiments were done according to the principles of the Declaration of Helsinki.
Neuropsychological assessment
Participants underwent a neuropsychological assessment using the following tests: Symbol Digit Modalities Test -SDMT28, Modified Five Point Test – MFPT29, short version of the Stroop Colour-Word Test30, Raven’s Advanced Progressive Matrices49, Phonological Word Fluency – FAS31, Beck Depression Inventory -BDI32, Hamilton Depression Rating Scale -HDRS33.
Short-term memory and long-term memory experimental tasks
Two parallel versions of Digit span, Corsi span, Supraspan verbal learning, and Supraspan spatial learning tasks were used.
The subjects had to memorize a series of digits (Digit-Span test), the position of cubes (Corsi-Block test), the sequences of digits (Supraspan verbal learning task), and the positions of cubes (Supraspan spatial learning task).
In the short-term memory tasks, a random series of auditory presented digits (Digit span) or a random series of visually presented spatial positions (cubes: Corsi span) were used. Subjects were asked to repeat the sequence (verbally in the Digit span task and with manual pointing in the Corsi span task) in the same order as it was presented. The participant’s span was considered the longest number of sequential digits or spatial positions the subjects could accurately remember. For the Digit Span and Corsi Span tests, the score range is 0–9.
In the long-term memory tasks, Supraspan spatial and verbal learning tasks were used.
In the Supraspan spatial learning tasks, the examiner presented the set series of 8 spatial positions (cubes) at the rate of one cube every 2 s. After the presentation of each series, the examiner asked the subject to reproduce it and then recorded the sequence of cubes touched by the subject. The sequence was repeated until the learning criterion was reached (three exact consecutive repetitions) or up to a maximum of 18 tests. Five minutes after the end of the last test, the reproduction of the sequence of cubes was requested without prior demonstration of the same. An interfering verbal activity, with subjects speaking to the experimenter, occupied the 5 min delay period.
For each test, all the cubes touched in the correct order, and their combinations (“chunks”) were computed, and the corresponding informational score was attributed to each test50. For the Supraspan Spatial learning test, the score range is 0-2951(see Table 9 supplementary materials).
In the Supraspan verbal learning tasks, the same experimental study, procedure, and scoring method were used except for the material (digits instead of cubes).
The duration of the memory tasks was approximately 25 min.
Prism adaptation procedure
The procedure for PA was the same as that adopted in previous studies10.
The L-PA group wore prismatic lenses inducing a leftward 10° shift of the visual field, and the R-PA group wore prismatic lenses inducing a rightward 10° shift of the visual field. The NL group wore neutral lenses, i.e. not inducing any shift of the visual field.
During PA, subjects were seated at a table in front of a box (height = 30 cm, depth = 34 cm at the centre and 18 cm at the periphery, width = 72 cm) open both on the side facing the subjects and on the opposite side facing the experimenter. The experimenter placed a visual target at the distal edge of the top surface of the box in one of three possible positions: a central position (0°), 21° to the left of the centre, and 21° to the right of the centre. Each position was randomly determined on each trial. Subjects had to maintain their right hand at the level of the sternum and then had to point toward the visual target using the index finger; the experimenter recorded the end position of the subject’s pointing direction.
In the invisible pointing trials (open-loop), the subjects’ arm was covered by a black sheet so that the subjects could not see any part of the arm’s trajectory.
In the visible pointing trials (closed-loop), the arm was covered only in the proximal part so that the subjects could see the last third of the trajectory of the pointing movement.
The pointing task was performed in three conditions: pre-exposure, exposure, and post-exposure. In the pre-exposure condition, 60 pointing trials were administered without prismatic lenses, 30 with vision of the pointing and 30 without vision of the pointing. In the exposure condition, 90 trials in visible pointing were administered to subjects wearing prismatic lenses that induced a 20° shift of the visual field to the right or the left. In the post-exposure condition, after the removal of the prisms, 30 trials in invisible pointing were administered52.
All the trials were equally and randomly distributed in the three marked positions of the panel.
The duration of the entire procedure was approximately 15 min (pre-exposure closed loop condition: 3 min; pre-exposure open loop condition: 3 min; exposure condition: 6 min; post-exposure condition: 3 min).
Experimental procedure
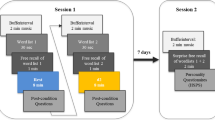
The L-PA, the R-PA and NL groups participated in two testing sessions over two separate days, with an interval of seven days between sessions in order to minimize the influence of task repetition on the results. The time of day (morning) and experimental conditions of the applied tasks were maintained the same in the two sessions (Fig. 10).
In the first testing session, subjects were given the cognitive tests and the experimental memory tasks consisting of Digit span, Spatial span, Supraspan verbal learning, and Supraspan spatial learning tasks.
In the second testing session, participants were first administered the PA procedure (L-PA, R-PA or NL), immediately followed by the parallel versions of experimental memory tasks.
The order of administration of the two versions of memory tasks was counterbalanced and randomly assigned to the recruited subjects.
Statistical analyses
Memory tasks
Behavioral data were analyzed with an ANOVA, with Group (L-PA, R-PA, NL) as a between-subjects factor and Session (pre-PA, post-PA) as a within-subjects factor.
Post-hoc analyses were conducted using Tukey’s test. Effect size is reported as partial eta square.
Prism adaptation
Aftereffect
To measure the aftereffect following the prisms’ removal, the dependent measure was the mean displacement (measured in degrees of visual angle) of the subjects’ invisible pointing responses in the post-exposure vs. pre-exposure conditions (Magnani et al., 2020). An ANOVA was conducted, with Group (L-PA; R-PA; NL) as between-subjects and Session (pre-exposure, post-exposure) as the within-subjects variable. Whenever necessary, post hoc comparisons were performed using Tukey’s test. Effect size is reported as partial eta square.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rossetti, Y., Kitazawa, S. & Nijboer, T. Prism adaptation: from rehabilitation to neural bases. Cortex J. Devoted Study Nerv. Syst. Behav. 111, A1–A6 (2019).
Bonnet, C., Poulin-Charronnat, B., Vinot, C., Bard, P. & Michel, C. Cross-modal aftereffects of visuo-manual prism adaptation: transfer to auditory divided attention in healthy subjects. Neuropsychology 36, 64–74 (2022).
Nijboer, T. C. W., McIntosh, R. D., Nys, G. M. S., Dijkerman, H. C. & Milner, A. D. Prism adaptation improves voluntary but not automatic orienting in neglect. Neuroreport 19, 293–298 (2008).
Frassinetti, F., Magnani, B. & Oliveri, M. Prismatic lenses Shift Time Perception. Psychol. Sci. 20, 949–954 (2009).
Oliveri, M., Magnani, B., Filipelli, A., Avanzi, S. & Frassinetti, F. Prismatic adaptation effects on spatial representation of time in neglect patients. Cortex J. Devoted Study Nerv. Syst. Behav. 49, 120–130 (2013).
Striemer, C. L. & Danckert, J. Dissociating perceptual and motor effects of prism adaptation in neglect. Neuroreport 21, 436–441 (2010).
Michel, C., Bonnet, C., Podor, B. & Bard, P. Poulin-Charronnat, B. wearing prisms to hear differently: after-effects of prism adaptation on auditory perception. Cortex J. Devoted Study Nerv. Syst. Behav. 115, 123–132 (2019).
Di Marco, J. et al. Regression of left hyperschematia after prism adaptation: a single case study. Cortex J. Devoted Study Nerv. Syst. Behav. 119, 128–140 (2019).
Schintu, S., Freedberg, M., Alam, Z. M., Shomstein, S. & Wassermann, E. M. Left-shifting prism adaptation boosts reward-based learning. Cortex J. Devoted Study Nerv. Syst. Behav. 109, 279–286 (2018).
Turriziani, P. et al. Improvement of phonemic fluency following leftward prism adaptation. Sci. Rep. 11, 7313 (2021).
Michel, C. Simulating unilateral neglect in normals: myth or reality? Restor. Neurol. Neurosci. 24, 419–430 (2006).
Michel, C. Beyond the Sensorimotor plasticity: cognitive expansion of prism adaptation in healthy individuals. Front. Psychol. 6, 1979 (2015).
Michel, C. et al. Simulating unilateral neglect in normals using prism adaptation: implications for theory. Neuropsychologia 41, 25–39 (2003).
Colent, C., Pisella, L., Bernieri, C., Rode, G. & Rossetti, Y. Cognitive bias induced by visuo-motor adaptation to prisms: a simulation of unilateral neglect in normal individuals? Neuroreport 11, 1899–1902 (2000).
Berberovic, N. & Mattingley, J. B. Effects of prismatic adaptation on judgements of spatial extent in peripersonal and extrapersonal space. Neuropsychologia 41, 493–503 (2003).
Clarke, S., Farron, N. & Crottaz-Herbette, S. Choosing sides: impact of prismatic adaptation on the lateralization of the Attentional System. Front. Psychol. 13, 909686 (2022).
Magnani, B., Caltagirone, C. & Oliveri, M. Prismatic adaptation as a novel tool to directionally modulate motor cortex excitability: evidence from paired-pulse TMS. Brain Stimulat 7, 573–579 (2014).
Bracco, M., Mangano, G. R., Turriziani, P., Smirni, D. & Oliveri, M. Combining tDCS with prismatic adaptation for non-invasive neuromodulation of the motor cortex. Neuropsychologia 101, 30–38 (2017).
Bracco, M., Veniero, D., Oliveri, M. & Thut, G. Prismatic adaptation modulates Oscillatory EEG correlates of Motor Preparation but not visual attention in healthy participants. J. Neurosci. Off J. Soc. Neurosci. 38, 1189–1201 (2018).
Schintu, S. et al. Paired-pulse parietal-motor stimulation differentially modulates Corticospinal excitability across hemispheres when combined with prism adaptation. Neural Plast. 5716179 2016 (2016).
Schintu, S., Gotts, S. J., Freedberg, M., Shomstein, S. & Wassermann, E. M. Effective connectivity underlying neural and behavioral components of prism adaptation. Front. Psychol. 13, 915260 (2022).
Redding, G. M. & Wallace, B. Generalization of prism adaptation. J. Exp. Psychol. Hum. Percept. Perform. 32, 1006–1022 (2006).
Geva, S. et al. Lesions that do or do not impair digit span: a study of 816 stroke survivors. Brain Commun. 3, fcab031 (2021).
Chechlacz, M., Rotshtein, P. & Humphreys, G. W. Neuronal substrates of Corsi Block span: lesion symptom mapping analyses in relation to attentional competition and spatial bias. Neuropsychologia. 64, 240–251 (2014).
De Renzi, E., Faglioni, P. & Previdi, P. Spatial memory and hemispheric locus of lesion. Cortex J. Devoted Study Nerv. Syst. Behav. 13, 424–433 (1977).
Mock, N. et al. Lesion-symptom mapping corroborates lateralization of verbal and nonverbal memory processes and identifies distributed brain networks responsible for memory dysfunction. Cortex J. Devoted Study Nerv. Syst. Behav. 153, 178–193 (2022).
Okamoto, M. et al. Process-specific prefrontal contributions to episodic encoding and retrieval of tastes: a functional NIRS study. NeuroImage 54, 1578–1588 (2011).
Nocentini, U., Giordano, A., Di Vincenzo, S., Panella, M. & Pasqualetti, P. The Symbol Digit modalities test - oral version: Italian normative data. Funct. Neurol. 21, 93–96 (2006).
Cattelani, R., Dal Sasso, F., Corsini, D. & Posteraro, L. The modified five-point test: normative data for a sample of Italian healthy adults aged 16–60. Neurol. Sci. 32, 595–601 (2011).
Carraffa, P., Vezzadini, G., Dieci, F., Zonato, F. & Venneri, A. Una versione abbreviata del test di Stroop: Dati normativi nella popolazione Italiana. Nuova Riv Neurol. 12, 111–115 (2002).
Carlesimo, G. A. et al. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur. Neurol. 36, 378–384 (1996).
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J. & Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571 (1961).
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62 (1960).
Jacquin-Courtois, S. et al. Rehabilitation of spatial neglect by prism adaptation: a peculiar expansion of sensorimotor after-effects to spatial cognition. Neurosci. Biobehav Rev. 37, 594–609 (2013).
Schintu, S. et al. The asymmetrical effect of leftward and rightward prisms on intact visuospatial cognition. Cortex J. Devoted Study Nerv. Syst. Behav. 97, 23–31 (2017).
Cremonini, W., De Renzi, E. & Faglioni, P. Contrasting performance of right- and left-hemisphere patients on short-term and long-term sequential visual memory. Neuropsychologia 18, 9 (1980).
Oliveri, M. et al. A novel digital approach for post-stroke cognitive deficits: a pilot study. Restor. Neurol. Neurosci. https://doi.org/10.3233/RNN-231305 (2023).
Danesin, L. et al. Prism adaptation in patients with unilateral lesion of the parietal or cerebellar cortex: a pilot study on two single cases using a concurrent exposure procedure. Neuropsychologia 184, 108557 (2023).
Luauté, J. et al. Dynamic changes in brain activity during prism adaptation. J. Neurosci. Off J. Soc. Neurosci. 29, 169–178 (2009).
Panico, F., Sagliano, L., Grossi, D. & Trojano, L. Cerebellar cathodal tDCS interferes with recalibration and spatial realignment during prism adaptation procedure in healthy subjects. Brain Cogn. 105, 1–8 (2016).
Pisella, L. et al. Preserved prism adaptation in bilateral optic ataxia: strategic versus adaptive reaction to prisms. Exp. Brain Res. 156, 399–408 (2004).
Ballard, H. K., Goen, J. R. M., Maldonado, T. & Bernard, J. A. Effects of cerebellar transcranial direct current stimulation on the cognitive stage of sequence learning. J. Neurophysiol. 122, 490–499 (2019).
Gheysen, F. et al. Taking the brakes off the learning curve. Hum. Brain Mapp. 38, 1676–1691 (2017).
Panico, F., Rossetti, Y. & Trojano, L. On the mechanisms underlying prism adaptation: a review of neuro-imaging and neuro-stimulation studies. Cortex J. Devoted Study Nerv. Syst. Behav. 123, 57–71 (2020).
Aleman, A., Van, T. & Wout, M. Repetitive Transcranial Magnetic Stimulation over the Right Dorsolateral Prefrontal Cortex disrupts Digit Span Task Performance. Neuropsychobiology. 57, 44–48 (2008).
Kaneko, H. et al. Hemodynamic changes in the Prefrontal Cortex during Digit Span Task: a Near-Infrared Spectroscopy Study. Neuropsychobiology. 63, 59–65 (2011).
Tulving, E., Kapur, S., Craik, F. I., Moscovitch, M. & Houle, S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc. Natl. Acad. Sci. U S A. 91, 2016–2020 (1994).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 39, 175–191 (2007).
Raven, J. C. Advanced Progressive Matrices (HK Lewis, 1962).
Spinnler, H. & Tognoni, G. Taratura E standardizazione italiana di test neuropsicologici. Italian Neurol. Sci. 7, 1–19 (1987).
Capitani, E., Grossi, D., Lucca, U., Orsini, A. & Spinnler, H. Spatial and color cues in a route-learning task. Acta Neurol. (Napoli). 2, 305–314 (1980).
Magnani, B., Musetti, A. & Frassinetti, F. Spatial attention and representation of time intervals in childhood. Sci. Rep. 10, 14960 (2020).
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript in a meaningful way. F.C., G.R.M., R.E.B. were salient in discussing and collecting data. P.T and M.O. were salient in conceptualizing and supervisioning the conduction of the experiment, the discussion of the results and the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Turriziani, P., Campo, F., Bonaventura, R.E. et al. Modulation of memory by prism adaptation in healthy subjects. Sci Rep 14, 25358 (2024). https://doi.org/10.1038/s41598-024-77027-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77027-z