Abstract
Dilated cardiomyopathy (DCM) is a progressive myocardial disorder characterized by impaired cardiac contraction and ventricular dilation. However, some patients with DCM improve when experiencing left ventricular reverse remodeling (LVRR). Currently, the detailed association between genotypes and clinical outcomes, including LVRR, particularly among children, remains uncertain. Pediatric patients with DCM from multiple Japanese institutions recorded between 2014 and 2023 were enrolled. We identified their DCM-related genes and explored the association between gene variants and clinical outcomes, including LVRR. We included 123 pediatric patients (62 males; median age: 8 [1–51] months) and found 50 pathogenic variants in 45 (35.0%) of them. The most identified gene was MYH7 (14.0%), followed by RYR2 (12.0%) and TPM1 (8.0%). LVRR was achieved in 47.5% of these patients. The left ventricular ejection fraction remained unchanged (31.4% to 39.8%, P = 0.1913) in patients with sarcomere gene variants and in those with non-sarcomere gene variants (33.4% to 47.8%, P = 0.0522) but significantly increased in those without gene variants (33.6% to 54.1%, P < 0.0001). LVRR was not uniform across functional gene groups. Hence, an individualized gene-guided prediction approach may be adopted for children with DCM.
Similar content being viewed by others
Introduction
Dilated cardiomyopathy (DCM) is a myocardial disorder which is characterized by impaired cardiac contraction and dilation of the left ventricle (LV) or both ventricles without clear etiologies, with a prevalence rate of approximately 1 in 2,500 adults1,2. In the pediatric population, the annual incidence rate is considerably lower, with estimates of 1 in 170,000 in the US, 1 in 140,000 in Australia, and 1 in 200,000 in Japan3,4. Although pediatric DCM has a lower annual incidence than that in adults, its clinical outcomes can be particularly severe, with the potential for heart failure and fatal outcome. Moreover, life-threatening cardiac outcomes were recorded to be 42% for heart transplants and accounted for 5% of deaths in the US5,6.
The occurrence of multiple DCM in patients within a single pedigree indicates genetic contributions7,8. Recently, approximately 30%–40% of DCM cases have been reported to be caused by pathogenic gene variants, with over 50 genes likely associated with the condition1,9,10. The pathophysiology of DCM involving genetic factors is linked to genes encoding sarcomere proteins, cellular cytoskeleton components, nuclear membrane proteins, and ion channels. However, most genetic studies have been limited to adults. Consequently, the genetic causes of primary DCM occurring during childhood remain largely unknown. Despite recommendations for genetic testing in pediatric cardiomyopathy, clinical practice varies because of the lack of larger-scale studies in children. The identification of these variants is especially important in pediatric cases, where the disease often presents more aggressively and with different genetic profiles compared to adult-onset DCM. By understanding the genetic underpinnings of pediatric DCM, clinicians can improve risk stratification, tailor medical therapy, and potentially develop new therapeutic targets that could alter the course of the disease.
Heart remodeling in response to myocardial stress is a characteristic feature of DCM. Recently, several cohort studies have demonstrated that a process generally referred to as left ventricular reverse remodeling (LVRR) can reverse DCM in some patients. Therefore, DCM may not follow an irreversible progression of myocardial damage but rather represent a reversible and dynamic disease if correctly treated11,12. Successful LVRR has also been reported in pediatric patients with DCM13,14,15. However, specific genotypes associated with LVRR in children remain unclear11.
Given that genetic data on pediatric cardiomyopathy are limited, this study aimed to investigate the genetic structure of pediatric-onset DCM through a nationwide survey in Japan and to examine the association between disease-causing genes and LVRR.
Results
Baseline clinical characteristics
This study enrolled 123 patients (62 males and 61 females; median age, 8 [1–51] months) (Table 1). The median follow-up duration was 25 (4.0–58) months. Figure 1 presents the distribution of age at DCM onset. Most patients were diagnosed under 1 year of age, and a minimal peak was observed during school age.
Table 1 details the characteristics of the included patients. DCM diagnosis was made through clinical symptoms in 89 (72.4%) patients and fetal screening in 10 (8.1%) patients. Perinatal abnormalities were observed in 14 (11.4%) patients. Furthermore, 86 (70.0%) patients showed electrocardiographic abnormalities. Ventricular tachycardia was observed in 3 (2.4%) patients and ventricular fibrillation in 1 (0.8%). None had supraventricular tachycardia or bradycardia, such as complete atrioventricular block and sick sinus syndrome.
A high cardiothoracic ratio and pulmonary congestion on chest X-ray were frequently observed. Patients with DCM had high levels of plasma brain natriuretic peptide (BNP) (191.4 [22.0–1,492.2 pg/mL]). Moreover, a reduced left ventricular ejection fraction (LVEF) (33.5% [23.0%–49.5%]) and high Z-scores in left ventricular diastolic diameter (LVDD) (5.1 [2.6–8.0]) were common.
Both LVEF and LVDD changed from baseline to follow-up. LVEF at follow-up significantly increased compared with that at baseline (33.5% [23.0%–49.5%] to 54.0% [28.2%–67.9%], P < 0.0001), as illustrated in Fig. 2. Similarly, the Z-scores of LVDD at follow-up significantly decreased compared with those at baseline (5.1 [2.6–8.0] to 1.8 [0.9–4.7], P < 0.0001), also depicted in Fig. 2.
Comparison of serial echocardiographic data for the entire cohort and among patients according to variants. Left ventricular ejection fraction (LVEF) from baseline to follow-up for the entire cohort (A) and among patients according to variants: sarcomere gene variant (n = 7), non-sarcomere gene variant (n = 12), and negative variant (n = 22). (B) Left ventricular diastolic diameter (LVDD) Z-score from baseline to follow-up for the entire cohort (C) and among patients according to variants: sarcomere gene variant (n = 7), non-sarcomere gene variant (n = 12), and negative variant (n = 22) (D).
The clinical features and anatomical characteristics of the infantile cases of LVNC (age at presentation less than 2 years) were compared with juvenile cases (age at presentation 2 years and older). The baseline characteristics, genetic testing results, and clinical outcomes for these age groups are detailed in Table 1. Our analysis revealed distinct differences in the prevalence of specific genetic variants and clinical outcomes across these age groups.
Genetic and phenotypic analyses
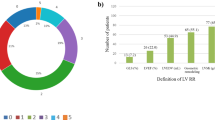
Figure 3 and Table S1 present the distribution of pathogenic variants. We found 50 pathogenic variants (41 missense, 3 deletions, 5 nonsense, and 1 splicing variants) in 45 patients (35.0%) with DCM. The most commonly identified gene was MYH7 (n = 7, 14.0%), followed by RYR2 (n = 6, 12.0%) and TPM1 (n = 4, 8.0%). Additionally, 5 (4.1%) patients had multiple variants.
To elucidate the genotype–phenotype correlation, we divided the patients into six groups according to gene function: (1) sarcomere group (patients with the sarcomere gene variants ACTC1, MYH6, MYH7, MYPN, TNNC1, TNNI3, TNNT2, TPM1, and TTN), (2) cytoskeleton group (patients with the cytoskeleton gene variants DES, DTNA, FLNC, SGCD, and VCL), (3) ion channel group (patients with the ion channel gene variant RYR2), (4) desmosomal group (patients with the desmosomal gene variants DSG2 and PKP2), (5) nuclear envelope group (patients with the heart development gene variants EMD and LMNA), and (6) other variants. Variants in genes associated with the sarcomere, cytoskeleton, ion channel, desmosomal, and nuclear envelope groups accounted for 46.0% (n = 23), 12.0% (n = 6), 12.0% (n = 6), 8.0% (n = 4), and 4.0% (n = 2), respectively, in all patients (Fig. 3).
Almost all baseline data did not reveal any differences between individuals with and without sarcomere gene variants, except for details regarding diagnostic chance, but patients with sarcomere gene variants had fewer symptoms and high fetal screening (Table 2). Most patients with variants in the MYH7 gene were diagnosed younger than the other patients (Table S2).
Notably, we identified a novel variant in the CASZ1 gene (NM_001079843.2 c.3356G > A, p. Trp1119Ter) (Table S1). One patient with DCM had an MYL3 variant (NM_000258.2 c.170C > G, p. Ala57Gly), which was previously reported in several patients with hypertrophic cardiomyopathy (HCM) (Table S1).
We then analyzed the distribution of these gene variants among the two age groups. In the group of children younger than 2 years, the variants in the sarcomere, cytoskeleton, ion channel, desmosomal, nuclear envelope, and other gene groups accounted for 46.0% (n = 14), 18.0% (n = 5), 10.0% (n = 3), 10.0% (n = 3), 0.0% (n = 0), and 16.0% (n = 5), respectively. In contrast, in the group of children 2 years or older, these variants accounted for 42.1% (n = 8), 15.8% (n = 3), 15.8% (n = 3), 10.5% (n = 2), 10.5% (n = 2), and 5.3% (n = 1), respectively. Further stratification within the pathogenic/likely pathogenic (PLP) variants group revealed that, in the younger than 2 years group, sarcomere gene variants were predominant at 75.0% (n = 9), with cytoskeleton variants at 16.7% (n = 2), and no detected ion channel, nuclear envelope, or other variants. On the other hand, the 2 years or older group exhibited a more diverse distribution, with sarcomere variants at 30.0% (n = 3), cytoskeleton variants at 20.0% (n = 2), ion channel variants at 10.0% (n = 1), and nuclear envelope and other variants at 20.0% (n = 2) each. In summary, the group of children younger than 2 years shows a higher prevalence of sarcomere and other gene variants, while the group of children 2 years or older has a higher occurrence of ion channel and nuclear envelope gene variants, with a more even distribution across different gene function categories. This data underscores the importance of age-based stratification in the analysis of genotype–phenotype correlations, particularly in pediatric cardiomyopathy, and suggests that genetic variation may influence clinical outcomes differently across age groups.
In addition, we classified the variants detected in our cohort according to the ACMG guidelines, focusing only on pathogenic and likely pathogenic variants while excluding variants of unknown significance (VUS). Table 2 provides a detailed breakdown of the baseline characteristics stratified by pathogenic and likely pathogenic variants, VUS, and variant-negative groups. As shown in Table 2, we observed that the exclusion of VUS from our analysis did not significantly alter the distribution of key clinical characteristics across the groups.
Interestingly, the sequential changes in LVEF and LVDD Z-scores between baseline and follow-up were specifically characterized according to gene variants. During the observation period, significant differences were observed in the LVEF changes for and the negative variant group ((33.4 [24.0–46.2] to 47.8 [28.3–61.7], P < 0.0001), while the sarcomere and non-sarcomere gene variant group did not show significant change (31.4 [17.2–53.0] to 39.8 [20.0–62.0] and 33.6 [27.0–55.6] to 54.1 [35.0–69.5], P = 0.1913 and P = 0.0522, respectively) (Fig. 2). Similarly, the LVDD Z-score at follow-up in patients with sarcomere gene variants and non-sarcomere gene variants remained unchanged during the observation period (5.35 [1.88–8.40] to 3.11 [− 1.24 to 4.54] and 4.42 [1.58–7.05] to 1.60 [0.95–5.12], P = 0.117 and P = 0.0593, respectively), whereas that in patients with negative gene variants significantly increased (5.37 [3.27–7.74] to 4.27 [2.51–7.63], P = 0.0057) (Fig. 2). The echocardiographic follow-up data, presented in Figure S1, showed that patients with pathogenic or likely pathogenic variants exhibited a less pronounced improvement in LVEF over time compared to those with VUS or those without any detected variants. Specifically, the LVEF in patients with pathogenic or likely pathogenic variants improved from 32.5% (16.0–54.0) at baseline to 33.2% (23.5–54.0) at follow-up (P = 0.8807). In contrast, patients with VUS and those without variants showed significant LVEF improvement (Figure S1).
At 1 year after the initial echocardiogram at baseline, LVRR developed in 47.5% of all patients, 45.0% of the variant-positive group, and 50.0% of the variant-negative group; however, the statistical difference was not significant because of the small sample size (P = 0.483) (Fig. 4). LVRR also differed among the functional gene groups, although the difference was not statistically significant (Fig. 4). The worst response was observed in the sarcomere gene group (43%), followed by the non-sarcomere gene group (46%); however, owing to the small sample size, the statistical difference was insignificant (P = 0.942). Analysis of LVRR, illustrated in Figure S2, indicated that patients with pathogenic or likely pathogenic variants were less likely to achieve LVRR compared to those with VUS or no variants. LVRR was observed in 43% of patients with pathogenic or likely pathogenic variants, while it was 50% in those without detected variants. However, these differences were not statistically significant, likely due to the limited sample size (P = 0.942). Likewise, LVRR showed no significant differences between the pharmacological therapies, including guideline-directed medical therapy (Figure S3).
Clinical outcomes
Among patients with DCM, 22 (20.4%) were hospitalized (Table 1). A total of 111 patients (90.2%) received treatment, with guideline-directed medical therapy (ACE inhibitor/angiotensin receptor blocker, β-blocker, and/or mineralocorticoid) being the most frequent.
Adverse events were noted in 23 (18.7%) patients, 18 of whom (14.6%) died because of cardiac death, and 6 (4.9%) patients underwent heart transplantation (Table 1). None of the patients had LV assist device implantation or appropriate implantable cardioverter defibrillator shock. Figure 5 illustrates the event-free survival from the date of DCM diagnosis. At 1 and 5 years after diagnosis, survival rates were 90% and 80%, and freedom from major adverse cardiac events (MACEs) including cardiac death, LV assist device implantation, heart transplantation, and appropriate implantable cardioverter-defibrillator (ICD) was 90% and 75%, respectively (Fig. 5A, B). As displayed in Figure S5, event-free survival was compared across the groups. The survival analysis revealed no significant difference in the incidence of MACE between the groups, with a slightly lower survival rate in the pathogenic/likely pathogenic variant group compared to the other groups, although this was not statistically significant (P = 0.6653). The Figure S6 illustrates the survival and event-free survival rates across these age groups, providing no significant difference between these groups.
Kaplan–Meier analysis and time from diagnosis to life-threatening cardiac outcome. Event-free survival (A) and freedom from major adverse cardiac events (MACE) (B) in patients with dilated myocardiopathy (DCM). Event-free survival in patients with DCM (C) according to age (D) and plasma BNP levels. Kaplan–Meier curves were compared using the log-rank test.
Early infantile patients with DCM had a poorer prognosis than older pediatric patients (P = 0.044) (Fig. 5C). High plasma BNP levels (more than 500 pg/mL) were found to be an independent risk factor for survival (odds ratio, 7.32; 95% confidence interval, 1.56–54.20; P = 0.0010) (Table 3 and Fig. 5D). Meanwhile, mortality or MACE did not significantly differ according to gene function (P = 0.6653 and P = 0.5787, respectively), pathogenic variants (P = 0.6799 and P = 0.5282, respectively), and age at diagnosis (P = 0.7529 and P = 0.5435, respectively) (Figure S4-6). The area under the curve was 0.7646 for BNP to predict survival.
Discussion
Genetic research has made significant strides in elucidating the genetic contributions of cardiomyopathy; however, these studies are largely limited to adults. The genetic structure of pediatric-onset DCM must also be evaluated. In this study, we established a cohort of 123 pediatric patients with DCM. First, we identified patient’s age at diagnosis and predicted the risk factors for survival. Second, we found clinically pathogenic variants in 35% of pediatric cases, with the most common genes not being shared between pediatric and adult cases. Third, we evaluated the genetic structure of pediatric DCM and identified LVRR according to the genetic status.
In this study, an analysis of genetic testing for cardiomyopathy in the largest pediatric cohort with genetically evaluated primary DCM identified disease-causing variants in 35% of patients. In this proband, the detection rate of causal variants is consistent with the results obtained from adult DCM studies and other pediatric cohorts16,17,18. Additionally, genetic studies that identified variants in patients with pediatric DCM revealed age-related differences in phenotype expression according to the affected genes19,20. Contrary to adult data, the prevalence of variants in sarcomere genes was high in this pediatric cohort. These variants are more evenly distributed among genes encoding cytoskeletal, nuclear, mitochondrial, and calcium-handling proteins21. The absence of hotspots or repetitive variants and a high prevalence of private variants have been reported. While LMNA, RBM20, and PLN are common in adults, RBM20 and PLN variants were noted in only 4% and 2% of our pediatric cases. However, we found no LMNA variants. MYH7 and RYR2 variants, which are frequently identified in other pediatric DCM cohorts, accounted for 26% of the disease-causing variants in our cohort. Patients with MYH7 variants all exhibited symptoms in infancy, whereas those with RYR2 variants demonstrated symptoms at two different periods. MYH7 variants have been reported in only 3%–4% of adult-onset DCM cases; however, in this pediatric cohort, 10% had MYH7 variants22,23. Interestingly, some variants are known to cause HCM24. MYH7-related DCM features include an early onset, which corroborates with the features observed in our cases25. In variant-positive pediatric DCM cases, two (4%) patients had TTN truncating variants; this proportion is lower than that reported in previous studies6,26,27. Several reports have indicated an association between TTN variants and pediatric DCM26,27. In one study, 9% of variant-positive pediatric DCM cases had TTN truncating variants6. Those who developed DCM in their teens were more likely to possess TTN variants by fourfold than those who were diagnosed at a younger age. Our data of a lower prevalence of TTN truncating variants may reflect an age difference in our study compared with another previous research. Furthermore, we identified two novel genes associated with DCM: a CASZ1 variant (NM_001079843.2 c.3356G > A, p. Trp1119Ter) and a MYL3 variant (NM_000258.2 c.170C > G, p. Ala57Gly). CASZ1 is a para-zinc finger transcription factor necessary for vertebrate heart development28. Currently, this gene has only three known variants, namely, Leu38Pro, Lys351Term, and Val815Profs*1529,30,31,32. All of these are loss-of-function variants located in the coding region and may be associated with DCM. CASZ1 variants may result in haploinsufficiency caused by nonsense-mediated decay, which is important in the pathological mechanism of DCM29. A variant in the MYL3 gene is a sarcomere gene that was previously reported in several HCM cases and was identified in a patient with DCM.
This study investigated the genetic basis of Japanese patients with DCM and explored the association between genotypes and phenotypes. Results revealed an association between LVRR and the genotype. LVRR occurs in approximately 40% of patients with DCM under medical treatment11,33. Recently, LVRR was observed with optimal oral treatment in five Japanese pediatric patients with early onset DCM34. While LVRR achievement is associated with a favorable prognosis in DCM cases, specific genotypes involved in LVRR are unknown in pediatric patients11. In our study, LVRR occurred more frequently in variant-negative patients than in variant-positive patients, and sarcomere genes showed a strong inverse correlation with LVRR. Recent studies have supported the finding that, compared with the favorable prognosis in the variant-negative group, LVRR occurrence is lower in the sarcomere and desmosome gene groups10,35. Other groups have studied LVRR incidence in patients with DCM by using different definitions. These criteria were based on LVEF and LVDD improvement11,36. Our study focused on pediatric patients, and LVDD varied with age. Thus, LVDD was deemed inappropriate, and this criterion was not used. Although the baseline and follow-up values of LVEF and LVDD significantly differed according to the genetic type, no significant difference was found in LVRR because of the limited sample size. Future studies should accumulate more cases for further validation.
The distribution of onset age dominated in the younger age group, especially those under 1 year of age. In Japan, heart disease screening is conducted in schools to determine those who need further examination and to manage school activities for those with underlying heart diseases37. School-based screening programs have been reported to be beneficial for the early diagnosis of arrhythmia and cardiomyopathies. Regarding left ventricular noncompaction (LVNC), school screening accounted for 41.9% of diagnosed patients in the overall pediatric patients and 59.5% of school children38. However, in the current study, while a minimal peak was observed in DCM cases that are likely detected in the school age period, it is not as pronounced as that observed with LVNC. This discrepancy may be explained by the differences in the proportion of patients manifesting clinical symptoms; patients with DCM tend to exhibit clinical symptoms at a younger age, whereas patients with LVNC are asymptomatic and incidentally diagnosed during school screenings. At 1 and 5 years after diagnosis in our cohort, the survival rates were high (90% and 80%, respectively), as well as the rates of freedom from death or heart transplantation (90% and 75%, respectively). A recent Japanese cohort study reported that the survival rates at 1 and 5 years after diagnosis were 81% and 75%, respectively, and the rates of freedom from death or heart transplantation were 76% and 64%, respectively39. The difference in outcomes between these two studies can be attributed to the fact that our study targeted patients from 2014 to 2023, whereas the previous Japanese cohort study included patients from 1990 to 2014, reflecting differences in medical practices over different time periods. These tendencies of the survival rates or heart transplantation were similar to the US data (84% and 76% at 1 and 5 years, respectively)40. In the Australian cohort study, the survival rates or heart transplantation at 1 and 5 years after diagnosis were 74% and 65%, respectively14,41. Unlike our study, patients with clear evidence of myocarditis were included in this Australian study. The long-term incidence of death or heart transplantation in the pediatric population is markedly higher than that in the adult population, as reported in the US, Australia, and Japan4,42,43,44. This result may be attributed to genetic causes; for instance, TTN variants were predominantly found in adult patients but were rare in pediatric patients in our study. Our report shows a better prognosis than previously reported results, emphasizing the importance of LVRR.
In our multivariate analysis, elevated plasma BNP levels were identified as an independent risk factor for survival. Additionally, a younger age may be a risk factor for survival. In pediatric cohorts, similar to N-terminal-pro-BNP, reduced LV function, older age, familial cardiomyopathy, and heart rate increase have been recognized as risk factors for survival in pediatric patients with DCM14,39,40,41,45,46. Meulen et al. conducted multivariate analysis and reported that N-terminal-pro-BNP was the sole independent predictor of adverse outcomes47. Mori et al. found that a reduced LV function at baseline was associated with an increased risk of death or heart transplantation in Japan, as well as in Australia and the US, supporting the results of our univariate analysis14,40,41. Older age and familial cardiomyopathy were also previously linked to an increased risk of death or heart transplantation14,40,41,45. However, in our study, both were not significant risk factors. The reasons for this difference remain unclear but may be multifactorial, including factors such as age distribution, racial differences, and differences in the study period. In another study using univariate analysis, an elevated heart rate was associated with an increased risk of death and the combined outcome of death or heart transplantation46. Unfortunately, we did not thoroughly evaluate the heart rate at baseline and follow-up in patients with DCM. Further research is required to validate these results.
While our study indicates that sarcomere gene variants did not lead to significant improvements in cardiac function among pediatric DCM patients, these findings highlight the complexity of genetic influences on disease progression. The presence of sarcomere gene variants, such as those in MYH7, may predispose patients to a less favorable response to standard heart failure therapies, suggesting the need for more targeted approaches. Understanding the role of these variants could lead to the development of individualized therapeutic strategies that better address the specific genetic makeup of each patient, potentially improving prognosis and offering new avenues for treatment. Furthermore, these insights could guide the future design of clinical trials focusing on gene-specific interventions, ultimately contributing to the advancement of precision medicine in pediatric cardiology.
Study limitations
This study had limitations in determining whether segregation analysis or de novo occurrence could explain certain variants, because some parental samples were unavailable. Some of our cases were registered over 5 years ago, and new treatments have been developed, potentially altering the outcomes. The selection of a next-generation sequencing panel targeting genes that are known to be associated with cardiac phenotype and development may have resulted in the oversight of unknown variants because information from the entire genome was not obtained. Furthermore, inadequate follow-up investigations are possible because of missing data in some cases. Given the small sample size, future large-scale studies are necessary to further elucidate genotype–phenotype correlations. Additionally, phenotype analysis through comprehensive diagnostics involving magnetic resonance imaging and pathological tissue examination would be desirable. In line with the current guidelines, our study focused on primary cardiomyopathy by excluding secondary forms such as metabolic, mitochondrial, and RASopathies. This exclusion may limit the generalizability of our findings, particularly in infants and toddlers, where these conditions are more prevalent. Future studies should aim to include these secondary cardiomyopathies to offer a more comprehensive view of the etiology and outcomes in young patients with cardiomyopathy.
Conclusions
Our results confirm that pediatric DCM exhibits marked genetic heterogeneity with a different landscape from adult DCM. DCM and risk factors for survival were predominant in younger patients. In addition, LVRR was not uniform across functional gene groups; thus, an individualized prediction approach in DCM on the basis of genetic features may be adopted.
Methods
Study population
From October 2014 to March 2023, a total of 123 Japanese probands with DCM were referred to our institution for genetic testing from 45 Japanese hospitals. The patients < 18 years of age and recently diagnosed with DCM. The exclusion criteria included children with secondary cardiomyopathies (e.g., neuromuscular, pulmonary, endocrine, rheumatic, and immunologic diseases; inborn errors of metabolism with multiple organ involvement; and congenital heart disease), cardiotoxic exposures, systemic hypertension, and those missing follow-up records.
Clinical data were retrieved from the patients’ medical records. The following clinical characteristics were collected during the initial presentation: (1) presence and nature of cardiac symptoms, (2) reason for referral, (3) presence of concomitant cardiac or extracardiac involvements, and (4) family history. Cardiac death, LV assist device implantation, heart transplantation, and appropriate ICD shock were classified as MACE.
According to institutional guidelines, informed consent was obtained from all patients or their parents. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by the a priori approval of the Research Ethics Committee of the University of Toyama, Japan.
Echocardiographic data
All echocardiographic data were analyzed by two independent reviewers (K.H. and S.O.) who were blinded to each other’s measurements. Echocardiography (2D, M-mode, and color Doppler) was used to evaluate cardiac structure, LV size and function (LVEF), and valve regurgitation. The thickness of the compacted layer in the LV and LVDD) were expressed as Z-scores, which were indexed to body surface area48.
DCM was defined as LV dilation with a decreased LV systolic function40,49. LV dilation was defined as an LV end‐diastolic dimension more than 2 standard deviations above the mean normal value for body surface area (LVDD Z score > 2), and LV systolic dysfunction was defined as an LVEF more than 2 standard deviations below the mean value for healthy children, which is the adjusted normal value for age (LVEF Z score of < 2, < 50%)40,49.
Given the nature of this study, we first identified patients diagnosed with DCM and then reviewed their echocardiograms to ensure that they met all inclusion criteria. HF was diagnosed based on clinical symptoms of feeding difficulty and tachypnea, decreased LVEF on echocardiography, and cardiomegaly on chest X-ray. Cardiomegaly was defined as a cardiothoracic ratio of ≥ 0.55 (≥ 0.60 for patients < 1 year old) on chest X-ray.
LVRR was defined as either LV normalization (LVEF improvement to ≧50% with a ≧5% LVEF increment on echocardiogram at 1 year after) or an absolute increase in LVEF by ≧10% on echocardiogram at 1 year after from initial echocardiogram at baseline, as described50,51.
Follow-up information
After the initial diagnosis and baseline evaluation, all patients were followed up regularly at intervals of 3 to 6 months, depending on the severity of their condition and the presence of clinical symptoms. Follow-up assessments included comprehensive clinical evaluations, echocardiography, electrocardiography, and laboratory testing, including measurements of BNP levels. During each follow-up visit, LVEF and LVDD Z-scores were measured to monitor cardiac function and structural changes over time. These measurements were compared with baseline values to assess the progression or regression of DCM. Any significant changes in these parameters were noted and correlated with clinical outcomes, including hospitalization, heart transplantation, and survival. Patients who exhibited significant clinical deterioration during follow-up were managed according to guideline-directed medical therapy and were re-evaluated more frequently to monitor the effectiveness of treatment. The follow-up data were meticulously recorded and used for statistical analysis to determine the impact of genetic variants on disease progression and outcomes.
Genetic testing using NGS
Genomic DNA was extracted from whole blood samples obtained from the patients using a QIAamp DNA Mini Kit (Qiagen, Redwood City, CA, USA). NGS of 203 cardiovascular disease–related genes associated with cardiomyopathies and channelopathies (Table S3) was conducted using the Ion PGM System (Thermo Fisher Scientific, Waltham, MA, USA). This custom panel used two separate polymerase chain reaction (PCR) primer pools, yielding a total of 6,138 amplicons and was used to generate target amplicon libraries. Genomic DNA samples were PCR-amplified using a custom panel and an Ion AmpliSeq Library Kit Plus (Thermo Fisher Scientific). Individual samples were labeled using the Ion Xpress Barcode Adapters Kit (Thermo Fisher Scientific) and then pooled at equimolar concentrations. Emulsion PCR and ion sphere particle enrichment were performed using the Ion PGM™ Hi-Q™ View OT2 Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Ion sphere particles were loaded onto a 318 chip and sequenced using the Ion PGM™ Hi-Q™ View Sequencing Kit (Thermo Fisher Scientific). The Torrent Suite and Ion Reporter Software version 5.0 (Thermo Fisher Scientific) were used to perform primary, secondary, and tertiary analyses, including the optimized signal processing, base calling, sequence alignment, and variant analysis.
Classification of pathogenicity
The variants were manually assessed based on detailed information obtained from ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php). They were classified according to the American College of Medical Genetics and Genomics guidelines.
Variant filtration
For subsequent filtering, minor allele frequencies were estimated using the Genome Aggregation Database (gnomAD) and the Japanese allele frequency in the Tohoku University Tohoku Medical Megabank Organization (Japanese Multi-omics Reference Panel [jMorp]; https://jmorp.megabank.tohoku.ac.jp). Filter impact ratings of the high (start loss, stop gain, stop loss, frameshift, and splice acceptor/donor) and moderate (missense and in-frame insertion/deletion and protein-altering) variants were then employed at a frequency of < 0.0005 in gnomAD, Tohoku University Tohoku Medical Megabank Organization 8.3KJPN (ToMMo 8.3KJPN), and a CADD score ≥ 20.
Selection of potential variants
The pathogenicity of possible variants was evaluated using five different in silico predictive algorithms: FATHMM, SIFT, Align GVGD, MutationTaster2, and PolyPhen-2 (Table S4). Subsequently, only variants identified as all “pathogenic” by 4 of the 5 in silico tools were selected.
Sanger sequencing
For all candidate pathogenic variants that satisfied the selection criteria, Sanger sequencing was used to validate the NGS results. Furthermore, the nucleotide sequences of the amplified fragments were analyzed through direct sequencing in both directions using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). Sequence analysis was performed using an ABI 3130xl automated sequencer (Applied Biosystems).
Statistical analysis
Continuous variables with normal distribution are expressed as mean ± standard deviation, and those with nonnormal distribution are expressed as median with interquartile range. Conversely, categorical variables are expressed as numbers and frequencies (%). To compare continuous variables, an unpaired t-test, nonparametric Mann–Whitney U test, or one-way analysis of variance was used, and χ2 statistics or Fisher’s exact test was used for the categorical variables. The data were analyzed using the Wilcoxon signed-rank test, a non-parametric method suitable for paired data and small sample sizes, to compare differences between baseline and follow-up measurements within each group.
Time-to-event data are presented as Kaplan–Meier estimates, which were compared using the log-rank test. Baseline variables considered clinically relevant or that showed significant univariate relationships with adverse events were included in the multivariable Cox proportional hazards regression models. Variables to be included were carefully selected, considering the number of events, to ensure parsimony of the final models. Statistical analyses were conducted using JMP software (version 17; SAS institute, Cary, NC, USA). To determine the optimal cutoff values of the number of derivations obtained from chest X-ray and echocardiographic data for predicting MACE and hospitalization, receiver operating characteristic curve analysis was performed. P < 0.05 was considered statistically significant.
Data availability
All data supporting the findings of this study are available within the paper and its supplementary information.
Abbreviations
- DCM:
-
Dilated cardiomyopathy
- ECG:
-
Electrocardiogram
- HF:
-
Heart failure
- LV:
-
Left ventricle
- LVRR:
-
Left ventricular reverse remodeling
- MACE:
-
Major adverse cardiac events
- NGS:
-
Next-generation sequencing
- PCR:
-
Polymerase chain reaction
- BNP:
-
Brain natriuretic peptide
References
Fatkin, D., Huttner, I. G., Kovacic, J. C., Seidman, J. G. & Seidman, C. E. Precision medicine in the management of dilated cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 74, 2921–2938. https://doi.org/10.1016/j.jacc.2019.10.011 (2019).
McKenna, W. J. & Judge, D. P. Epidemiology of the inherited cardiomyopathies. Nat Rev Cardiol 18, 22–36. https://doi.org/10.1038/s41569-020-0428-2 (2021).
Mori, H. et al. CORRIGENDUM: Outcomes of dilated cardiomyopathy in japanese children - A retrospective cohort study. Circ J 86, 916–917. https://doi.org/10.1253/circj.CJ-66-0204 (2022).
Lipshultz, S. E. et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med 348, 1647–1655. https://doi.org/10.1056/NEJMoa021715 (2003).
Kirk, R. et al. The International Society for Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: Executive summary. [Corrected]. J Heart Lung Transplant 33, 888–909, https://doi.org/10.1016/j.healun.2014.06.002 (2014).
Khan, R. S. et al. Genotype and cardiac outcomes in pediatric dilated cardiomyopathy. J Am Heart Assoc 11, e022854. https://doi.org/10.1161/JAHA.121.022854 (2022).
Tayal, U., Ware, J. S., Lakdawala, N. K., Heymans, S. & Prasad, S. K. Understanding the genetics of adult-onset dilated cardiomyopathy: What a clinician needs to know. Eur Heart J 42, 2384–2396. https://doi.org/10.1093/eurheartj/ehab286 (2021).
Haas, J. et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur Heart J 36, 1123–1135a. https://doi.org/10.1093/eurheartj/ehu301 (2015).
Verdonschot, J. A. J. et al. Implications of genetic testing in dilated cardiomyopathy. Circ Genom Precis Med 13, 476–487. https://doi.org/10.1161/CIRCGEN.120.003031 (2020).
Escobar-Lopez, L. et al. Association of genetic variants with outcomes in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol 78, 1682–1699. https://doi.org/10.1016/j.jacc.2021.08.039 (2021).
Merlo, M. et al. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol 57, 1468–1476. https://doi.org/10.1016/j.jacc.2010.11.030 (2011).
Hoshikawa, E. et al. Effect of left ventricular reverse remodeling on long-term prognosis after therapy with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers and beta blockers in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 107, 1065–1070. https://doi.org/10.1016/j.amjcard.2010.11.033 (2011).
Shaddy, R. E. et al. Carvedilol for children and adolescents with heart failure: A randomized controlled trial. JAMA 298, 1171–1179. https://doi.org/10.1001/jama.298.10.1171 (2007).
Daubeney, P. E. et al. Clinical features and outcomes of childhood dilated cardiomyopathy: Results from a national population-based study. Circulation 114, 2671–2678. https://doi.org/10.1161/CIRCULATIONAHA.106.635128 (2006).
O’Sullivan, J. J. et al. Recovery of heart function in children with acute severe heart failure. Transplantation 85, 975–979. https://doi.org/10.1097/TP.0b013e318168fe3c (2008).
Herkert, J. C. et al. Toward an effective exome-based genetic testing strategy in pediatric dilated cardiomyopathy. Genet Med 20, 1374–1386. https://doi.org/10.1038/gim.2018.9 (2018).
Vasilescu, C. et al. Genetic basis of severe childhood-onset cardiomyopathies. J Am Coll Cardiol 72, 2324–2338. https://doi.org/10.1016/j.jacc.2018.08.2171 (2018).
Ouellette, A. C. et al. Clinical genetic testing in pediatric cardiomyopathy: Is bigger better?. Clin Genet 93, 33–40. https://doi.org/10.1111/cge.13024 (2018).
Pugh, T. J. et al. The landscape of genetic variation in dilated cardiomyopathy as surveyed by clinical DNA sequencing. Genet Med 16, 601–608. https://doi.org/10.1038/gim.2013.204 (2014).
Ellepola, C. D., Knight, L. M., Fischbach, P. & Deshpande, S. R. Genetic testing in pediatric cardiomyopathy. Pediatr Cardiol 39, 491–500. https://doi.org/10.1007/s00246-017-1779-2 (2018).
McNally, E. M., Golbus, J. R. & Puckelwartz, M. J. Genetic mutations and mechanisms in dilated cardiomyopathy. J Clin Invest 123, 19–26. https://doi.org/10.1172/JCI62862 (2013).
Rossano, J. W. et al. The Registry of the International Society for Heart and Lung Transplantation: Nineteenth Pediatric Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 35, 1185–1195. https://doi.org/10.1016/j.healun.2016.08.018 (2016).
Kayvanpour, E. et al. Genotype-phenotype associations in dilated cardiomyopathy: meta-analysis on more than 8000 individuals. Clin Res Cardiol 106, 127–139. https://doi.org/10.1007/s00392-016-1033-6 (2017).
Walsh, R. et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med 19, 192–203. https://doi.org/10.1038/gim.2016.90 (2017).
de Frutos, F. et al. Natural history of MYH7-related dilated cardiomyopathy. J Am Coll Cardiol 80, 1447–1461. https://doi.org/10.1016/j.jacc.2022.07.023 (2022).
Brown, E. E. et al. Genetic dilated cardiomyopathy due to TTN variants without known familial disease. Circ Genom Precis Med 13, e003082. https://doi.org/10.1161/CIRCGEN.120.003082 (2020).
Zaklyazminskaya, E. et al. Low mutation rate in the TTN gene in paediatric patients with dilated cardiomyopathy - a pilot study. Sci Rep 9, 16409. https://doi.org/10.1038/s41598-019-52911-1 (2019).
Christine, K. S. & Conlon, F. L. Vertebrate CASTOR is required for differentiation of cardiac precursor cells at the ventral midline. Dev Cell 14, 616–623. https://doi.org/10.1016/j.devcel.2008.01.009 (2008).
Qiu, X. B. et al. CASZ1 loss-of-function mutation contributes to familial dilated cardiomyopathy. Clin Chem Lab Med 55, 1417–1425. https://doi.org/10.1515/cclm-2016-0612 (2017).
Guo, J. et al. A novel de novo CASZ1 heterozygous frameshift variant causes dilated cardiomyopathy and left ventricular noncompaction cardiomyopathy. Mol Genet Genomic Med 7, e828. https://doi.org/10.1002/mgg3.828 (2019).
Huang, R. T. et al. CASZ1 loss-of-function mutation associated with congenital heart disease. Gene 595, 62–68. https://doi.org/10.1016/j.gene.2016.09.044 (2016).
Orlova, A., Guseva, D. & Ryzhkova, O. Identification of a Novel de Novo Variant in the CASZ1 Causing a Rare Type of Dilated Cardiomyopathy. Int J Mol Sci 23, https://doi.org/10.3390/ijms232012506 (2022).
Kubanek, M. et al. Novel predictors of left ventricular reverse remodeling in individuals with recent-onset dilated cardiomyopathy. J Am Coll Cardiol 61, 54–63. https://doi.org/10.1016/j.jacc.2012.07.072 (2013).
Tsuda, E., Negishi, J., Noritake, K., Iwasa, T. & Abe, T. Left ventricular reverse remodeling with infantile dilated cardiomyopathy and pitfalls of carvedilol therapy. J Cardiol 67, 147–152. https://doi.org/10.1016/j.jjcc.2015.08.022 (2016).
Dal Ferro, M. et al. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart 103, 1704–1710. https://doi.org/10.1136/heartjnl-2016-311017 (2017).
Morimoto, R. et al. Myocardial contractile reserve predicts left ventricular reverse remodeling and cardiac events in dilated cardiomyopathy. J Cardiol 70, 303–309. https://doi.org/10.1016/j.jjcc.2017.02.005 (2017).
Sawada, H. et al. Detection of pediatric pulmonary arterial hypertension by school electrocardiography mass screening. Am J Respir Crit Care Med 199, 1397–1406. https://doi.org/10.1164/rccm.201802-0375OC (2019).
Hirono, K. et al. A significance of school screening electrocardiogram in the patients with ventricular noncompaction. Heart Vessels 35, 985–995. https://doi.org/10.1007/s00380-020-01571-7 (2020).
Mori, H. et al. Outcomes of dilated cardiomyopathy in Japanese children - A retrospective cohort study. Circ J 86, 109–115. https://doi.org/10.1253/circj.CJ-20-1239 (2021).
Towbin, J. A. et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 296, 1867–1876. https://doi.org/10.1001/jama.296.15.1867 (2006).
Alexander, P. M. et al. Long-term outcomes of dilated cardiomyopathy diagnosed during childhood: Results from a national population-based study of childhood cardiomyopathy. Circulation 128, 2039–2046. https://doi.org/10.1161/CIRCULATIONAHA.113.002767 (2013).
Nugent, A. W. et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med 348, 1639–1646. https://doi.org/10.1056/NEJMoa021737 (2003).
Puggia, I. et al. Natural history of dilated cardiomyopathy in children. J Am Heart Assoc 5, https://doi.org/10.1161/JAHA.116.003450 (2016).
Tobita, T. et al. Genetic basis of cardiomyopathy and the genotypes involved in prognosis and left ventricular reverse remodeling. Sci Rep 8, 1998. https://doi.org/10.1038/s41598-018-20114-9 (2018).
Rusconi, P. et al. Differences in presentation and outcomes between children with familial dilated cardiomyopathy and children with idiopathic dilated cardiomyopathy: A report from the pediatric cardiomyopathy registry study group. Circ Heart Fail 10, https://doi.org/10.1161/CIRCHEARTFAILURE.115.002637 (2017).
Rossano, J. W. et al. Elevated heart rate and survival in children with dilated cardiomyopathy: A multicenter study from the pediatric cardiomyopathy registry. J Am Heart Assoc 9, e015916. https://doi.org/10.1161/JAHA.119.015916 (2020).
van der Meulen, M. et al. Predicting outcome in children with dilated cardiomyopathy: The use of repeated measurements of risk factors for outcome. ESC Heart Fail 8, 1472–1481. https://doi.org/10.1002/ehf2.13233 (2021).
Kampmann, C. et al. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart 83, 667–672 (2000).
Everitt, M. D. et al. Recovery of echocardiographic function in children with idiopathic dilated cardiomyopathy: Results from the pediatric cardiomyopathy registry. J Am Coll Cardiol 63, 1405–1413. https://doi.org/10.1016/j.jacc.2013.11.059 (2014).
Akhtar, M. M. et al. Clinical phenotypes and prognosis of dilated cardiomyopathy caused by truncating variants in the TTN gene. Circ Heart Fail 13, e006832. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006832 (2020).
Verdonschot, J. A. J. et al. Titin cardiomyopathy leads to altered mitochondrial energetics, increased fibrosis and long-term life-threatening arrhythmias. Eur Heart J 39, 864–873. https://doi.org/10.1093/eurheartj/ehx808 (2018).
Acknowledgements
The authors wish to acknowledge Hitoshi Moriuchi, Haruna Hirai, and Eriko Masuda for their expert technical assistance.
Author information
Authors and Affiliations
Contributions
K.H. designed the study, the main conceptual ideas, and the proof outline. K.T., S.T., M.O., H.N., K.I., S.O., J.M., K.Y., K.U., H.O., T.M., K.B., A.K., H.S., N.K., M.H., S.B., M.M., H.I., Y.H., Y.I., H.S., S.U., K.T., E.K., S.O., Y.H., S.H., T.F., N.M., M.N., K.O., M.F., Y.Y., Y.F., S.I., K.T., Y.S., R.I., J.M., Y.H., T.F., H.N., S.F., K.N., A.K., N.K., S.K., and K.W. collected the data. Y.H., S.I., N.N., Y.A., and Y.K. aided in interpreting the results and worked on the manuscript. F.I. supervised the project. K.H. wrote the manuscript with support from T.I. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hirono, K., Hata, Y., Ichimata, S. et al. Sarcomere gene variants did not improve cardiac function in pediatric patients with dilated cardiomyopathy from Japanese cohorts. Sci Rep 14, 30469 (2024). https://doi.org/10.1038/s41598-024-77360-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77360-3