Abstract
The presence of mineral pollutants, combined with bacterial contamination, has significantly impacted groundwater quality and led to various health-related issues in mining-impacted areas. Therefore, we measured the concentration of fluoride (F-), phosphate (PO43-), sulphate (SO42-), ammonium (NH4+), nitrate (NO3-), the total coliforms (TCs), and physiochemical characteristics in groundwater samples of South Khorasan, Eastern Iran. For this, we collected water samples from 100 wells in spring and autumn across this mining-impacted area. We then measured the concentrations of mineral pollutants and assessed their associated health risks to children and adults using the Environmental Protection Agency (EPA) models and spatiotemporal zoning maps in ArcGIS. The concentrations of PO43-, NH4+, SO42-, NO3-, and F- were 0.70 ± 0.34, 0.82 ± 0.9, 175.45 ± 123, 15.26 ± 9.41, and 0.53 ± 0.68 mg/L in spring, and 0.71 ± 3.18, 4.68 ± 31, 306.72 ± 615.80, 19.30 ± 15.61, and 0.72 ± 0.65 mg/L in autumn, respectively. PO43-, NH4+, and SO42- exceeded both the World Health Organization (WHO) and EPA, but NO3- exceeded only EPA standards. TCs in both seasons exceeded the standards set by the EPA and WHO. The hazard quotient (HQ) values indicated non-carcinogenic risks for F⁻ and NO3⁻, while posing no risks NH4⁺ and PO43⁻ in both adults and children during autumn and spring.Hazard index (HI) was greater than 1 for all minerals in both children and adults in autumn and spring. No correlation was observed between mineral compounds and TCs in the study area, yet the water samples were highly contaminated by coliform with a significant risk to adults and children. In essence, both mineral pollutants and TCs potentially pose serious risks to human, and more efforts are required to improve the quality of water in this area.
Similar content being viewed by others
Introduction
Groundwater plays a crucial role in supplying water for different purposes such as consumption, industrial use, and agriculture throughout the world. It represents a suitable option in regions with low availability of surface water, particularly in arid and semi-arid areas1. Groundwater contributes to supplying approximately 20% of the freshwater demand and stands as the sole source of potable water in many nations. Therefore, ensuring its quality is imperative for the well-being of human populations2. In the context of drinking water, mineral compounds such as sulphate, phosphate, and fluoride are ubiquitous in the groundwater, in exceeding concentrations, could act as pollutants. Such compounds can cause health-related issues in target consumers and impact the health of the population. The presence of fluoride in underground water sources represents a global issue, with a considerable portion of the world’s population facing exposure to water rich in fluoride content. Fluoride predominantly occurs in natural water bodies in the form of unbound ions, typically originating from the dissolution of minerals like fluorite (CaF2) within groundwater3. The prevalence of sulphate- and phosphate-rich water has given rise to issues in the arid areas of many countries, including India, Pakistan, South Africa, Bangladesh, and Nigeria. Although the permissible concentration of mineral compounds has been legitimated by the World Health Organization (WHO) and Environmental Protection Agency (EPA), in exceeding values can potentially pose risks to humans.
Groundwater quality in Iran is influenced by several critical factors. Anthropogenic activities have led to significant depletion of Iran’s aquifers, contributing to a broader water crisis4. This depletion is further exacerbated by the hydrochemical changes occurring in Iran’s groundwater systems, where increased salinity and contamination from agricultural runoff are notable concerns. The decline in groundwater recharge has been linked to both natural variability and human-induced factors, including unsustainable water extraction practices5. Additionally, the renovation of Iran’s irrigation network has inadvertently deepened the water crisis by promoting inefficient water use and exacerbating the depletion of groundwater reserves6.
The hazards of nitrate7, sulphate8, fluoride9, phosphate, and ammonium10 to consumers have been investigated in the groundwater of many areas around the World. In accordance with the regulations established by the Institute of Standards and Industrial Research of Iran, a defined range of permissible fluoride levels in drinking water has been instituted. Specifically, the prescribed guidelines dictate that fluoride concentrations should fall within the range of 0.5 to 1.5 mg/l, establishing a threshold that both defines acceptable limits and underscores the need for vigilance in monitoring fluoride levels in drinking water. Exceeding the permissible limit of 1.5 mg/l, as outlined in has risen many issues in consumers. Nitrate is frequently detected in groundwater and exert an influence on its quality, which is characterized by its significant solubility, mobility, and stability in aqueous settings. Chemical fertilizers1, wastewater irrigation11, industrial effluent discharge12, and landfill seepage13 have a major contribution to nitrate pollution in the groundwater. Consequently, nitrate in high enough concentrations can cause serious health-related problems in consumers.
The ingestion of elevated mineral pollutants, including nitrate, fluoride, and sulphate results in issues in the digestive, endocrine, and nervous systems14. Nitrate and fluoride have been shown to be carcinogenic, causing adverse effects on neonatal health, and other health implications in vulnerable individuals15. The maximum allowable concentration for nitrate (45 mg/L) and fluoride (1.5 mg/L) has been stated by the Iranian Standard Organisation (ISO)16. If the concentration of these compounds exceeds the acceptable limits, they can interact with amines and form secondary metabolites that are toxic to individuals. In addition, the interaction of mineral contaminants with microorganisms in the groundwater could potentially cause other health issues in vulnerable consumers, including children, pregnant women, and elderly people17. Total coliforms (TCs), a group of bacteria characterized by specific properties, serve as water quality indicators and are employed in estimating water quality18,19. TCs can contaminate groundwater by reproducing in a short period of time and therefore pollute the drinking water. The adverse effects of TCs-contaminated water have been investigated in many studies3,7. Thus, the potential interactions between mineral pollutants and TCs could be used as a useful tool to measure the health-related risks to consumers, especially in mining-polluted arid and semi-arid areas relying on groundwater.
Health risk assessment methods play a pivotal role in evaluating the potential effects of exposure to contaminants in drinking water1. The EPA defines human health risk assessment as a systematic approach to appraising the potential health repercussions of exposure to harmful substances in polluted ecosystems20. This method provides a systematic framework for quantitatively or semi-quantitatively describing the detrimental effects of exposure to harmful substances, allowing the assessment of risk across various age groups. The health risks associated with the environmental pollutants in mining areas have studied in various parts of Iran, where most of the regions suffer from the lack of surface water to supply fresh water. In particular, South Khorasan has been facing challenges not only because of the freshwater shortages but mainly due to the overloaded mining activities. The highest percentage of drinking water is provided from the wells, qanats, and springs that have been affected by mining developments.
Many studies have investigated the potential risks of heavy metals to consumers in this area, but much less attention has been paid to the risk of mineral pollutants and TCs in two different seasons in this area. There is a lack of knowledge on the impacts of TCs and mineral pollutants and their interactions in the groundwater resources in this study area. We use a spatiotemporal analysis of the health risk of minerals as the chemical contaminant and TCs as the biological contaminant to measure the associated health risks to the target groups in two different seasons. More importantly, this is the first study uses a wide range of groundwater sampling sites in a mining-impacted area to find the impacts of both chemical and biological pollutants on consumers and warn the responsible authorities for the potential hazards. Therefore, in the present study, we aimed to (i) measure the concentration of five mineral compounds (i.e. PO42-, NH4+, SO42-, NO3-, and F-) and TCs in groundwater samples collected in spring and autumn, (ii) assess the associated non-carcinogenic risks in adults and children using spatiotemporal zooning map, (iii) study the correlation between physicochemical parameters in two seasons, and (iv) compare the results in the context of national and international standards.
Materials and methods
Study location
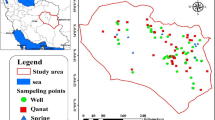
The study area, South Khorasan Province, is located in Eastern Iran, which lies between 32.5176° N latitude and 59.1042° E longitude and encompasses an area of about 151. 193 km 21 (Fig. 1). Aquifer and groundwater are abundant in this region to supply water for a total of 11 counties consisting of a population of 768.898 people. The rural areas of South Khorasan Province are primarily concentrated in mountainous regions and foothills. The province experiences an average annual rainfall of 152 mm, with daily minimum and maximum temperatures averaging 7°C and 26°C, respectively. Among the meteorological stations, Qaen stands out as the coldest area, with an average temperature of 14°C and a record low of − 27°C. On the other hand, Tabas is the warmest, with an average temperature of 24°C and an absolute high of 49°C. South Khorasan is known for its unique mining resources as well as qanats as wondrous inventions relating to ancient Iranians. Having a heavy load of active mines, including iron, chromite, magnesium, copper, dolomite, marmorite, potassium, barite, and bentonite, South Khorasan has a major contribution to the gross national product of Iran (Table 4). Groundwater is the main source of water in arid and semi-arid regions of Eastern Iran. There have been different types of groundwater in Eastern Iran, including qanat, wells, and springs. Such aquifers play a pivotal role in supplying water for different consumptions like drinking and irrigation.
Sampling
Water samples were taken from 100 wells from March to May for spring and from September to November for autumn in 2021. Sampling sites were strategically selected based on their proximity to populated areas, industrial zones, and known sources of pollution, reflecting a range of conditions related to population density, industrial activity, and pollution status. However, due to data limitations, specific quantitative details on these parameters were not available to visually show these variations.. Water samples were taken thrice in each well and collected in 500-ml polypropylene bottles (APHA, 201221). Bottles were acid-washed and rinsed with ultrapure water before sampling. In each sampling well, a portable multi-parameter instrument (SensoDirect 150, Lovibond, Germany) was used to measure physicochemical parameters e.g. temperature (°C), pH, electrical conductivity (EC; mS cm−1), total dissolved solids (TDS; mgL−1 and dissolved oxygen (DO; mg L−1). To avoid changes in the samples, an ice box was used to transfer the samples to the lab. Total hardness (mg L −1 CaCo 3), total alkalinity (mg L −1 CaCo3), bicarbonate (mg L −1 CaCo3), NH+4; mg L −1 and sulphate were measured in the lab (APHA, 200521). All samples were stored at 4°C in a temperature-controlled facility to prevent degradation of the sample integrity. The storage duration ranged from 5 to 7 days, ensuring compliance with recommended standards for preserving the analytes of interest.
Mineral compounds analyses
To remove impurities, particles, and organic materials in the samples, Millipore filters (0.45 μm) were used. Water samples were prepared by adding five drops (~ 250 µL) of nitric acid (HNO3) (Merck, Germany, ultra-pure grade). Inductively coupled plasma mass spectrometry (ICP-MS) (HP-4500 USA, equipped with autosampler Asx-520) was used to detect the mineral compounds concentrations as per22. The blank sample was a laboratory blank made from ultra-pure water (Milli-Q), prepared under sterile conditions to ensure no contamination. This blank was used to verify the accuracy of the analytical instruments. Limits of detection (LOD) and relative standard deviation (RSD) were examined below 5% for all samples as per Kazemi et al.1. The estimated analytical uncertainties were calculated based on the RSD of duplicate samples, with an RSD of approximately 4.95% for all measurements. This percentage indicates a high level of precision in the analysis. Duplicate samples were analysed at regular intervals to ensure consistency across all measurements. The LOD and limit of quantification (LOQ) for the analytical instruments were determined following the standard procedures of our laboratory. The LOD for ICP-MS was 0.1 µg/L, while the LOQ was 0.5 µg/L. These values were established by repeatedly measuring low-concentration standards and observing the signal-to-noise ratio. These thresholds ensured that the results remained well within detectable and quantifiable ranges for all tested samples.Quality assurance and control procedures included calibration of instruments before analysis, use of replicates to verify consistency, and the inclusion of both positive and negative controls throughout the analysis.
TCs analyses
Pour plate method was used to determine the total coliforms in the water samples collected from different stations in two seasons23. To test this, the water samples underwent serial dilution processes, creating dilutions at 1:10 and 1:100 in Ringer’s solution. This was done to ensure that the number of colonies fell within the range of 20 to 100 colonies, which allows for accurate counting. Subsequently, around 0.1 mL of each of these serially diluted water samples was carefully dispensed onto sterile agar plates. The samples were evenly spread across the entire surface of the agar. These prepared agar plates were then placed in an incubator at 37 °C for a duration of 24 h. After incubation, the total count of coliform colonies was meticulously determined, and the mean value was calculated based on three separate replicates. To confirm the presence of coliforms as identified through the conventional method, additional testing was conducted using BioMérieux’s API® identification test kits, specifically API 20E. This comprehensive approach was validated through the inclusion of negative, positive, and sterility controls, ensuring the reliability and accuracy of the results.
National and international standard limits
The concentration of mineral compounds was compared to the Iran National Standards Organization (INSO), EPA, and WHO. All concentrations were reported as mean ± SE with the maximum and minimum values.
Relationships between physicochemical parameters and TCs
Person’s correlation test was conducted using heatmap analysis in R (version 2023.9.0.463). We compared the TCs in water samples with five studied mineral compounds as well as hadrochemical parameters.
Health risk assessments
The oral route was used as the main exposure pathway to the potential contaminants in the drinking water collected from the wells, therefore, dermal, and respiratory exposure routes were ignored in this study. We assessed the risks of PO43-, NH4+, SO42-, NO3-, and F- in adults and children. To do this, we first calculated the chronic daily intake (CDI) of each pollutant as per24 (Eq. 1).
where CDI is a chronic dose of contaminant (mg/kg/d), C is the concentration of contaminant water samples (mg/l), IR is ingestion rate (drinking water) (L/d), EF is the exposure frequency, ED is the exposure duration (day), BW is average body weight (Kg), AT is the average time (year).
As the studied pollutants are not carcinogenic, we calculated the non-carcinogenic effects which is known as hazard quotient (HQ) following Ludwicki et al.25 (Eq. 2).
where HQ is the hazard quotient and RFD is the reference dose (mg/kg-d). We also used the most recent reginal screening tables (USEPA, 2023) for the values required to calculate the HQ. We used the background levels of (PO43⁻) typically ranges from 0.01 to 0.5 mg/L, ammonium (NH4⁺) is generally below 0.1 mg/L, sulfate (SO42⁻) usually falls between 2 to 10 mg/L, nitrate (NO₃⁻) often remains below 0.5 mg/L in natural conditions but can reach 10 to 20 mg/L in agricultural areas, and fluoride (F⁻) concentrations range from 0.1 to 0.5 mg/L. We used the common threshold to determine the potential non-carcinogenic risk of pollutants as per3. HQ values smaller than 1 are considered as no health risks, while those greater than 1 considered with non-carcinogenic risks to the consumers studied. To measure the combined health risks of pollutants, we also used hazard index (HI) following Eq. 3.
where HQP, HQN, HQS, HQF, and HQA are the calculated hazard quotients of phosphate, nitrate, sulphate, fluoride, and ammonium, respectively. The constant values used in calculations are presented in Table 1.
Statistical analyses
All the statistical analyses were conducted in R (version 2023.9.0.463). Data arrangement was conducted in MS Excel (2021). Mean concentrations of pollutants were presented with standard errors, maximum, and minimum values in each season. Pearson correlation analyses were conducted using a heatmap for two seasons studied. Spatiotemporal zoning maps were created in ArcGIS (version 10.3; ESRIInc., Redlands CA). Graphs were plotted using ggplot2 package in R. Principal Component Analysis (PCA) was employed to assess the relationships between water quality parameters for both spring and autumn seasons, using the prcomp() function in R (version 2023.9.0.463) with scaling to normalize the variables. PCA simplifies the complex relationships among multiple water quality variables by reducing the dataset into fewer uncorrelated principal components (PCs) that capture the most variation. The first two principal components (PC1 and PC2) were used for interpretation, and biplots were created to visualize the contributions of individual variables and their relationships in each season.
Results and discussion
Physicochemical characteristics of water samples
In spring, the groundwater samples showed an average dissolved oxygen (DO) concentration of 6.64 ± 1.18 mg/L, which increased slightly to 7 ± 1.10 mg/L in autumn. The pH values remained relatively stable across both seasons, averaging around neutral to slightly alkaline conditions (7.8 ± 0.30 in spring and 7.88 ± 0.27 in autumn). Electrical conductivity (EC) was notably higher in autumn (2.13 ± 3 mg/L) compared to spring (1.40 ± 0.7 mg/L), indicating potential seasonal variations in dissolved solids. Chemical Oxygen Demand (COD) and Biological Oxygen Demand (BOD) showed increased concentrations in autumn, suggesting a rise in organic matter during this period, which is consistent with findings in other mining-impacted areas like Silva et al.26. Total Hardness (TH) and Total Dissolved Solids (TDS) values also demonstrated higher levels in autumn, with TH reaching 394.13 ± 315.48 mg/L and TDS peaking at 265.12 ± 134.56 mg/L, indicating a potential increase in mineral concentrations during the post-monsoon period, aligning with Golaki et al.3, who observed similar seasonal variations in water quality. These fluctuations suggest that the presence of active mining operations, as highlighted by Sharifi et al.27, may be contributing to the observed variations, particularly in the higher EC and mineral concentrations in autumn.
For the spring season, the PCA biplot revealed that PC1 and PC2 captured the majority of the variation in water quality parameters (Fig. 2A). Variables such as BOD, NH4+, and total hardness were positively correlated with each other, while dissolved oxygen was negatively correlated with these factors. This suggests that areas with higher biological oxygen demand, ammonium, and total hardness experienced lower levels of dissolved oxygen, possibly indicating organic pollution. The clustering of stations around the center of the plot indicates similar water quality across most sampling points, with few outliers showing localized variations. The autumn PCA biplot demonstrated stronger correlations between SO42-, NO3-, and total hardness, indicating that these parameters are positively linked, while dissolved oxygen, pH, and coliform formed another group of associated variables (Fig. 2B). The autumn plot shows greater variability in water quality across stations, with some outliers reflecting localized differences, possibly due to seasonal changes or increased anthropogenic activities. The autumn results suggest that lower dissolved oxygen and higher microbial contamination may correspond with areas of increased sulfate, nitrate, and hardness levels.
Mineral compounds and TCs relative to the standards
The physicochemical characteristics of water samples are presented in Table 2. The concentrations of PO43⁻, NH4⁺, SO42⁻, NO3⁻, and F⁻ were 0.70 ± 0.34, 0.82 ± 0.9, 175.45 ± 123, 15.26 ± 9.41, and 0.53 ± 0.68 mg/L in spring, and 0.71 ± 3.18, 4.68 ± 31, 306.72 ± 615.80, 19.30 ± 15.61, and 0.72 ± 0.65 mg/L in autumn, respectively (Table 3). The probable and confirmed total coliforms (TCs) showed 554.50 ± 245.50 and 120.56 ± 300 in spring, and 401.02 ± 450.22 and 133.33 ± 330 in autumn, respectively (Table 2). A comparison of these findings with ISO standards indicated that nitrate (45 mg/L) and fluoride (1.5 mg/L) concentrations were lower than national standards, while TCs exceeded this limit in both seasons studied (Table 3).
In terms of EPA standards, except for fluoride, all other minerals and TCs surpassed international limits in autumn, while they were below the standards except for nitrate and TCs in spring. Phosphate in both seasons and sulfate in autumn exceeded the WHO’s acceptable limits. Golaki et al.3 observed that nitrate and nitrite levels were higher than the acceptable limits set by ISO, WHO, and EPA in the groundwater samples from urban areas. Our findings in the autumn samples are consistent with those reported by Tiwari et al.28, where sulfate concentrations exceeded WHO limits. The elevated levels of TCs also indicate microbial contamination at all studied stations in both seasons. In agreement with our findings, Affum et al.23 reported that TCs exceeded WHO guidelines in most groundwater samples.
The variation in the concentrations of analyzed elements may be attributed to several factors, including local geological conditions, anthropogenic influences, and seasonal changes. For instance, mining activities in South Khorasan have been linked to increased concentrations of mineral pollutants in groundwater26, and the presence of active mines correlates with higher mineral concentrations in the surrounding water sources. Moreover, seasonal variations in precipitation can influence runoff patterns, potentially altering the concentrations of these elements27. Agricultural practices and the use of fertilizers may also contribute to elevated nutrient levels, particularly in rural areas, leading to fluctuations in concentration levels over time.
The distribution of different mines is presented in Table 4. The active mines in South Khorasan show the highest concentration of minerals in the groundwater samples, emphasizing the impact of mining activities on water quality. Silva et al.26 found a positive relationship between mining activity and the presence of mineral pollutants in groundwater, which aligns with our findings in South Khorasan, a mining area with more than 600 active mines. Similarly, previous studies have indicated that groundwater contamination in rural areas is often associated with agricultural activities, household chemicals, runoff, and faulty septic systems27.
Relationships between studied parameters and TCs
The Pearson’s correlation heatmap showed similar correlations between the physicochemical parameters studied in spring and autumn (Fig. 3). In both seasons, findings showed no correlation between the five mineral pollutants (i.e. nitrate, phosphate, sulphate, ammonium, and fluoride) and TCs, but they had positive correlations in response to DO (dissolved oxygen), BOD (biological oxygen demand), and COD (chemical oxygen demand). TCs also showed negative correlation with TDS, TH (total hardness), and EC (electrical conductivity). The strongest positive relationship was observed between sulphate, TDS, and EC in the water samples collected in both spring and autumn. The correlation between physicochemical parameters and TCs was found by Appah et al.20 with the negative correlations in response to phosphate and pH. Another study showed a positive relationship between TCs and nitrate and DO29. Our findings agree with those found by Kolawole and Afolayan with no correlation between physicochemical factors and TCs. The supportive role of hadrochemical factors such as nitrate, ammonium, DO, and BOD has been proven in groundwater30, therefore, it is possible that these factors increased TCs in our study area. TDS and EC could affect the TCs as they have inhibitory effects on some sensitive microorganisms existing in the groundwater31. In a similar study, Armah18,19 found that electrical conductivity, total dissolved solids, turbidity, nitrates, and some heavy metals were significant predictors of E. coli in groundwater in mining areas, which is not consistent with our findings. One possible explanation is the toxicity impacts of chemicals on the microorganism like bacteria in the groundwater. It is possible that in mining-impacted areas, chemical compositions are released considerably to the groundwater and reduce the quantity of bacterial communities.
Health risk assessment
HQs in adults
Spatiotemporal distribution of the non-carcinogenic risk of ammonium showed no risks to the consumers in spring (2.77E-03–1.08E + 00) and autumn (1.09E-03–1.38E-01) with approximately 100% locations had HQ < 1 (Fig. 4A and B). Fluoride showed potential risks (about 30% of the whole area) to consumers in the east and south of the study area in both seasons (0–2.7E + 00) (Fig. 4C and D). Nitrate had risks in 20% and 30% of the sites in spring (6.23E-03–2.10E + 00) and autumn (6.38E03-1.30E + 00), respectively (Fig. 5A and B). Contaminated groundwater with phosphate showed 80% and 85% of the studied wells with the highest HQ values but below 1, indicating no risks to consumers in spring (5.46E-04–1.50E-02) and autumn (2.00E-03–1.98E-02), respectively (Fig. 5C and D). Sulphate had risks in 10% and 25% of the locations in spring (1.35E-01–9.49E + 01) and autumn (1.38E-01–1.28E + 01), respectively (Fig. 6A and B). The Mean of HQs also showed the highest risks of sulphate for adults relative to other studied mineral pollutants, with the values in autumn greater than in spring. The health risks of nitrate to adults were in line with the results obtained by Karunanidhi et al.32 as most of the studied stations had HQ greater than 1. Although the concentrations of phosphate and ammonium exceeded the standard limits in some stations in both seasons, they had no risks to adults in the present study. Our results are in line with what found by Rahman & Bodrud-doza33 in the same region in eastern Iran in terms of the risks of nitrate and fluoride to adults with HQ greater than 1. In our previous study, we found a positive relationship between the chromium contamination of groundwater in South Khorasan and associated risks to adults1. The majority of wells with potential health risks were found in the central areas of South Khorasan, indicating the role of intensive mining activity as well as population. Similarly, Rezvani Ghalhari et al.34 found that the HQ of mineral pollutants in the groundwater of Kashan (Central Iran) was at the highest level (HQ > 1) for the infants and children but for adults the risk was considered as negligible. Our findings regarding no risks to adults in spring are in line with Amiri et al.35, finding 80% of the samples with no non-carcinogenic risks to adults in the rural areas of Fereidan in the West of Iran. Our findings highlight that in contaminated areas with lower amount of annual precipitation the risk of mineral contaminants is higher than non-contaminated areas with higher precipitation.
HQs in children
In children, ammonium revealed 10% of risks in spring (4.9E-03–1.91E + 00) but no sites showed HQ values greater than 1 in autumn (3.40E-03–2.43E-01), indicating no risks of ammonium in children (Fig. 7A and B). Approximately 23% and 25% of sampled wells showed non-carcinogenic risks of fluoride to children in spring (0–4.7E + 00) and autumn (1.08E-06–4.92E + 00), respectively (Fig. 7C and D). Nitrate showed 12% and 35% of sites with risks to children in spring (1.19E-02–3.72E + 00) and autumn (1.13E-02–2.30E + 00) (Fig. 8A and B), but phosphate had no risks to children in both seasons studied (Fig. 8C and D). In terms of sulphate, 11% and 46% of studied sites in the study area had risks to children in spring (2.38E-01–1.68E + 02) and autumn (2.44E-01–2.26E + 01), respectively (Fig. 9A and B). Similar to adults, the mean of HQs showed sulphate with the heist risks among other compounds that was higher in autumn than spring (Fig. 10A and B). The risk of nitrate showed HQ values greater than those found in adults, indicating a higher risk of nitrate in children than in adults. These findings agree with the results found by Ahada and Suthar36. Our findings also agree with those found by Shalyari et al. (2019) in terms of the sensitivity of children relative to other groups in response to nitrate.
Our study found that nitrate posed significant risks to children, with 12% and 35% of sites showing risks in spring and autumn, respectively. This is consistent with the findings of Noori et al.37, who utilized a non-threshold model to estimate carcinogenic risks from nitrate, indicating elevated health risks in similar settings. Gheshlagh et al.38 also reported significant risks from nitrate and nitrite contamination in various regions, aligning with our observation of higher nitrate risks in autumn compared to spring. Additionally, Weber et al. (2022) highlighted the impact of industrial pollutants on groundwater quality in the Middle East, which supports our finding of high risks associated with sulphate and fluoride, especially in industrially influenced areas. These studies underscore the importance of considering both seasonal variations and regional pollution sources when assessing health risks from drinking water contaminants. Our results contribute to this body of knowledge by providing localized data specific to South Khorasan and highlighting seasonal differences in the risks of pollutants. The difference between the risks of nitrate to children in spring and autumn could be attributed to the hydrochemical characteristics of the water samples in the two seasons. Our results showed the highest risk of fluoride to children in autumn which is in line with those explored by Mirzabeygi et al.39. There are many active mines in South Khorasan contributing to the pollution of groundwater resources. The health risks of trace metals to children in South Khorasan were found in our previous studies in this region1. Children are classified as vulnerable individuals who are at risk of contaminants in industrial areas. Therefore, our findings confirm that children are more susceptible to studied mineral compounds than adults.
Hazard index
The cumulative risk of studied mineral compounds showed that adults are at risk in approximately 15% and 57% of the stations studied in spring (3.44E-01—9.64E + 01) and autumn (2.96E-01–1.37E + 01), respectively (Fig. 11A and B). In children, 17% and 58% of the wells had risks of mineral pollutants in spring (6.08E-0–1.71E + 02) and autumn (5.23E-01–2.42E + 01), respectively (Fig. 11C and D). Autumn was the most dangerous season in both children and adults in the study area. The sum of individual risk of mineral compounds showed values higher than 1 in both children and adults, especially in autumn in 60% of the studied wells. These findings are in accordance with3 who found HI values more than 1 in 56% of locations in children. Rahman & Bodrud-doza33 explored similar results with HI values higher than 1 in most of the sampled sites, showing the high potential of health risks to consumers.
Policy implications
Given the significance of our findings regarding the contamination levels of groundwater in South Khorasan Province, it is imperative to consider policy implications and preventive measures to mitigate pollution in the study area. Policymakers should implement stricter regulations on agricultural runoff, as excessive use of fertilizers and pesticides can significantly contribute to elevated levels of nitrates and phosphates in groundwater27. Additionally, enforcing best management practices in mining operations is essential to reduce the release of mineral pollutants, as demonstrated by Silva et al.26, which highlighted the correlation between mining activities and groundwater contamination. Furthermore, community awareness programs should be established to educate residents on the proper disposal of household chemicals and the maintenance of septic systems, thereby minimizing their impact on water quality23. By adopting a multi-faceted approach that combines regulation, education, and community engagement, it is possible to significantly improve the water quality in this vulnerable region and protect public health.
Limitations
One of the primary limitations of this study is the lack of detailed climate data, such as monthly precipitation, temperature, and groundwater recharge rates, which would have provided a more comprehensive understanding of the factors influencing groundwater quality in the study area. Additionally, data on groundwater abstraction rates were not available, making it challenging to fully assess the impact of human activities on the aquifer system. Furthermore, while we selected sites based on their proximity to populated areas, cities, mines, and industrial zones, specific data on population density, industrial activity, and pollution status were not available to create a detailed figure. The geological characteristics of the region, which are critical for understanding the distribution of minerals and pollutants in groundwater, were also not considered due to the absence of specific data. Future research should aim to incorporate these important environmental variables and provide more detailed data to enhance the analysis of groundwater contamination dynamics and inform more effective mitigation strategies.
Conclusion
The present study investigated the spatiotemporal distribution and potential health risks of ammonium, sulfate, nitrate, fluoride, and phosphate in the groundwater of South Khorasan during spring and autumn. Our findings revealed that while concentrations of these minerals were below national standards, some exceeded the WHO and EPA thresholds, particularly sulfate, which posed the highest risk to both adults and children. The groundwater contamination is largely influenced by the province’s extensive mining activities, with over 600 active mines contributing to elevated mineral levels. Physicochemical parameters, including TDS, EC, and hardness, also displayed seasonal variations, with autumn exhibiting higher concentrations, indicating increased mineral dissolution during the post-monsoon period. Furthermore, the analysis of health risk indices, such as hazard quotients, showed higher risks during autumn, emphasizing the seasonal impact of mining and environmental factors on water quality. Notably, TCs consistently exceeded ISO, WHO, and EPA standards across all stations, though no significant correlation was observed between TCs and the five mineral pollutants. These results highlight the need for targeted water quality management and risk mitigation strategies to protect the population from groundwater pollution in this arid mining region. We recommend future research to explore the potential risks of other pollutants in other groundwater sources, including qanat and spring in this study area.
Data availability
The authors confirm that all of the data that supports the conclusions of this study can be found within the article.
References
Kazemi, A., Esmaeilbeigi, M., Sahebi, Z., & Joorabian, S. Hydrochemical evaluation of groundwater quality and human health risk assessment of trace elements in the largest mining district of South Khorasan , Eastern Iran. Environ. Sci. Pollut. Res. 0123456789. https://doi.org/10.1007/s11356-022-21494-2 (2022)
Parang, H. & Esmaeilbeigi, M. Total mercury concentration in the muscle of four mostly consumed fish and associated human health risks for fishermen and non-fishermen families in the Anzali Wetland, Southern Caspian Sea. Region. Stud. Mar. Sci. 52, 102270. https://doi.org/10.1016/j.rsma.2022.102270 (2022).
Golaki, M., Azhdarpoor, A., Mohamadpour, A., & Derakhshan, Z.. Health risk assessment and spatial distribution of nitrate , nitrite , fluoride , and coliform contaminants in drinking water resources of kazerun , Iran. Environ. Res. 203, 111850. https://doi.org/10.1016/j.envres.2021.111850 (2022)
Noori, R., Maghrebi, M., Mirchi, A. & Madani, K. Anthropogenic depletion of Iran’s aquifers. Proc. Natl. Acad. Sci. 118(25), e2024221118. https://doi.org/10.1073/pnas.2024221118 (2021).
Noori, R., Maghrebi, M., Jessen, S., Bateni, S. M., Heggy, E., Javadi, S., Noury, M., Pistre, S., Abolfathi, S., & AghaKouchak, A. Decline in Iran’s groundwater recharge. Nat. Commun. 14, Article 6674. https://doi.org/10.1038/s41467-023-16774-9 (2023).
Maghrebi, M., Noori, R. & AghaKouchak, A. Iran: Renovated irrigation network deepens water crisis. Nature 618(7964), 238. https://doi.org/10.1038/d41586-023-01851-y (2023).
Kumar, L., Kiran, D., Panga, K., Dhakate, R. & Himabindu, V. Health risk assessment of nitrate and fluoride toxicity in groundwater contamination in the semi - arid area of Medchal, South India. Appl. Water Sci. 12(1), 1–21. https://doi.org/10.1007/s13201-021-01557-4 (2022).
Adimalla, N., & Li, P. Human and ecological risk assessment : An international occurrence , health risks , and geochemical mechanisms of fluoride and nitrate in groundwater of the rock-dominant semi-arid region , Telangana State , India. Hum. Ecol. Risk Assess. 1–23. https://doi.org/10.1080/10807039.2018.1480353 (2018).
Raja, V. & Neelakantan, M. A. Pollution and noncarcinogenic health risk levels of nitrate and fluoride in groundwater of Ramanathapuram district, Tamil Nadu, India. Int. J. Environ. Anal. Chem. 00(00), 1–16. https://doi.org/10.1080/03067319.2021.1890063 (2021).
Raja, V., & Neelakantan, M. A. Evaluation of Groundwater Quality with Health Risk Assessment of Fluoride and Nitrate in Virudhunagar District , Tamil Nadu , India (2021).
Wang, J., Teng, Y., Zhai, Y., Zhang, C., Pan, Z., & Yue, W. Influence of surface-water irrigation on the distribution of organophosphorus pesticides in soil-water systems, Jianghan Plain, central China. J. Environ. Manag. 281(2020). https://doi.org/10.1016/j.jenvman.2020.111874 (2021).
Lajmanovich, R. C. et al. Acute toxicity of colloidal silicon dioxide nanoparticles on amphibian larvae: Emerging environmental concern. Int. J. Environ. Res. 12(3), 269–278. https://doi.org/10.1007/s41742-018-0089-8 (2018).
Jarsjö, J. et al. Projecting impacts of climate change on metal mobilization at contaminated sites: Controls by the groundwater level. Sci. Total Environ. 712, 135560. https://doi.org/10.1016/j.scitotenv.2019.135560 (2020).
Sarkar, S., Singh, S., Ghanshyam, G., Gupta, D., & Verma, S. K. (2022). Water toxicants : a comprehension on their health concerns , detection , and remediation. Environ. Sci. Pollut. Res. 53934–53953. https://doi.org/10.1007/s11356-022-20384-x
Xiao, Y. et al. Investigating sources, driving forces and potential health risks of nitrate and fl uoride in groundwater of a typical alluvial fan plain. Sci. Total Environ. 802, 149909. https://doi.org/10.1016/j.scitotenv.2021.149909 (2022).
Radfard, M., Gholizadeh, A., Azhdarpoor, A. & Badeenezhad, A. Health risk assessment to fluoride and nitrate in drinking water of rural residents living in the Bardaskan city, arid region, southeastern Iran. Desalin. Water Treat. 145, 249–256. https://doi.org/10.5004/dwt.2019.23651 (2019).
Odiyo, J. O., Mathoni, M. M., & Makungo, R. Health risks and potential sources of contamination of groundwater used by public schools in Vhuronga 1 , Limpopo Province , South Africa. Int. J. Environ. Res. Public Health 17(6912) (2020).
Armah, F. A. Relationship between coliform bacteria and water chemistry in groundwater within gold mining environments in Ghana. Water Qual. Expo Health 5(3), 183–195. https://doi.org/10.1007/s12403-014-0110-1 (2014).
Armah, F. A. Relationship Between Coliform Bacteria and Water Chemistry in Groundwater Within Gold Mining Environments in Ghana. 183–195. https://doi.org/10.1007/s12403-014-0110-1 (2014).
Appah, S., Id, A., Id, B. M. S., & Lartey, P. O. Comparative assessment of the relationship between coliform bacteria and water geochemistry in surface and ground water systems. Plos One. 1–17. https://doi.org/10.1371/journal.pone.0257715 (2021).
APHA. Standard Methods for the Examination of Water and Wastewater 23rd edn. (American Public Health Association, 2017). https://doi.org/10.2105/AJPH.51.6.940-a.
Griboff, J., Wunderlin, D. A., & Monferran, M. V. Metals, As and Se determination by inductively coupled plasma-mass spec- trometry (ICP-MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem. J. https://doi.org/10.1016/j.microc.2016.09.013 (2016).
Affum, A. O. et al. Total coliforms, arsenic and cadmium exposure through drinking water in the Western Region of Ghana : Application of multivariate statistical technique to groundwater quality. Environ. Monit. Assess https://doi.org/10.1007/s10661-014-4167-x (2015).
Moazeni, M., Heidari, Z., Golipour, S., Ghaisari, L., Sillanpää, M., & Ebrahimi, A. Dietary intake and health risk assessment of nitrate , nitrite , and nitrosamines : A Bayesian analysis and Monte Carlo simulation. Environ. Sci. Pollut. Res. 45568–45580 (2020).
Ludwicki, J. K. et al. Hazard quotient pro fi les used as a risk assessment tool for PFOS and PFOA serum levels in three distinctive European populations. Environ. Int. 74, 112–118. https://doi.org/10.1016/j.envint.2014.10.001 (2015).
Silva, V. H. C., Francisco, H., Santana, C. S., Montalv, D. M., Luzardo, M., Velasco, F. G., & Jesus, R. M. De. Assessment of water resources pollution associated with mining activity in a semi-arid region. J. Environ. Manag. 273. https://doi.org/10.1016/j.jenvman.2020.111148 (2020).
Sharifi, Z. & Safari Sinegani, A. A. Assessment of arsenic, nitrate and phosphorus pollutions in shallow groundwater of the rural area in Kurdistan Province (Iran). Curr. World Environ. 7(2), 233–241 (2012).
Tiwari, R. N., Mishra, S. & Pandey, P. Study of major and trace elements in groundwater of Birsinghpur Area, Satna District Madhya Pradesh, India. Int. Jo. Water Resour. Environ. Eng. 5, 380–386. https://doi.org/10.5897/IJWREE2012.0408 (2013).
Gomaa, F. A. Spatial distribution and hydrogeochemical factors influencing the occurrence of total coliform and E . coli bacteria in groundwater in a hyperarid area, Ad-Dawadmi , Saudi Arabia. Water 14(3741) (2022).
Sojobi, A. O. Evaluation of groundwater quality in a rural community in North Central of Nigeria. Environ. Monit. Assess https://doi.org/10.1007/s10661-016-5149-y (2016).
Semanka, T., Seifu, E. & Sekwati-monang, B. Effects of Moringa oleifera seeds on the physicochemical properties and microbiological quality of borehole water from Botswana. Water Sanit. Hyg. Dev. 12(9), 659–670. https://doi.org/10.2166/washdev.2022.100 (2022).
Karunanidhi, D., Aravinthasamy, P., Subramani, T., Wu, J. & Srinivasamoorthy, K. Potential health risk assessment for fluoride and nitrate contamination in hard rock aquifers of Shanmuganadhi River basin, South India. Hum. Ecol. Risk Assess. 25, 250–270. https://doi.org/10.1080/10807039.2019.1568859 (2019).
Rahman, M. & Bodrud-doza, A. I. Spatio-temporal assessment of groundwater quality and human health risk : A case study in Gopalganj, Bangladesh. Exposure Health 10(3), 167–188. https://doi.org/10.1007/s12403-017-0253-y (2018).
Rezvani Ghalhari, M., Ajami, B., Ghordouei Milan, E., Zeraatkar, A. & Mahvi, A. H. Assessment of non-carcinogenic health risk of nitrate of groundwater in Kashan, Central Iran. Int. J. Environ. Anal. Chem. 101(23), 4641–4653. https://doi.org/10.1080/03067319.2021.1931157 (2021).
Amiri, V., Sohrabi, N., Li, P. & Amiri, F. Groundwater quality for drinking and non-carcinogenic risk of nitrate in urban and rural areas of Fereidan, Iran. Exposure Health 15, 807–823. https://doi.org/10.1007/s12403-022-00493-z (2023).
Ahada, C. P. S. & Suthar, S. Groundwater nitrate contamination and associated human health risk assessment in southern districts of Punjab, India. Environ. Sci. Pollut. Res. 25, 25336–25347. https://doi.org/10.1007/s11356-018-2581-2 (2018).
Noori, R. et al. A non-threshold model to estimate carcinogenic risk of nitrate-nitrite in drinking water. J. Clean. Prod. 363, 132432. https://doi.org/10.1016/j.jclepro.2022.132432 (2022).
Seifi-Nigje Gheshlagh, F., Ziarati, P., & Arbabi Bidgoli, S. Seasonal fluctuation of heavy metal and nitrate pollution in groundwater of farmlands in Talesh, Gilan, Iran. Int. J. Farm. Allied Sci. (2013).
Mirzabeygi, M. et al. The concentration data of fluoride and health risk assessment in drinking water in the Ardakan city of Yazd province, Iran. Data Br. 18, 40–46. https://doi.org/10.1016/j.dib.2018.02.069 (2018).
Albertini, R. et al. The use of biomonitoring data in exposure and human health risk assessments. Environ. Health Perspect. 114(11), 1755–1762. https://doi.org/10.1289/ehp.9056 (2006).
Ortiz, M. E., Marco, A., Saiz, N. & Lizana, M. Impact of ammonium nitrate on growth and survival of six European amphibians. Arch. Environ. Contamin. Toxicol. 47(2), 234–239. https://doi.org/10.1007/s00244-004-2296-x (2004).
Environmental Protection Agency (USEPA). Regional Screening Level Tables. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (2023).
Weber, G. & Kubiniok, J. Spring waters as an indicator of nitrate and pesticide pollution of rural watercourses from nonpoint sources: Results of repeated monitoring campaigns since the early 2000s in the low mountain landscape of Saarland, Germany. Environ. Sci. Eur. 34(1), 53. https://doi.org/10.1186/s12302-022-00632-0 (2022).
Salehi, F., Sharafi, S., Kazemi, A., & Esmaeilbeigi, M. Unveiling hidden threats: Assessing the risks ofpotentially toxic elements (PTEs) in contaminated fish of the Anzali Wetland. Reg. Stud. Mar. Sci. 79, 103853. (2024).
Kazemi, A., Ebrahimpour, E., Esmaeilbeigi, M., et al. Optimizing oxytetracycline removal from aqueoussolutions using activated carbon from barley lignocellulosic wastes with isotherms and thermodynamic studies. Sci.Rep. 14, 23281. (2024).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Ali Kazemi: supervision, investigation. Milad Esmailibegi: data analysis, writing first draft, data visualisation. Mohammad Javad Nahvinia: graphical analysis, ArcGIS. Parisa Sahebi: graphical abstract and data analysis. Zahra Sahebi: data curation, data collection. Maral Ahadi: manuscript revision. Ali Ghanavati Asl: software and graphical.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kazemi, A., Esmaeilbeigi, M., Nahvinia, M.J. et al. Mineral pollutants and coliform contamination in groundwater pose health risks to consumers: a spatiotemporal study in a mining-impacted area. Sci Rep 14, 26293 (2024). https://doi.org/10.1038/s41598-024-77721-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77721-y