Abstract
Chitinase-like proteins have multiple biological functions that promote tumor growth, angiogenesis and metastasis. Expression of CHI3L2, which is similar in structure to CHI3L1, is detected in glioma cells and tumor-associated macrophages (TAMs) in glioma and breast cancer. However, its exact role remains unclear. We analyzed the expression of CHI3L2 in 74 invasive ductal breast carcinoma (IDC) tumors, breast cancer and macrophages cell cultures using immunohistochemistry, immunofluorescence, Western blot and PCR methods. Clinicopathologic data were included in the analysis. The results obtained show that CHI3L2 expression decreases with increasing degree of tumor grade and negative status of estrogen (ER) and progesterone receptors (PR). Furthermore, CHI3L2 is significantly and positively correlated with phosphorylation of STAT-3 and ERK1/2 signaling pathways, but negatively correlated with macrophage infiltration. CHI3L2 is expressed both in the cytoplasm of cancer cells and in macrophages and may regulate STAT-3 and ERK1/2 phosphorylation in breast cancer cell lines. Analysis of the clinicopathologic data revealed that CHI3L2 levels had no effect on patient survival. CHI3L2 expression may be specific for cancer cells in IDC and involved in cross-talk with the tumor microenvironment. Our study has shown that IDC cancer cells express the CHI3L2 protein, possibly indicating a novel function of this protein.
Similar content being viewed by others
Introduction
CHI3L2 (chitinase 3-like 2 protein; YKL-39) is a 39 kDa lectin that belongs to the chitinase-like protein family and shows high homology and structural similarity to CHI3L1. Of the chitinases found in human tissues, only mammalian acid chitinase and chitotriosidase exhibit chitinase activity. However, chitinase-like proteins, including CHI3L1, CHI3L2 and SI-CLP (stabilin-1 interacting chitinase-like protein), have biological functions that contribute to stimulation of cell proliferation, support of angiogenesis and tumor invasion, cell interactions and immunomodulation1,2,3,4,5,6,7. The best-known protein in this family is undoubtedly CHI3L1, also known as YKL-40. Its established role in promoting angiogenesis and involvement in development of various cancers, such as breast cancer, colorectal cancer and glioblastoma is well documented8,9,10,11. In addition, it is involved in bypassing the cancer immune system and in pathological inflammation in various diseases, including Alzheimer’s and Parkinson’s disease, multiple sclerosis and even asthma12,13,14,15,16,17,18.
Elevated levels of the CHI3L1 protein have been detected in the serum of cancer patients, including those with gliomas, colorectal, lung and breast cancer as well as leukemia. These elevated levels are usually associated with poor prognosis, tumor growth and metastasis6. In our previous study, we have shown that increased CHI3L1 expression in IDC tumors can promote angiogenesis and be associated with the growth of triple-negative tumors19. Furthermore, our results show a correlation between the expression of CHI3L1 and Nogo-A in IDC and their involvement in angiogenesis in this cancer subtype20. Recent studies also indicate increased levels of CHI3L1 in bronchoalveolar lavage fluid and serum of critical COVID-19 patients, suggesting the possible involvement of this protein in the pathogenesis of the disease and its importance as a biomarker21,22,23.
The significance of CHI3L2 in breast cancer is still largely unknown. There are only a limited number of articles pointing to its importance in the expression of TAMs. Recent studies emphasize the central role of this protein in promoting angiogenesis and supporting distant metastasis3,24. In fact, it is more likely that the source of CHI3L2 protein in breast tumor tissue is macrophages.
In this article, the expression of CHI3L2 in the cytoplasm of IDC and breast cancer cell lines is presented for the first time, and the influence of CHI3L2 protein on the phosphorylation of STAT-3 and ERK1/2 signaling pathways is discussed. Furthermore, the correlation between CHI3L2 expression and clinicopathological data of patients diagnosed with IDC was analyzed. Our results reveal a novel role for chitinase-like proteins. CHI3L2 may have a protective function against tumors that differs from CHI3L1 despite the structural similarity of the two proteins.
Materials and methods
Cell lines
Human cancer cell lines were cultured from various clinicopathological breast tumors, including MCF-7, SK-BR-3, T-47D, BT-474, B-T549, MDA-MB-231, MDA-MB-436, MDA-MB-468, and BO2 (all from ATCC, American Type Culture Collection ATCC, Old Town Manassas, VA, USA)25,26. The MCF10A cell line, which is derived from fibrocystic breast disease, and the HME1-hTERT (Me16C) (ATCC) cell line, which is normal mammary epithelial cells immortalized with hTERT, were also used. Cells were cultured in appropriate media: α-MEM (Sigma-Aldrich) for MCF-7, BO2 and BT-474, L-15 medium (ATCC) for MDA-MB-231 and MDA-MB-468, EMEM (Lonza) for SK-BR-3, L-15 (ATCC) supplemented with 10 µg/ml insulin and 16 µg/ml glutathione (both from Sigma-Aldrich) for MDA-MB-436, RPMI-1640 (Gibco, Thermo-Fisher Scientific) supplemented with 0.1% insulin (Sigma-Aldrich) for BT-549. The human acute monocytic leukemia cell line THP-1 (ATCC) was cultured in RPMI-1640 medium (Gibco, ThermoFisher Scientific, Wilmington, DE, USA). All media were supplemented with fetal bovine serum (FBS) up to 10% (Sigma-Aldrich) and penicillin–streptomycin solution up to 1% of the final volume (Sigma-Aldrich). For MCF10A and hTERT (Me16C), the MEGM ™ bullet kit (Lonza) was used for cell culture. Cell passaging was performed with TrypLE (Gibco, ThermoFisher Scientific) at a maximum confluence of 70%. The media were changed twice a week. Cell culture was performed under standard conditions in a humidified atmosphere with 5% CO2. The molecular characteristics of the individual breast cancer lines according to Kao et al.27 are listed in Table 1.
Monocyte differentiation
THP-1 cells were cultured for 24 h in complete RPMI-1640 medium (Gibco), supplemented with 100 nM PMA (phorbol 12-myristate 13-acetate) (Sigma-Aldrich)28,7. Subsequently, adherent macrophages were identified at the bottom of the vessel.
Co-cultures
Macrophages were cultured for one week in conditioned media of MCF-7, MDA-MB-468, MCF 10A and MDA-MB-231 cells. Macrophage pellets were then harvested and frozen at -80°C for protein expression analysis.
Transfections experiment
In turn, effect of CHI3L2 siRNA on ERK1/2 and STAT-3 patchways phosphorylations were investigated in two cell lines characterised by expresion of this protein: BT-549 and MDA-MB-231. Cells were seed on 6-well plate in amount 3 × 105 per well. Next day, transfection were provided with 2 µg siRNA CHI3L2 (cat. AM16708, ID: 119,204, ThermoFischer Scientific) and X-tremeGENE siRNA Transfection Reagent (Roche, Mannheim, Germany) (proportion 1:5) in 2 ml of Opti-MEM medium (Gibco). Cells without siRNA were used as controls. After 24 and 48h, cells were harvested for molecular analysis and stored in −80 °C.
CHI3L2 recombinant protein
Infuence of exogenous CHI3L2 were next analysed on breast cancer cell lines without expression of this protein. First, MD-MB-468 and BT-474 cells were seeded on 6-well plates in amount 3 × 105 in dedicated media. Next day, media were removed and CHI3L2 human recombinant protein (cat. 5112-CH, R&D Systems, Minneapolis, MN, USA) was added to cell culture in amount 3 µg per well (1.5 µg/ml), in 2 ml of Opti-MEM medium (Gibco). After 24 and 48h, cells were harvested for molecular analysis and stored in -80 °C.
Patients and tumors
The 74 samples of IDC used in this study were archival material from patients diagnosed and operated on at the Lower Silesian Oncology Center (Wroclaw, Poland) between 1999 and 2009. As a control, 12 non-malignant breast tissue lesions (NBTL) were included in the study. Breast cancer samples were graded according to the WHO system29,30 and tissue samples were either embedded in paraffin or stored at −80 °C.
Immunohistochemical reactions
Immunohistochemistry was performed on 4 µm sections of paraffin-embedded tissue samples using the Dako Autostainer Link48 (Dako, Glostrup, Denmark). Paraffin removal and antigen retrieval were performed in PT-Link (Dako) with basic EnVision FLEX Target Retrieval Solution (97 °C, 20 min; Dako) for immunostaining of CHI3L2, CD68 and CD206, CHI3L1, VEGFA, VEGFD, CD31, CD34, estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). The VEGFC and Ki-67 antibodies were treated with Dako’s acidic EnVision FLEX Target Retrieval Solution. Dako’s EnVision FLEX Peroxidase Blocking Reagent was used to block endogenous peroxidase for 5 min. The EnVision FLEX + Mouse, High pH System (Dako) was used to visualize IHC reactions by mouse monoclonal antibodies: CHI3L2 (1:100 with linker, MAB5116, R&D, Minneapolis, Minnesota, USA), CD206 (1:100, MAB25341, R&D), CD31 (RTU, Dako; 20 min), CD34 (RTU, Dako; 20 min), VEGFA (1:50, Dako 18 h, 4°C), Ki-67 (RTU, Dako, 20 min), ER (RTU, Dako, 20 min) and PR (RTU, Dako, 20 min).
The LSAB + detection system (Dako) was used to visualize IHC reactions obtained with goat polyclonal antibodies against CHI3L1 (1:100, AF2599, 20 min; R&D Systems, Minneapolis, MN USA), VEGFC (1:100, 18 h, 4°C; ReliaTech GmbH, Braunschweig, Germany) and VEGFD (1:100, 18 h, 4°C; ReliaTech GmbH). HER2 expression was measured using the HercepTest kit (Dako). Counterstaining with hematoxylin (5 min) was performed (EnVision FLEX Hematoxylin). The visualization systems were used according to the manufacturer’s instructions. A secondary HRP-conjugated rat antibody was applied at a dilution of 1:400 (Jackson ImmunoResearch) and incubated for 1 h at room temperature (RT). EnVision FLEX/HRP was also applied and incubated for 1 h at RT (Dako). EnVisionFLEX Substrate Buffer was then added and incubated for 10 min at RT (Dako)19,20,26,31,32.
Evaluation of the immunohistochemical reactions
The immunohistochemical reactions for CHI3L2, CHI3L1, VEGFA, VEGFC and VEGFD were evaluated using the 12-point immunoreactive score (IRS) developed by Remmele and Stegner33 (Table 2).
For the evaluation of the pan-macrophage marker CD68 and the anti-inflammatory macrophage CD206, the positively tested cells were counted in five hotspots with a × 200 magnification and the average value was given. The Chalkley’s ophthalmoscope (Pyser Inc., Edenbridge, UK) was used to score CD31- and CD34-positive blood vessels based on the number of grid points (n = 1–25) and to estimate the relative microvessel count (MVC)34,35,36. Methodologically, the basis for the relative microvessel density (MVD) was determined by taking the mean of the three hotspots with the highest vascularization (magnification × 200), according to the approach of Fox and Harris36. A semi-quantitative scale from 0 to 4 was used for Ki-67 expression: 0 for 0% of positive cells, 1 for 1–10% of positive cells, 2 for 11–25% of positive cells, 3 for 26–50% of positive cells and 4 for 51–100% of positive cells37. For this marker, high expression was defined as values > 25% of positive cells, while low expression was considered ≤ 25%. For estrogen (ER), progesterone (PR) and human epidermal growth factor receptor 2 (HER2), a semi-quantitative scale of 0–3 was used: 0 means 0% of positive cells, 1 means 1–10% of positive cells, 2 means 11–50% of positive cells and 3 means 51–100% of positive cells. For HER2, a positive response was detected when more than 10% of cancer cells showed an intense membrane response (score of 3)38. CHI3L2 protein expression and its correlation with the clinicopathological data of the patients are shown in Table 3. For the markers CHI3L1, CHI3L2, VEGFA, VEGFC, VEGFD, CD31, CD34, CD68 and CD206, the median expression values were used as cut-off points for the analysis of low and high expression (Table 4). The immunohistochemical reactions were analyzed using a BX41 light microscope (Olympus, Tokyo, Japan).
Immunofluorescence reactions
Immunofluorescence reactions were performed on 4 µm thick tissue sections. Deparaffinization was performed with a graded alcohol series at room temperature, and epitope retrieval was performed at 90 °C for 15 min at pH = 9.0. For blocking, a 1% BSA solution in PBS/0.1% Tween 20 was used for 30 min at RT. Slides were then incubated overnight at 4 °C with anti-CHI3L2 anti-rat antibody (1:5, #18H10)24 and mouse monoclonal antibody (1:500, MAB5116, R&D). Incubation with secondary anti-rat and anti-mouse antibodies conjugated to the fluorochromes Alexa568 (Jackson ImmunoResearch) and Alexa 488 (ab150113, Abcam, Cambridge, UK) was performed for 1 h at RT (dilution 1:2000 in 1% BSA in PBS/0.1% Tween20). Slides were coverslipped with FluoroShield mounting medium with DAPI (Abcam, Cambridge, UK, ab104139). A confocal microscope (Olympus Fluoview FV3000, Tokyo, Japan) and imaging software (Olympus Cell Sense) were used to analyze the immunofluorescence reactions26,39.
Western blot
Tissue samples were digested in T-PER Tissue Protein Extraction Reagent using a TissueRuptor homogenizer (Qiagen, Hilden, Germany), while cell lysates were homogenized in RIPA buffer. For protein isolation, 5 mM PMSF (phenylmethanesulfonyl fluoride), EDTA and Heat™ Protease Inhibitor Coctail × 100 (all Thermo Scientific, Wilmington, DE, USA) were added. The bicinchoninic acid assay (Pierce BCA Protein Assay Kit) was used to determine total protein content, which was measured using the NanoDrop1000 (Thermo Fisher). Samples were denatured in a buffer consisting of 250 mM TRIS at pH 6.8, 40% glycerol, 20% (v/v) β-mercaptoethanol, 0.33 mg/ml bromophenol blue and 8% sodium dodecyl sulfate (SDS) for 10 min at 95 °C. To perform SDS-PAGE, a Mini Protean 3 instrument (Bio-Rad, Hercules, CA, USA) was used with a 10% polyacrylamide gel for total protein and 12% for VEGFA and VEGFC; 30 μg of protein40,41 was applied per lane. We then performed a wet transfer in Tris–glycine buffer containing 20% methanol and 0.05% SDS onto a PVDF 0.45 µm (polyvinylidene difluoride membrane) (Immobilon, Millipore, Bedford, MA, USA) for a 1h transfer at 140 V to separate the proteins. For VEGFA and VEGFC proteins, 0.20 µm nitrocellulose membranes (Bio-Rad) were used at transfer conditions of 0.5 h at 70 V. The blocker used was 5% skim milk in 0.05% TBST or 5% BSA in 0.05% TBST for CHI3L1.
Incubation with primary antibodies was performed overnight at 4 °C at the following concentrations: CHI3L2 (mouse monoclonal antibody, MAB5116, R&D, 1:1000 in buffer group 1 recommended by producer), STAT-3 (rabbit polyclonal antibody, 1:1000 in 0.1%, ab68153, Abcam, Cambridge, UK), p-STAT-3 (rabbit monoclonal antibody, 1:2000 in 5% milk in TBST 0.05%, ab76315, Abcam), ERK1/2 (rabbit monoclonal, 1:10 000, ab184699, Abcam), pERK1/2 (rabbit monoclonal, 1:1000, ab201015, Abcam), β-tubulin (rabbit polyclonal, 1:1000, ab6046, in 0.1% BSA in 0. 1% TBST, ab6046, Abcam,), VEGFA (mouse monoclonal, 1:1000, M7273, Dako, Glostrup, Denmark), VEGFC (mouse monoclonal, 101-M90, ReliaTech, Wolfenbüttel, Germany), VEGFD (mouse monoclonal, MAB286, R&D)7.
For the secondary HRP-conjugated antibodies, incubation was performed for 1 h at RT. Donkey anti-mouse (1:3000), donkey anti-rabbit (1:6000), both in 5% milk in 0.05% TBST (Jackson ImmunoResearch, Suffolk, UK) or donkey anti-goat (1:10,000 in TBST, Jackson ImmunoResearch) were used. Super-Signal West Femto chemiluminescence substrate from ThermoFisher was used for the chemiluminescence reaction. Visualization was performed using Bio-Rad’s ChemiDocTM MP system with Bio-Rad’s ImageLab software (exposure time from 1 s to 3 min). Densitometric analysis was based on independent triplicates using ImageLab software (Bio-Rad). Reference protein was β-tubulin, except for pSTAT-3 and pERK1/2, where STAT-3 and ERK1/2 levels, respectively, served as reference42.
The Droplet Digital PCR™ (ddPCR)
For determination of the absolute number of genes mRNA copies in the analyzed cells, the Droplet digital PCR (ddPCR) method was applied. Isolation of total RNA was provided with RNeasy Mini kit (Qiagen, Hilden, Germany), and reverse transcription (RT-PCR) with iScript™ Reverse Transcription Supermix for RT-qPCR (Bio-rad, Hercules, 345 CA, USA). From each sample, 70 ng of RNA was reverse transcribed with the C1000 Touch Thermal Cycler (Bio-Rad). Reactions were provided in the conditions as follows: priming (5 min, 25°C), reverse transcription (20 min, 46°C), inactivation of reverse transcriptase (1 min, 95°C). The ddPCR reaction mixtures were consisted of 2.3 μl of RT product, 1 μl of TaqMan specific probe (Applied Biosystems, Foster City, CA, USA), 6.7 μl of molecular biology-grade water and 10 μl of 2X ddPCR™ MasterMix for Probes (Bio-Rad). In the experiment, the TaqMan-specific probes were applied to assessed mRNA expression of investigated gene Hs00970220_m1 (CHI3L2). Next, 20 μl of the reaction mixtures were transferred into a plastic cartridge (Bio-Rad) with addition of 50 μl of Droplet Generation Oil for Probes (Bio-Rad) in the QX100 Droplet Generator (Bio-Rad). Finally, droplets get from each sample were loaded on 96-well PCR plate (Eppendorf, Hamburg, Germany). PCR amplifications were provided in a C1000 Touch Thermal Cycler (Bio-Rad) with conditions: enzyme activation (10 min, 95°C), followed by 40 cycles of denaturation (30 s, 94°C) and annealing/extension (1 min, 60°C), enzyme deactivation (10 min, 98°C), ending (10 min, RT). At the end, the plate was transferred to Droplet Reader (Bio-Rad) and automatically reading were applied. The absolute quantification of each mRNA was obtained from the number of positive counts per panel with the Poisson distribution. The quantification of the target mRNA was visualised as the number of copies/μl (AQ) in the PCR reaction mixture7,41.
mRNA expression profile
For the transcriptomic analysis of tumor material we used the database GSE3494 of the Gene Expression Omnibus Portal. This database contains clinical data and a complete microarray evaluation of gene expression for 251 breast cancer cases (G1: N = 67, G2: N = 128, G3: N = 54) and uses the GPL96 [HG-U133A] Affymetrix Human Genome U133A Array (Thermo Fischer)43. Patients underwent surgery in Sweden between 1987 and 1989. The raw gene expression data were normalized using the global mean approach, where the logarithmic signal values were transformed and scaled with log 500 as the target signal value. In our study, we examined CHI3L2 expression data from breast cancer tumors with different tumor grades (G1, G2, G3) and estrogen (ER) and progesterone receptor (PR) expression.
Institutional Review Board Statement
The study was conducted in compliance with the Declaration of Helsinki and was approved by the Bioethics Committee of WROCLAW MEDICAL UNIVERSITY (No. KB-517/2017, approved on July 22, 2017; KB-217/2022 and No. KB-216/2022, both approved on March 15, 2022).
Statistical analysis
The normal distribution was analyzed using the Shapiro–Wilk test. As the variables had a non-normal distribution, a non-parametric test was used for the statistical analysis. The expression of the markers was analyzed using Spearman’s and Kendall’s tau -b correlation, the Kruskal–Wallis test with post-hoc Dunn test and the Mann–Whitney U test. The Breslow, Kaplan–Meier and Cox hazard regression models were used to examine the survival rates. The proportionality assumption was met for all predictors except pT, which was converted to a categorical variable in the results. For analysis of results obtained with ddPCR, Kruskal–Wallis test with post-hoc Dunn test was applied. Statistically significant p-values were defined at a level ≤ 0.05. Statistical analyzes were performed using Statistica 13.3 (Tibco, Palo Alto, CA, USA).
Results
CHI3L2 expression in cancer cells is higher in lower tumors grade ER + and PR + and is not associated with angiogenesis and macrophage infiltration
The clinicopathologic data and the results of the immunohistochemical reactions are shown in Table 3.
Cytoplasmic expression of CHI3L2 in cancer cells in IDC was detected by immunohistochemistry. Low expression of this protein occurred in 52.7% of cases, while high expression occurred in 47.3% of cases (Table 4).
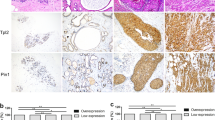
The differentiation of the expression levels of CHI3L2 and other markers detected by immunohistochemical reactions is shown in Fig. 1.
The expression level of CHI3L2 was highest in non-malignant breast tissue lesions (NBTLs) and decreased significantly with higher tumor grades. Conversely, the expression of CD68, indicative of macrophage infiltration, and CD206 (specific for an anti-inflammatory macrophage phenotype) increased with malignancy grade (Fig. 2).
The angiogenesis markers VEGFA, VEGFC and VEGFD were expressed in the cytoplasm of the cancer cells. No significant Spearman correlations were found between CHI3L2 expression and angiogenesis markers, including CD31 (r = −0.094, p = 0.42), VEGFC (r = 0.02, p = 0.9) and VEGFD (r = −0.12, p = 0.98) (data not shown). CHI3L2 showed an almost statistically significant correlation with CD34 (T = 0.14, p = 0.064) and VEGFA (T = 0.14, p = 0.082), while there were no correlations with CHI3L1 (Fig. 3A), as shown by the Kendall’s Tau-b correlations. Spearman correlation showed a significant negative association between CHI3L2 and CD68 expression (Fig. 3B), but no significant correlation was found between CHI3L2 and CD206 expression. The expression of CD68 and CD206 showed a significant moderate positive correlation (Fig. 3B).
The cases with the highest CHI3L2 expression showed positive ER and PR expression, which was significantly correlated, while the lowest CHI3L2 expression was associated with positive HER2 expression (Fig. 4).
Analysis of immunohistochemical expression and its correlation with estrogen receptor (ER; ER+ N = 45, ER- N = 21), progesterone receptor (PR, PR+ N = 40, ER- N = 26) and human epidermal growth factor receptor 2 (HER2, HER2+ N = 40, HER2- N = 26) status was performed using the Mann–Whitney U test. Expression was expressed as 0 for negative and 1 for positive receptors. Statistically significant p-values were < 0.05.
The immunofluorescence reaction proves the presence of CHI3L2 in the cytoplasm of cancer cells in IDC tumors. In addition, CHI3L2 expression was also observed in CD206-positive TAMs (Fig. 5).
Expression of CHI3L2 in IDC tumors. Immunofluorescence reactions visualized by confocal microscopy showed the expression of CHI3L2 (green; yellow arows) in cancer cells and CD206 (red; white arroheads) as a characteristic feature of TAMs. Co-expression of CHI3L2 and CD206 was also observed (magenta arrows). The nucleus was stained with DAPI (blue). Images from single fluorescent channels is showed in Fig. 1S supplemental material.
CHI3L2 is not a prognostic factor for patient survival
The analysis of patient survival using the proportional Cox hazards model showed no influence of CHI3L2 expression on the survival of patients with IDC (p = 0.52, Cox parameter = 0.11). However, an association was found with pT status (tumor size) and PR status (p = 0.011, Cox parameter = −1.5 and p = 0.0098, Cox parameter = 1.2, respectively) (Table 5). Other investigated clinicopathologic factors, such as ER, HER2 status, pN, Ki-76, age at treatment initiation, menopausal status and G, showed no significant associations. However, overall patient survival was found to be significantly influenced by pT, which had an unfavorable prognostic value, and PR, which had a favorable prognostic value. Table 5 shows the results of the Cox proportional hazard analysis.
Expression of CHI3L2 positively correlates with the phosphorylation of ERK1/2 and STAT-3 in IDC tumors
In tumor material, the highest expression of CHI3L2 was observed in G1 IDC tumors, and it decreased with the progression of tumor grades (see Fig. 6A, B). Immunoblotting also showed negative cases for CHI3L2 expression. No correlation was found between CHI3L2 expression and CHI3L1, VEGFA, VEGFC and VEGFD (data not shown). The highest level of CHI3L2 showed a positive and significant correlation with the phosphorylation of STAT-3 and ERK1/2 (Fig. 7).
Western blot analysis of CHI3L2 expression in IDC in A tumors of different malignancy grade: G1 (n = 3), G2 (n = 9) and G3 (n = 10), with expressions of pSTAT-3, STAT-3, pERK1/2 and ERK1/2; B statistical analysis (data from densitometric analysis of Western blots experiments provided in triplicates) on CHI3L2, pSTAT-3 and pERK1/2 expressions in G1-G3 grade tumors (Kruskal–Wallis test, post-hoc Dunn’s test). Raw data from densitrometric analysis is showed in Fig. 2S supplemental material.
Breast cancer cells produce CHI3L2 and may stimulate macrophages to express CHI3L2
Expression of CHI3L2 protein was observed in breast cancer cells BT-549, MDA-MB-436 and BO2, as well as in normal breast epithelium and cells with fibrocystic disease: HME1-hTERT (Me16C) and MCF10A (Fig. 8). All analyzed cells expressing CHI3L2 were negative for ER, PR and HER2 and are considered representative of the basal subtype of breast cancer, except for the BO2 cells with metastatic adenocarcinoma features, as shown in Table 1. Macrophages expressed CHI3L2 at low levels, but the expression of this protein was higher in macrophage cocultures with conditioned medium from CHI3L2-negative cancer cells (MCF-7 and MDA-MB-468), and also with CHI3L2-positive MDA-MB-231 cells. Interestingly, macropgahes co-culture with CHI3L2-positive normal epithelial cells (HME1-hTERT (Me16C) didn’t provide to CHI3L2 expresion in macrophages. In addition, the levels of pSTAT-3 and pERK1/2 in breast cancer cells and macrophages were not dependent on CHI3L2 expression (Fig. 8).
Western blot analysis of CHI3L2, pSTAT-3, STAT-3, pERK1/2 and ERK1/2 in breast cancer cell lines, macrophages (M) and macrophages in co-culture with MCF-7 (M/MCF7), MDA-MB-468 (M/M468), Me16C cells (M/Me16C) and MDA-MB-231 (M/M231) cells (the characteristics of breast cancer cell lines are shown in Table 1).
Changes in CHI3L2 levels affect ERK1/2 and STAT-3 phosphorylation
Transfection experiments with CHI3L2 siRNA resulted in a decrease of pERK1/2 in BT-549 cells at 24h and 48h, but no difference was observed in MDA-MB-231 cells. In the case of STAT-3 phosphorylation, the lowest expression levels were detected in BT-549 cells only at 24h post-transfection, whereas an increase was observed at 48h in MDA-MB-231 cells (Fig. 9A). The analysis of transfection with CHI3L2 mRNA levels in MDA-MB-231 and BT-549 cells was confirmed by ddPCR technique (Fig. 9B, C). Consistent with these findings, in experiments where CHI3L2 recombinant protein was added to MDA-MB-468 and BT-474 cell culture media (CHI3L2 status is negative in these two cell lines), an increase in pERK1/2 levels was observed at 24 and 48h compared to control cultures (Fig. 9D). No changes were observed in pSTAT-3 levels.
Breast cancer cells models with different status of CHI3L2 after transfection with CHI3L2 siRNA: (A) changing in STAT-3 and ERK1/2 phosphorylation; Western blot analysis; (B) analysis of CHI3L2 mRNA in MDA-MB-321 and (C) BT-549 cells during trnasfection experiments provided in triplicates in ddPCR analysis; and (D) with presence of recombinant CHI3L2 protein in MDA-MB-468 and BT474 cells ; Western blot analysis; Kruskal–Wallis test followed by Dunn test
CHI3L2 mRNA expression was not significantly different in non-specific breast cancers
Transcriptome analysis performed in breast cancer cases revealed that mRNA levels of CHI3L2 were highest in G3, although there were no significant differences between tumors with different tumor grades. A tendency for the highest CHI3L2 mRNA level was observed in tumors without ER or PR expression. This correlation was not significant, but a trend was observed (Fig. 10).
PCR analysis of CHI3L2 mRNA expression level in breast tumors. Different tumor grades: G1 (n = 67), G2 (n = 168), G3 (n = 54); G1 vs G2 p = 0.083; G2vsG3 p = 0.12, G1vs G3 p = 0.9 (Kruskal–Wallis test, post-hoc Dunn’s test); status of estrogen (ER), progesterone (PR) and HER2 receptor; 0: negative expression, 1: positive expression of the receptors; CHI3L2 vs PR p = 0.44, CHI3L2 vs ER p = 0.46 (Mann–Whitney U-test); statistically significant p-values were < 0.05.
Discussion
CHI3L1 and CHI3L2 proteins are both chitinase-like proteins with lack of enzymatic activity. Some proteins in this family have chitinase activity: chitotriosidase and acidic mammalian chitinase (AMCase). In recent years, the role of this chitinases in cancerogenesis has been discussed. CHI3L1 is one of the best known chitinase proteins and is currently known to have a pro-tumor and pro-angiogenic effect in many types of cancer, including breast cancer. On the other hand, little is known about CHI3L2, structural similarity with CHI3L1 in size, nucleotide and amino acid sequences is 47% and 51% homology, respectively, without cross-reactivity43. In addition, these proteins share the same NH2-terminal amino acid residues as noted in their alternative names (YKL-40 and YKL-39). Some authors have pointed out that simultaneous production of CHI3L1 and CHI3L2 does not occur and that these proteins have different biological functions, also in ERK patchway phosphorylation44.
In fact, only few papers have reported the expression of CHI3L2 in breast cancer, suggesting a possible role of this protein in carcinogenesis. Previous studies have demonstrated the expression of CHI3L2 in cancer cells, including glioblastoma tumors and cell lines such as U-87 MG and HEK29344,45.
Indeed, CHI3L2 was expressed in glioblastoma tumors in cancer cells and microglia, and its expression was associated with poor patient prognosis24,45. The presence of this protein in macrophages infiltrating nonspecific breast cancer tumors and its highest levels in developing metastases were reported by Liu and colleagues24. In addition, the authors show that the highest concentration of CHI3L2 after neoadjuvant therapy is an unfavorable indicator of treatment efficacy and also stimulates angiogenesis24.
In contrast, our team’s results indicate that CHI3L2 is expressed in breast cancer cells in in vitro cultures as well as in cancer cells in tumor material from patients with IDC. In our study, we found evidence that CHI3L2 expression in cancer cells is specific for this type of breast tumor.
In our studies performed on commercially available cancer cell lines, the expression of CHI3L2 was detected in triple negative cells lacking ER, PR and HER2, indicating a basal B breast cancer subtype. Our screening conducted on ten breast cancer cell lines showed that cells characterised as invasive ductal breast carcinoma basal subtype may expressed CHI3L2 protein, like BT-549 cells, and also may be CHI3L2 negative, like luminal BT-474 cells. Moreover, basal B subtype breast cancer cells, like MDA-MB-231, BO2, and MCF10A, which represent different breast cancer tumor type (metastatic adenocarcinoma, and fibrocystic disease), were also CHI3L2+. This finding could have clinical implications, and indicate, that CHI3L2 expression may be characteristic for basal B breast cancer subtype. Moreover, our results obtained in functional in vitro model suggest, that CHI3L2 is involved in IDC basal B breast cancer cells, represent in our studies by BT-549 cells, in modulation of ERK1/2 and STAT-3 phosphorylation patchways. Hovewer this findings may be general, due to broad spectrum of proces uder regulation of this signalling patchways, may have far reaching implication in our deeper understanding of IDC biology and therefore may open new areopag for precision medicine. It is worth noting that patients with triple-negative breast cancer have still a poorer prognosis, especcially triple-negative status, from all breast cancer diagnosis.
In contrast to breast cancer cells in in vitro culture, the expression of CHI3L2 in tumors was highest in tumors positive for estrogen receptor (ER) and progesterone receptor (PR) and lowest in HER2 positive tumors.
However, the results from the monolayer in vitro model of cancer cells may not reflect the complex system that occurs in tumors, where interactions between cancer cells, macrophages and stromal cells play a role and dynamics of this interaction are changing during tumor development. This difference in the expression pattern of CHI3L2 between in vitro models and patient material could be due to the above-mentioned interaction.
Although the differences in CHI3L2 mRNA levels were insignificant, this analysis was performed in breast cancer tumors without distinguishing between cancer subtype diagnoses. Analysis of clinicopathologic data showed that CHI3L2 expression had no effect on patient survival or event-free survival. Our analysis of tumor material also showed a decrease in CHI3L2 expression with higher tumor grades (G). It is possible that the overexpression of CHI3L2 in low-grade malignant tumors suggests that the protein may be involved in inhibiting tumor growth or promoting tumor growth only at early stages. Our results are different from those of previous studies, such as Liu et al. and Kzhyshkowska3,24,which suggest that CHI3L2 is rather an unfavorable prognostic factor in cancer cells. In our opinion, these discrepancies could be related to different forms of breast cancer, which might differ in terms of CHI3L2 expression. Therefore, it is worth investigating the role of CHI3L2 protein in different types of breast cancer.
Our hypothesis about the positive role of CHI3L2 in patient prognosis is noteworthy, especially considering that CHI3L1 plays a significant role as a poor prognostic factor in various cancers such as glioma and breast cancer45. Our previous study in IDC tumors19 shows that CHI3L1 supports VEGFD-mediated angiogenesis in this type of cancer46. Hovewer in these studies, no significant correlations were found between CHI3L2 and angiogenesis markers even with CHI3L1, suggesting that CHI3L2 is not involved in angiogenesis in IDC and this two similar protein: CHI3L1 and CHI3L2 appear to differ significantly in their function.
Activation of the ERK1/2 kinase pathway, a component of the mitogen-activated protein kinase pathway (MAPK), usually promotes tumor growth, metastasis and angiogenesis. High concentrations of pERK1/2 are observed in various types of cancer such as breast, colon and lung cancer. The combination of RAF-ERK inhibitors may have anti-cancer effects47,48. On the other hand, the anti-apoptotic function of pERK1/2 has also been documented in tumor suppressive mechanisms47. Certain anticancer drugs such as cisplatin, etoposide or paclitaxel and other sources of damage such as γ-irradiation, UV radiation and natural plant substances can trigger the anti-apoptotic function of pERK1/2.
From this perspective, CHI3L2 could serve as a promising subject to investigate its influence on ERK pathway activation in IDC47,49,50. Mechanistically, CHI3L2 has been found to activate ERK1/245,44. Consistent with these reports, our results also suggest that CHI3L2 increases pERK1/2 and pSTAT-3 in IDC tissue. Interestingly, opposite roles of pERK1/2 are possible and have been described by Areshkov and team. For example, CHI3L1 leads to a proliferation of cancer cells, while CHI3L2 appears to inhibit the cell cycle44. Reults obtained in our transfection studies showed, that decreasing CHI3L2 mRNA lead to decreasing phosphorylation of ERK1/2 in invasive ductal breast cancer cells BT-549, but no effect was in metastatic adenocarcinoma cells MDA-MB-231. Moreover, addition of exogenous CHI3L2 protein incresed level of pERK1/2 in CHI3L2- metastatic adenocarcinoma MDA-MB-436 cells, and also in invasive ductal breast carcinoma CHI3L2- BT-474 cells. This results are consistence, and suport our hypothesis on specific role of CHI3L2 protein in ERK1/2 phosphorylation, depending of breast cancer subtype, and desribed new role of CHI3L2 protein in invasive ductal breast carcinoma. Consisted with our findings from in vitro studies, we showed for the first time positive and signifficant correlation of CHI3L2 expression and phosphorylation of ERK1/2 in IDC tumors. Hovewer, the exact role of CHI3L2-mediated activation of the ERK1/2 signaling pathway remains to be investigated more deeply, and further studies are neddedd to fully understand mechanisms regulated by CHI3L2 in invasive ductal breast carcinoma, also in context of molecular differentated another subtypes of breast cancer tumors.
Another signal transducer and activator of transcription, STAT-3, in turn plays a multifaceted role in carcinogenesis. The promotion of migration, invasion, metastasis, angiogenesis and immune escape of tumors characterizes the tumor-promoting effect of STAT-3 phosphorylation. Conversely, the antitumor effect of pSTAT3 is mediated in part by the reduced efficacy of IL-8 observed in glioblastomas51,52. In breast cancer, activation of pSTAT-3 could contribute to cancer cell survival. In the review publication, written by Tolomeo and Cascio52, the importance of STAT-3 activation for the mechanisms of carcinogenesis is analyzed in detail. Currently, the investigation of the role of CHI3L2 in the effects of pSTAT-3 in IDC is an interesting question to be explored.
In our studies, transfected MDA-MB-231 with CHI3L2 siRNA cells showed increased level of pSTAT-3, contrary to BT-549; addition of recombinant CHI3L2 protein has no effect of pSTAT-3 level in MDA-MB-468 and BT-474. This result suggest, that CHI3L2 may has diferent role in STAT-3 activation, depending of breast cancer subtype, hovewer currently is not enough information in this area. Our results, obtained from analysis of IDC tumors showed positive, signiffcance correlation of CHI3L2 expression level and pSTAT-3, and this findings are in line with results obtained from functional in vitro experiments with BT-549 cells, suggesting for the first time regulation role of STAT-3 activation in this type of breast cancer tumors.
In addition, we also observed the expression of CHI3L2 in TAMs, which is consistent with the results of another study on breast cancer and glioma tumors45. Moreover, CHI3L2 was expressed at low levels in macrophage cells in vitro, but its expression was enhanced in co-cultures designed to mimic the tumor microenvironment and TAMs. Interestingly, in our simple co-culture model, CHI3L2-negative cancer cells were found to stimulate macrophage production of this protein, whereas CHI3L2-positive normal breast epithalial cells not. These results showed plasticity of CHI3L2 expession in macrophages, and suggest that CHI3L2 may be involved in communication between cancer cells and immune cells, but further studies are needed to confirm this hypothesis. The specific function of CHI3L2 in the interaction between cancer cells and macrophages remains unclear at this moment. Our results help to clarify previous observations by Liu and colleagues, who detected CHI3L2 expression exclusively in TAMs but not in cancer cells24 and indicate, that this situation may also occured. Furthermore, we observed a negative correlation between CHI3L2 expression in tumor cells and infiltration of macrophages, but not exactly TAMs. It is also conceivable that CHI3L2 expression in breast cancer cells and macrophages could promote different pathways of tumor growth. Our team will focus on this question in the future to clarify the mechanism of the role of CHI3L2 in the communication between TAMs and cancer cells in breast tumors, which could be a new key factor in IDC tumor progression53. This interaction could be important for predicting breast cancer treatment and categorizing breast cancer types, however, it is crucial to substantiate this hypothesis.
Conclusions
In this studies, we present for the first time the expression of CHI3L2 protein in cancer cells in IDC and also in breast cancer cells in in vitro culture, and also confirmed CHI3L2 expression possibility in tumor–assiociated macrophages. Previously, CHI3L2 expression in breast tumors had only been observed in TAMs. Higher expression of CHI3L2 was observed in tumors with lower malignancy grade or in non-malignant lesions and higher levels of CHI3L2 protein were detected in ER + and PR + tumors, which may indicate protective, anti-tumor role of this protein. Moreover, positive, significant correlation of CHI3L2 expression with pSTAT-3 and pERK1/2 level was detected in tumor material and also regulation of ERK1/2 and STAT-3 phosphorylation in in vitro studies by CHI3L2 protein was observed, suggesting role of CHI3L2 in this signalling pathways activation in IDC. Conducted research showed that CHI3L2 protein is not involved in angiogenesis and has a different function than CHI3L1. Additionally, provided in vitro experiments showed that CHI3L2-negative breast cancer cells can induce macrophages to produce this protein, which may suggest a potential role of CHI3L2 protein in IDC, possibly contributing to tumor-microenvironment cross-talk, as well as an antitumor protective function.
Taken together, this work presents for the first time a novel role of the CHI3L2 protein in IDC biology, which opens further direction of possibility for development of these findings in precision medicine areas.
Study limitation
This study analyzed 74 tumor samples from patients IDC diagnosed with and followed their progress over time. A larger study with complete treatment data will validate these results and evaluate the significance of CHI3L2 protein as a predictor of IDC outcome.
Data availability
The datasets for this study can be found in the POLISH PLATFORM FOR MEDICAL RESEARCH https://doi.org/10.60956/j7rs-6p79; https://doi.org/10.60956/sase-tr74.
Change history
12 September 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-18786-1
References
Kzhyshkowska, J., Gratchev, A. & Goerdt, S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights 2, 128–146 (2007).
Riabov, V. et al. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 5, 1–13 (2014).
Kzhyshkowska, J., Larionova, I. & Liu, T. YKL-39 as a potential new target for anti-angiogenic therapy in cancer. Front. Immunol. 10, 1–10 (2020).
Yin, S. et al. SI-CLP inhibits the growth of mouse mammary adenocarcinoma by preventing recruitment of tumor-associated macrophages. Int. J. Cancer 146, 1396–1408 (2020).
Zheng, Y. & Graeber, M. B. Microglia and brain macrophages as drivers of glioma progression. Int. J. Mol. Sci. 23, 15612 (2022).
Zhao, T., Su, Z., Li, Y., Zhang, X. & You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Target. Ther. 5, 1–20. https://doi.org/10.1038/s41392-020-00303-7 (2020).
Rusak, A. et al. Multimodal study of CHI3L1 inhibition and its effect on angiogenesis, migration, immune response and refractive index of cellular structures in glioblastoma. Biomed. Pharmacother. 161, 114520 (2023).
Cintin, C. et al. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer 95, 267–274 (2002).
Shao, R. et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 28, 4456–4468 (2009).
Francescone, R. et al. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J. Biol. Chem. 286, 15332–15343 (2011).
Rusak, A., Jabłońska, K. & Dzięgiel, P. The role of YKL-40 in a cancerous process. Postepy Hig. Med. Dosw. (Online) 70, 1286–1299 (2016).
Xu, N. et al. Chitinase-3-like-1 promotes M2 macrophage differentiation and induces choroidal neovascularization in neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 60, 4596–4605 (2019).
Johansen, J. High serum YKL-40 level in patients with small cell lung cancer is related to early death. Lung. Cancer 46, 333–340 (2004).
Lee, C. G. & Elias, J. A. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy. Asthma Immunol. Res. 2, 20–27 (2010).
Tizaoui, K. et al. The role of YKL-40 in the pathogenesis of autoimmune diseases: a comprehensive review. Int. J. Biol. Sci. 18, 3731–3746 (2022).
Russo, C., Valle, M. S., Casabona, A. & Malaguarnera, L. Chitinase signature in the plasticity of neurodegenerative diseases. Int. J. Mol. Sci. 24, 6301 (2023).
Li, F., Liu, A., Zhao, M. & Luo, L. Astrocytic Chitinase-3-like protein 1 in neurological diseases: Potential roles and future perspectives. J. Neurochem. 165, 772–790 (2023).
Kim, J.-S. Protein biomarkers in multiple sclerosis. Encephalitis 3, 54–63 (2023).
Rusak, A. et al. The role of CHI3L1 expression in angiogenesis in invasive ductal breast carcinoma. Anticancer Res. 38, 3357–3366 (2018).
Rusak, A. et al. Correlation of expression of CHI3L1 and Nogo-A and their role in angiogenesis in invasive ductal breast carcinoma. Anticancer Res. 39, 2341–2350 (2019).
Zeng, H. L. et al. Proteomic characteristics of bronchoalveolar lavage fluid in critical COVID-19 patients. FEBS J. 288, 5190–5200 (2021).
Ebihara, T. et al. Combination of WFDC2, CHI3L1, and KRT19 in plasma defines a clinically useful molecular phenotype associated with prognosis in critically Ill COVID-19 patients. J. Clin. Immunol. https://doi.org/10.1007/s10875-022-01386-3 (2022).
Parlak, E. & Laloğlu, E. Analysis of chitinase-3-like protein 1, IL-1-Alpha, and IL-6 as Novel inflammatory biomarkers for COVID-19. J. Interf. Cytokine Res. 42, 536–541 (2022).
Liu, T. et al. Tumor-associated macrophages in human breast cancer produce new monocyte attracting and pro- angiogenic factor YKL-39 indicative for increased metastasis after neoadjuvant chemotherapy. Oncoimmunology 7, 1–17 (2018).
Górnicki, T. et al. Expression of RBMS3 in breast cancer progression. Int. J. Mol. Sci. 24(3), 2866 (2023).
Kątnik, E. et al. BCL11A Expression in Breast Cancer. Curr. Issues Mol. Biol. 45, 2681–2698 (2023).
Kao, J. et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One 4, e6146 (2009).
Balon, K. & Wiatrak, B. Pc12 and thp-1 cell lines as neuronal and microglia model in neurobiological research. Appl. Sci. 11, 1–17 (2021).
Lakhani SR, Ellis IO, S. Sj. al. WHO Classification of tumors Editorial Board. Endocrine and Neuroendocrine TUMORS vol. 10 (2022).
Pareja, F. & Dalfonso, T. M. Neuroendocrine neoplasms of the breast: A review focused on the updated World Health Organization (WHO. Breast J. 26, 1160–1167 (2020).
Popiel-Kopaczyk, A. et al. The expression of testin, Ki-67 and p16 in cervical cancer diagnostics. Curr. Issues Mol. Biol. 45, 490–500 (2023).
Zadka, L. et al. Association between interleukin-10 receptors and the CD45-immunophenotype of central nervous system tumors: A preliminary study. Anticancer Res. 37, 5777–5783 (2017).
Remmele, W. & Stegner, H. E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8, 138–40 (1987).
Vermeulen, P. B. et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur. J. Cancer 38, 1564–1579 (2002).
Van der Auwera, I. et al. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br. J. Cancer 95, 1611–1625 (2006).
Fox, S. B. & Harris, A. L. Histological quantitation of tumour angiogenesis. Apmis 112, 413–430 (2004).
Królicka, A. et al. Comparison of metallothionein (MT) and Ki-67 antigen expression in benign and malignant thyroid tumours. Anticancer Res. 30, 4945–4950 (2010).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/ college of American pathologists clinical practice guideline focused update. J. Clin. Oncol. 36, 2105–2122 (2018).
Kmiecik, A. M. et al. Metallothionein-3 increases triple-negative breast cancer cell invasiveness via induction of metalloproteinase expression. PLoS One 10, 1–25 (2015).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Olbromski, M. et al. MicroRNAs modulate the expression of the SOX18 transcript in lung squamous cell carcinoma. Oncol. Rep. 36, 2884–2892 (2016).
Sun, Y. et al. Curcumin inhibits the proliferation and invasion of MG-63 cells through inactivation of the p-JAK2/ p-STAT3 pathway. Onco. Targets. Ther. 12, 2011–2021 (2019).
Miller, L. D. et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc. Natl. Acad. Sci. U. S. A. 102, 13550–13555 (2005).
Areshkov, P. O., Avdieiev, S. S., Balynska, O. V., LeRoith, D. & Kavsan, V. M. Two closely related human members of chitinase-like family, CHI3L1 and CHI3L2, activate ERK1/2 in 293 and U373 cells but have the different in-fluence on cell proliferation. Int. J. Biol. Sci. 8, 39–48 (2011).
Liu, L. et al. CHI3L2 is a novel prognostic biomarker and correlated with immune infiltrates in gliomas. Front. Oncol. 11, 1–13 (2021).
Bokhari, S. M. Z. & Hamar, P. Vascular endothelial growth factor-D (VEGF-D): An angiogenesis bypass in malignant tumors. Int. J. Mol. Sci. 24, 1–19 (2023).
Sugiura, R., Satoh, R. & Takasaki, T. Erk: A double-edged sword in cancer. erk-dependent apoptosis as a potential therapeutic strategy for cancer. Cells 10, 2509 (2021).
Morante, M., Pandiella, A. & Crespo, P. Immune checkpoint inhibitors and RAS–ERK pathway-targeted drugs as combined therapy for the treatment of melanoma. Biomolecules 12, 1562 (2022).
Nissan, M. H., Rosen, N. & Solit, D. B. Views in the spotlight erk pathway inhibitors: How low should we go ?. Cancer Disc. 3, 719–721. https://doi.org/10.1158/2159-8290.CD-13-0245 (2013).
Smalley, I. & Smalley, K. S. M. VIEWS eRK Inhibition : A new front in the war against MAPK pathway-driven cancers ?. Cancer Disc. 8, 140–142. https://doi.org/10.1158/2159-8290.CD-17-1355 (2018).
Guanizo, A. C., Fernando, C. D., Garama, D. J. & Gough, D. J. STAT3: A multifaceted oncoprotein. Growth Factors 36, 1–14 (2018).
Tolomeo, M. & Cascio, A. The multifaced role of stat3 in cancer and its implication for anticancer therapy. Int. J. Mol. Sci. 22, 1–25 (2021).
Zadka, Ł, Grybowski, D. J. & Dzięgiel, P. Modeling of the immune response in the pathogenesis of solid tumors and its prognostic significance. Cell. Oncol. https://doi.org/10.1007/s13402-020-00519-3 (2020).
Acknowledgements
The authors would like to thank Bożena Przygodzka, Elżbieta Połejko and Agnieszka Barańska for their fantastic technical assistance in the preparation of tissue sections at the Wroclaw Medical University, Poland. Great thanks are dedicated for Monika Mrozowska and Klaudia Krawczyńska for their support. Special thanks to Helena Rusak.
Funding
This research was partially funded by the National Science Center, Poland (NCN), under the Miniatura 5 grant (Grant Number 2021/05/X/NZ2/01698) to Agnieszka Rusak entitled “CHI3L1 and CHI3L2 as Proteins with Potential Prognostic and Therapeutic Significance in Breast Carcinoma and Glioblastoma”. In addition, the Wroclaw Medical University founded materials and reagents in projects (grant number: STM.A110.17.025) entitled “The Role of YKL-39 (CHI3L2) in Breast Cancer”. For the purpose of open access, the author has applied a CC-BY public copyright license to any Author Accepted Manuscript (AAM) version resulting from this submission.
Author information
Authors and Affiliations
Contributions
Conceptualization: AR; Methodology: AR; Investigation: AR, EK, TG, AP, CS, AW; Resources: AR, PD, JK; Validation: AR; Visualization AR, AK, KRW, AP; Formal Analysis: AR, KK; Writing–Original Draft: AR; Writing–Review & Editing: AR, PD, JK; Supervision: PD, JK; Project Administration: AR; Funding Acquisition: AR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in Table 1. Full information regarding the corrections made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rusak, A., Kątnik, E., Górnicki, T. et al. New insights into the role of the CHI3L2 protein in invasive ductal breast carcinoma. Sci Rep 14, 28529 (2024). https://doi.org/10.1038/s41598-024-77930-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77930-5