Abstract
Sublingual immunotherapy (SLIT) has received considerable attention as a method for allergen immunotherapy (AIT). However, the mechanism of SLIT, especially its timing, has not been thoroughly investigated. We evaluated therapeutic and prophylactic SLIT in an allergic airway inflammation model and evaluated their efficacies. Mice were intranasally exposed to Dermatophagoides farinae (Der f) extract and received SLIT before (prophylactic model) and after (therapeutic model) intranasal exposure of Der f. We investigated airway responsiveness, airway inflammation, allergen-specific antibodies, lung histology and single-cell RNA sequencing (scRNA-seq) and T-cell receptor sequencing were also investigated. SLIT in the therapeutic model was effective; however, the effects of SLIT in the prophylactic model were stronger and immune tolerance was maintained for three months. ScRNA-seq of lung CD4+CD25+ T cells revealed that the expansion of induced T regulatory (iTreg) cells was greater in the prophylactic model than that in the therapeutic model. Additionally, the TCR repertoire of iTregs from the prophylactic model was abundant, sharing many clones with the TCR repertoire of effector T cells. These data suggest that the prophylactic model of AIT is extremely effective and persistent, and may respond to allergen diversity, and provide evidence for the clinical recommendation of preventive AIT.
Similar content being viewed by others
Introduction
Allergen immunotherapy (AIT) involves the administration of specific allergens to patients with IgE-mediated conditions. The major objectives of AIT are to reduce responses to allergic triggers, decrease the inflammatory response, and prevent the development of persistent disease. Furthermore, AIT is the only curative treatment for allergic diseases, and subcutaneous immunotherapy (SCIT) has been clinically effective in treating asthma and rhinitis caused by allergic sensitization1. However, it requires inconvenient injections and is associated with potentially severe systemic reactions. Thus, sublingual immunotherapy (SLIT) was developed to avoid systemic reactions and has recently gained attention for its potential clinical application in AIT2.
However, the timing of AIT initiation has not yet been fully investigated. Early allergen sensitization, especially multiple allergen sensitization, is considered the most significant risk factor for subsequent asthma development3. AIT is generally used to treat established diseases; however, the National Institute of Health’s Immune Tolerance Network has proposed prophylactic AIT with the expectation of an early intervention effect because AIT suppresses new allergen sensitization4. Some studies have discovered that prophylactic immunotherapy can reduce new allergen sensitization in high-risk infants and young children5,6,7,8,9. Several analyses of prophylactic AIT in animal models have been reported10,11; however, few reports have examined the efficacy differences between prophylactic and therapeutic AIT in detail.
The mechanisms of immunotherapy include an increase in regulatory T cells (Treg) and B cells (Breg), accompanied by an increase in suppressive cytokines such as IL-10 and TGF-β, suppression of Th2 and Th9 cell responses, shifting from Th2 to Th1 (immune-deviation), a decrease in IgE production, an increase in the production of allergen-neutralizing antibodies such as IgG4 and IgA, and suppression of IgE-dependent activation of mast cells and basophils12,13,14. Among these factors, Treg expansion is crucial15,16 and hypomethylation of the foxp3 promoter in antigen-induced Tregs has been reported in AIT for patients with peanut allergy17,18. However, there have been few reports on the differences in the Treg characteristics during AIT administration, that is, the differences between prophylactic and therapeutic AIT and the pathophysiological significance of prophylactic AIT are unclear.
In this study, we aimed to evaluate the efficacy of therapeutic and prophylactic SLIT in an allergic airway inflammation model by comparing the mechanisms of the two models. We also examined the long-term effects of SLIT by comparing the two models. Furthermore, T cell receptor (TCR) repertoire analysis and single-cell RNA sequencing (scRNA-seq) of lung CD4+CD25+ T cells were performed to examine the differences in Tregs between the two models.
Results
Changes in lung resistance, airway inflammation, lung tissue, and Treg cells in a therapeutic and prophylactic SLIT model
Mice were intranasally exposed to Der f extract for 3 weeks before receiving SLIT of Der f for 2 weeks, followed by another week of exposure to Der f (Fig. 1A). Compared to control mice that received SLIT in saline, mice that received SLIT in Der f had decreased lung resistance to methacholine, total cell, and eosinophil count in BALF (Fig. 1B,C). Preliminary experiments of dose determination showed a dose-dependent decrease in airway hyperresponsiveness and in total cell count and eosinophil count in BALF (Supplemental Fig. 1A and B). Regarding cytokine levels in BALF, IL-5 levels were significantly lower, whereas IL-10 levels were higher than those in the control mice (Fig. 1D). Decreased IL-5 levels may be due to fewer cells infiltrating the airway from the result of intracellular cytokine staining (Supplemental Fig. 1C). Cytokine measurements of lung cell and peribronchial lymph node MNC culture supernatants revealed no significant changes compared to the normal saline (NS) SLIT group (Supplemental Fig. 1D,E). Regarding histological features, mice that received SLIT had decreased inflammatory cell infiltration and goblet cell metaplasia (Fig. 1E). Der f-specific IgA levels were significantly higher than that in control mice, whereas serum Der f-specific IgE, IgG1, and IgG2a were not significantly different between groups (Fig. 1F,G and Supplemental Fig. 1F,G). The proportion of Tregs in SLIT mice increased significantly, whereas the Breg proportion remained unchanged (Fig. 1H and Supplemental Fig. 1H).
The effects of therapeutic sublingual immunotherapy (SLIT) model. (A) Experimental protocols. (B) Changes in lung resistance with increasing concentrations of nebulized methacholine. (C) Changes in the cellular composition of bronchoalveolar lavage fluid (BALF). (D) Levels of indicated cytokines in BALF. (E) Representative haematoxylin and eosin staining (a, b) and periodic acid-Schiff (PAS) staining (c, d), performed in lung sections 24 h after the last challenge (100 × magnification). NS SLIT (a, c) and Der.f SLIT (b, d). Quantitative analysis of PAS-positive cells in bronchial tissue (e). (F) The levels of Der f-specific IgE in serum. (G) The levels of Der f-specific IgA in BALF. (H) Representative data of lung regulatory T cells by flow cytometry. Lung CD4+Foxp3+ in CD25+CD45+ (gated) were expressed in NS SLIT (a), and Der f SLIT (b). Percentages of CD25+Foxp3+ fraction in lung CD4 cells (c). Data presented as mean ± standard error of the mean (SEM). *P < 0.05 or **P < 0.01 compared to the NS SLIT group. i.n., intranasal; NS, normal saline; Der f, Dermatophagoides farina; Mac, macrophages; Lym, lymphocytes; Neu, neutrophils; Eos, eosinophils; BM, basement membrane.
In the prophylactic model, mice received two weeks of prophylactic SLIT of Der f, followed by four weeks of intranasal Der f (Fig. 2A). Compared to control mice, SLIT-treated mice had significantly decreased lung resistance to methacholine, total cell, and eosinophil number in BALF (Fig. 2B,C). In preliminary experiments, the 1ug group effectively reduced airway hyperresponsiveness and eosinophil counts in BALF (Supplemental Fig. 2A, B). Regarding cytokine production in BALF, IL-5 and IL-13 levels were significantly lower, whereas IL-10 and IFN-γ levels were significantly higher (Fig. 2D). In this model, decreased IL-5 levels may contribute not only to the reduced number of cells infiltrating the airways, but also to the reduced production capacity of CD4 + T cells, based on the results of intracellular cytokine staining results (Supplemental Fig. 2C). Cytokine measurements of the culture supernatants of lung cells and peribronchial lymph node MNCs revealed a decrease in type 2 cytokines and an increase in IFN-γ compared to the NS SLIT group (Supplemental Fig. 2D, E). Regarding histological features, SLIT-treated mice had decreased infiltration of inflammatory cells and goblet cell metaplasia (Fig. 2E). The Der f-specific IgE levels in serum and Der f-specific IgA levels in BALF were significantly lower between the groups (Fig. 2F,G), whereas serum Der f-specific IgG1 and IgG2a were not significantly different between the groups (Supplemental Fig. 2F, G). The proportion of Tregs in SLIT-treated mice significantly increased, whereas the proportion of Bregs did not differ (Fig. 2H, Supplemental Fig. 2H).
The effects of prophylactic SLIT model. (A) Experimental protocols. (B) Changes in lung resistance with increasing concentrations of nebulized methacholine. (C) Changes in the cellular composition of BALF. (D) Levels of indicated cytokines in BALF. (E) Representative haematoxylin and eosin staining (a, b) and periodic acid-Schiff (PAS) staining (c, d), performed in lung sections 24 h after the last challenge (100 × magnification). NS SLIT (a, c) and Der f SLIT (b, d). Quantitative analysis of PAS-positive cells in bronchial tissue (e). (F) The levels of Der f-specific IgE in serum. (G) The levels of Der f-specific IgA in BALF. (H) Representative data of lung regulatory T cells by flow cytometry. Lung CD4+Foxp3+ in CD25+CD45+ (gated) were expressed in NS SLIT (a), and Der f SLIT (b). Percentages of CD25+Foxp3+ fraction in lung CD4 cells (c). Data presented as mean ± standard error of the mean (SEM). *P < 0.05 or **P < 0.01 compared to the NS SLIT group. i.n., intranasal; NS, saline; Der f, Dermatophagoides farina; Mac, macrophages; Lym, lymphocytes; Neu, neutrophils; Eos, eosinophils; BM, basement membrane.
These results indicated that the prophylactic model could achieve a greater effect with fewer allergens than the therapeutic model. The common mechanism in both models was an increase in Tregs.
Changes in lung resistance, airway Inflammation, lung tissue, immunoglobulins, and regulatory cells in a therapeutic and prophylactic SLIT model with time interval
Mice were intranasally exposed to Der f extract for 3 weeks before receiving SLIT of Der f for 2 weeks, followed by a 3-month break and one more week of Der f exposure (Fig. 3A). Interestingly, lung resistance after methacholine inhalation was not different between the mice that received Der f SLIT and those that received NS SLIT (Fig. 3B). The total cell and eosinophil numbers in BALF and cytokine production in BALF and lung cells and peribronchial lymph node MNCs of Der f-SLIT mice were like those in NS-SLIT mice (Fig. 3C,D, and Supplemental Fig. 3A, B). Histological findings, such as inflammatory cell accumulation and goblet cell metaplasia, were also similar between the groups (Fig. 3E). Der f-specific IgE, IgG1, and IgG2a levels in serum, as well as Der f-specific IgA in BALF, were not significantly different between the groups (Fig. 3F,G and Supplemental Fig. 3C, D). The proportions of Tregs and Bregs in SLIT were not significantly different (Fig. 3H and Supplementary Fig. 3E).
The effects of therapeutic SLIT model with time interval. (A) Experimental protocols. (B) Changes in lung resistance with increasing concentrations of nebulized methacholine. (C) Changes in the cellular composition of BALF. (D) Levels of indicated cytokines in BALF. (E) Representative haematoxylin and eosin staining (a, b) and periodic acid-Schiff (PAS) staining (c, d), performed in lung sections 24 h after the last challenge (100 × magnification). NS SLIT (a, c) and Der f SLIT (b, d). Quantitative analysis of PAS-positive cells in bronchial tissue (e). (F) The levels of Der f-specific IgE in serum. (G) The levels of Der f-specific IgA in BALF. (H) Representative data of lung regulatory T cells by flow cytometry. Lung CD4+Foxp3+ in CD25+CD45+ (gated) were expressed in NS SLIT (a), and Der f SLIT (b). Percentages of CD25+Foxp3+ fraction in lung CD4 cells (c). Data presented as mean ± standard error of the mean (SEM). *P < 0.05 or **P < 0.01 compared to the NS SLIT group. i.n., intranasal; NS, saline; Der f, Dermatophagoides farina; Mac, macrophages; Lym, lymphocytes; Neu, neutrophils; Eos, eosinophils; BM, basement membrane.
In contrast, the prophylactic model with a three-month interval (Fig. 4A) demonstrated a significant reduction in lung resistance to methacholine and total cell and eosinophil numbers in BALF compared to NS-SLIT mice (Fig. 4B,C). Regarding cytokine production in BALF, IL-5 and IL-13 levels were significantly lower, whereas IL-10 and IFN-γ were higher than that in NS-SLIT mice (Fig. 4D). Cytokine measurements of the culture supernatants of lung cells revealed an increase in IFN-γ compared to the NS SLIT group (Supplemental Fig. 4A, B). Regarding histological features, mice that received SLIT had decreased infiltration of inflammatory cells and goblet cell metaplasia (Fig. 4E). The levels of Der f-specific IgE, IgG1, and IgG2a in serum, as well as Der f-specific IgA in BALF, were lower than those in NS-SLIT mice (Fig. 4F,G and Supplemental Fig. 4C,D). The percentages of Treg, but not of Breg, in Der f-SLIT mice, were consistently higher than that in NS-SLIT mice (Fig. 4H, Supplemental Fig. 4E).
The effects of prophylactic SLIT model with time interval. (A) Experimental protocols. (B) Changes in lung resistance with increasing concentrations of nebulized methacholine. (C) Changes in the cellular composition of BALF. (D) Levels of indicated cytokines in BALF. (E) Representative haematoxylin and eosin staining (a, b) and periodic acid-Schiff (PAS) staining (c, d) performed in lung sections 24 h after the last challenge (100 × magnification). NS SLIT (a, c) and Der f SLIT (b, d). Quantitative analysis of PAS-positive cells in bronchial tissue (e). (F) The levels of Der f-specific IgE in serum. (G) The levels of Der f-specific IgA in BALF. (H) Representative data of lung regulatory T cells by flow cytometry. Lung CD4+Foxp3+ in CD25+CD45+(gated) were expressed in NS SLIT (a), and Der.f SLIT (b). Percentages of CD25+Foxp3+ fraction in lung CD4 cells (c). Data presented as mean ± standard error of the mean (SEM). *P < 0.05 or **P < 0.01 compared to the NS SLIT group. i.n., intranasal; NS, saline; Der f, Dermatophagoides farina; Mac, macrophages; Lym, lymphocytes; Neu, neutrophils; Eos, eosinophils; BM, basement membrane.
These results suggest that the effect of immunotherapy is sustained in the prophylactic model and that Tregs increased by SLIT have an effect even after a period has elapsed.
Effects of transfer of Lung CD4+CD25+ T cells on lung resistance, airway inflammation, and lung tissue
To analyze the allergen-specificity of lung Tregs, we isolated lung CD4+CD25+ T cells from mice treated with prophylactic and therapeutic SLIT of Der f and transferred them to OVA-sensitized mice, followed by an OVA challenge (Fig. 5A). In contrast to the lack of effects on airway resistance, airway inflammation, and goblet cell metaplasia in lung CD4+CD25+ T cells from mice treated with therapeutic SLIT, those from mice treated with prophylactic SLIT revealed a decrease in lung resistance, suppression of eosinophilic airway inflammation, type 2 cytokine production, and goblet cell metaplasia (Fig. 5B–E). Moreover, the inhibitory effects of the above indices were observed when both types of lung CD4+CD25+ T cells were transferred to mice sensitized with Der f (Supplemental Fig. 5). These results suggest that lung CD4+CD25+ T cells from mice subjected to prophylactic SLIT may retain allergen-non-specific anti-inflammatory effects.
The effects of transfer of lung CD4+CD25+ T cells from SLIT model to OVA primed model. (A) Experimental protocols. (B) Changes in lung resistance with increasing concentrations of nebulized methacholine. (C) Changes in the cellular composition of BALF. (D) Levels of indicated cytokines in BALF. (E) Representative haematoxylin and eosin staining (a—c) and periodic acid-Schiff (PAS) staining (d–f), performed in lung sections 24 h after the last challenge (100 × magnification). NS SLIT (a, d) therapeutic SLIT Treg transfer (b, e) and prophylactic SLIT Treg transfer (c, f). Quantitative analysis of PAS-positive cells in bronchial tissue (g). Data from three independent experiments (n = 9) are presented as means + standard error of the mean (SEM). *P < 0.05 or **P < 0.01 compared to the PBS transfer group. i.n., intranasal; NS, saline; Der f, Dermatophagoides farina; Mac, macrophages; Lym, lymphocytes; Neu, neutrophils; Eos, eosinophils; BM, basement membrane.
ScRNA-seq and scTCR-seq with lung Treg cells of the prophylactic and therapeutic models
Based on the results of the murine model, we speculated that there was a difference in the characteristics of Tregs induced by prophylactic and therapeutic SLIT. Therefore, we purified lung CD4+CD25+ T cells from both models and performed scRNA-seq and scTCR-seq to investigate the differences between the two models. As only a small number of lung CD4+CD25+ cells can be collected from a single animal, each model required 6–8 mice for single-cell analysis.
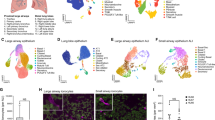
ScRNA-seq of CD4+CD25+ T cells derived from lungs and UMAP identified four clusters based on gene expression (Fig. 6A, B, and supplemental Fig. 6: naïve CD4 T cells, naturally occurring Tregs (nTregs), inducible Tregs (iTregs), and effector T cells). A comparison of the distribution of lung CD4+CD25+ T cells in both models revealed an increased an absolute number of iTregs in the prophylactic model (p < 0.001; Chi-square test) (Fig. 6C). Differences in the genetic analysis of each fraction were compared. Th2-related genes such as Il4, Il13, and Il1rl1 in effector T cells as well as Treg-related genes, such as Il10, Tgfb1, Ebl3, and Il12a in regulatory T cells, were expressed similarly in the therapeutic and prophylactic models (Fig. 6D).
The data of scRNA-seq of lung CD4+CD25+ T cells. (A) UMAP of unsupervised clustering of 21,892 cells integrated from prophylactic and therapeutic model according to similarity of transcriptome resulting in four different color-coded clusters. (B) Feature plots combining all samples of expression distribution for cluster-specific marker genes. Intensity of expression levels for each cell is color-coded (blue) and overlaid onto UMAP plots. (C) Number of four different color-coded clusters in prophylactic and therapeutic model. (D) Dot plot of Th-related and Treg-related genes expression in various cell type for two experimental protocol.
TCR repertoire analysis (TCR β) revealed that iTregs in the prophylactic model had a higher Shannon’s index and a lower DE50 (Fig. 7A,B). These data indicated that iTregs in the prophylactic model were more diverse and had higher clonality. The distribution of the top 10 clones also revealed that iTregs were more abundant in the prophylactic model than in the therapeutic model (Fig. 7C,D). Furthermore, an analysis of TCR clone sharing between effector T cells and iTregs revealed that they shared significantly more clones in the prophylactic model (Fig. 7E). The proportion of clones shared with iTregs among effector T cells was significantly higher in the prophylactic model (prophylactic, 32.9%; therapeutic, 17.1%; odds ratio 2.30 (1.61–3.28), p < 0.001).
The data of scTCR-seq of lung effector T cells and iTreg cells. (A) TCRβ repertoire diversity determined as Shannon index. (B) TCRβ repertoire clonality determined as DE50 (Diversity Evenness 50 score). (C) The top 10 clonotypes (color-coded) in bulk were plotted and overlaid onto UMAP plots. (D) Proportion of top 10 clonotypes in iTreg and effector T cell from prophylactic and therapeutic model. (E) Venn diagram of TCRβ clonotypes between iTreg and effector T cells in prophylactic and therapeutic model.
Discussion
This study demonstrated that the timing of SLIT resulted in an obvious difference in its effectiveness. We created a therapeutic model after allergen sensitization and a prophylactic model before sensitization; the latter significantly reduced lung resistance to methacholine, eosinophilic airway inflammation, and elevated type 2 cytokines at low antigen doses. These effects were sustained for long after SLIT, and lung Treg cells transferred from mice receiving prophylactic SLIT suppressed allergic inflammation in different allergen models. Repertoire analysis of Tregs in prophylactic and therapeutic models revealed TCR diversity and clonality, indicating that Tregs can respond to various antigens.
One advantage of AIT is that it suppresses the establishment of new allergen sensitization19. Even if sensitization has been established, AIT may reduce disease onset20. In addition, birth cohort studies have demonstrated that IgE production without symptoms is common early in life, followed by allergic symptoms after repeated exposure to allergens21,22. Therefore, immunotherapy at an early stage may be more effective23. However, the details of this mechanism are unknown, but this study speculates that the epitopes recognized by iTregs, which prophylactic AIT produces, may be diverse, resulting in cross-reactivity to other allergens.
Recently, several studies have analyzed patients with allergies using single-cell transcriptomes. Seumois et al. purified CD4 + helper T cells (TH) and Treg reactive to HDM from the peripheral blood of HDM-positive patients with asthma and performed a single-cell transcriptome analysis. Compared to HDM-negative cases, interferon response signature (IFNR)-expressing TH and Tregs are decreased in HDM-positive cases, suggesting that these cells may play a role in suppressing allergen-specific inflammation24. In our study, an increase in IFN-γ levels in BALF was evident in the prophylactic model (Figs. 2 and 4). Similarly, we compared the IFNR expression but observed no obvious changes. Another study of temporal changes in gene expression after oral AIT in patients with peanut allergy revealed a decrease in the expression of Th2 signatures in the effector Th2 fraction in patients with immune tolerance to AIT. However, Tregs had no significant changes in gene expression25. In this study, the expression of cytokine and chemokine genes related to type 2 inflammation in effector T cells were not different between the two models.
This study highlights the unique characteristics of the TCR repertoire of iTregs in both models. In the prophylactic model, the TCR clonality of iTregs was more diverse, and the TCR clones of effector T cells were shared with iTregs compared to the therapeutic model. This TCR repertoire signature may be the mechanism by which the prophylactic model suppressed inflammation, even though the expression of type 2 inflammation-related genes in effector T cells was not reduced. In an inflammatory bowel disease model report, IFN-γ-positive and IL-17-positive CD4 T cells shared the same TCR clonotype but not with the TCR of Tregs26. The ability of lung CD4+CD25+ T cells from a prophylactic model to suppress inflammation after transfer to a different allergen model suggests that the diversity of TCR clonality is also crucial. Another explanation for this finding is that the scRNA-seq data showed a higher percentage of Tregs in the CD4 + CD25 + T-cell population of the prophylactic model (Fig. 6C). It is possible that more Tregs were included in the CD4 + CD25 + T cells from the prophylactic model, thus enhancing the effects.
The difference in the mechanism of AIT in both models is particularly notable in this study: although Treg expansion was similar, an increase in IFN-γ, i.e. immune deviation, was only observed in the prophylactic model. In contrast, in the therapeutic model, a local increase in allergen-specific IgA is more likely to be involved in immune tolerance. While it is difficult to explain the reasons underlying these differences in detail, we speculate that differences in SLIT dose, duration, and airway inflammation models may play a role. There have been reports indicating no increase in IFN-γ with AIT27,28, and this outcome may be influenced by various protocols and other factors. Nevertheless, it can be inferred that Th2 inflammation is suppressed, resulting in relative immune deviation. Furthermore, in the therapeutic model, allergen-specific IgA is effectively induced consistent with a previous report29. In contrast, no increase in IgA was seen in the prophylactic model. This disparity may be due to the early production of immune deviation and regulatory T cells that respond to broader clones, inducing a strong immune tolerance. Consequently, this may not evoke the induction of blocking antibodies such as allergen-specific IgA.
A comparison of the allergen doses used in SLIT revealed a clear difference between the two models: the prophylactic model required a small dose of allergen (1 μg/day) to establish significant effects, whereas the therapeutic model required a large dose of allergen (1,000 μg/day). In our previous report, we discovered that the treatment model requires more allergens30. In preliminary experiments, the allergen dose in the prophylactic model was fixed to 1 μg/day because the effect tended to diminish at higher doses (Supplemental Fig. 1). It is unclear whether this is the optimal dose or if a technical factor is involved. In contrast, in the therapeutic model, a large amount of allergen is required to induce immune tolerance after a strong immune system has been established, as observed in clinical AIT31,32,33. From this perspective, early induction of AIT should be effective.
Furthermore, the duration of intranasal exposure and SLIT is also crucial in analyzing this model. As mentioned earlier, comparing 3 weeks of intranasal exposure to 5 weeks, the 5 week-model requires a greater amount of allergen for SLIT30. In contrast, a shorter intranasal exposure may lead to milder airway reactivity and eosinophilic airway inflammation. Hence, a 4-week intranasal exposure was adopted to demonstrate a well-designed asthma model. The SLIT period of 2 weeks was also chosen because 1 week was deemed insufficiently effective. Furthermore, the use of low concentrations of allergoids rather than house dust mite extracts, is reported to have better suppression effects on allergic inflammation and Th2-type cytokine production from the regional lymph nodes. Although there is a difference between subcutaneous and sublingual administration of allegoids, its potential therapeutic effect at low doses is very encouraging34. Further analysis is needed to determine how the use of allergoids in prophylactic models affects induction of tolerance.
This study had several limitations. First, antigen-presenting cells, particularly dendritic cells, were not analyzed. The properties of dendritic cells may play a role in the induction of distinctive iTregs. Miyanaga et al. reported that in a mouse allergic airway inflammation model using OVA, conventional dendritic cells of the submandibular lymph nodes are important for the induction of Tregs in the mechanism of SLIT35. Second, the role of increased IFN-γ in the prophylactic model remains unknown. Immuno-deviation, Th2 to Th1 deviation, has been reported as a mechanism of immunotherapy12. An increase in IFN-γ-positive CD8 + T cells in the lungs has been reported as an outcome of prophylactic AIT in a mouse model of allergic airway inflammation36. However, the role of IFN-γ upregulation in immune tolerance and its effects on iTregs remain unknown. Third, T-cell exhaustion, which is one mechanism of AIT, has not yet been analyzed. The expression of inhibitory markers (PD-1 and CTLA-4) in AIT models has been reported to vary depending on the experimental protocol37,38,39, and future analysis of the expression patterns over time may help clarify the mechanism of action of AIT. Fourth, we have been unable to identify the IL-10-producing cells. CD4+Tregs could be considered as a source cell of IL-10, since IL-10+Bregs were not significantly involved in this study. As a mechanism of AIT, an early increase in IL-10+B cells is important for subsequent tolerance and clinical improvement40. However, there may be presumably other mechanisms, which need to be further elucidated. Finally, protocols should be considered during clinical applications. It is unrealistic to initiate immunotherapy in purely naïve individuals. In terms of early exposure to infants with risk factors, the early initiation of early exposure in this study has not been evaluated. The purpose of this study was to compare the mechanisms of action at different initiation times. The above points should be clarified in future studies.
In summary, we demonstrated that the timing of SLIT significantly influenced its effectiveness. AIT is generally introduced after sensitization has been established (therapeutic model); however, the effects of AIT conducted in the absence of sensitization (prophylactic model) are stronger, and immune tolerance is maintained for a long time. Interestingly, CD4+CD25+ T cells were purified from the lungs of mice undergoing prophylactic SLIT with Der f extract and transferred to mice sensitized with different allergens, which attenuated airway inflammation. This indicates that the prophylactic model can induce immune tolerance in an antigen-non-specific manner. To observe differences in the properties of pulmonary iTregs after SLIT, scRNA-seq and scTCR-seq were performed. The prophylactic model had greater iTreg expansion than the therapeutic model, and the TCR repertoire of iTregs from the prophylactic model was abundant, sharing many clones with the TCR repertoire of effector T cells, implying a combination of diversity and clonality. These data suggest that the prophylactic model of AIT is extremely effective and efficient, and provides evidence for the clinical recommendation of preventive AIT.
Materials and methods
Animals
This study used 8-week-old female BALB/c mice free of murine-specific pathogens (CLEA Japan Inc., Tokyo, Japan). All experiments followed a protocol approved by the Niigata University Ethics Committee for Animal Experiments (SA00590). The authors confirm that animal experiment procedures complied with LANUV regulations and the ARRIVE 2.0 guidelines41. Details of the procedures are provided in the online supplementary file.
Dermatophagoides farinae-induced asthma model and SLIT protocol
Without immunization, mice were intranasally exposed to Dermatophagoides farinae (Der f) extract (Torii Pharmaceutical Co. Ltd., Tokyo, Japan). We established two protocols for SLIT: SLIT after Der f intranasal administration (therapeutic model) and SLIT before Der f intranasal administration (prophylactic model). The procedures are described in detail in the online supplementary file.
Determination of airway responsiveness (AR)
We assessed AR by measuring changes in total lung resistance in response to increasing doses of inhaled methacholine using the FlexiVent system (SCIREQ, Montreal, Quebec, Canada)42. The details of the procedures are provided in the online supplementary file.
Measurement of cytokine levels in bronchoalveolar lavage fluid (BALF) and culture supernatants of peribronchial lymph node and lung cells.
Following the assessment of AR, bronchoalveolar lavage (BAL) was performed using a tracheal tube, as described previously43. Mononuclear cells (MNCs) of peribronchial lymph nodes and lung cells were cultured on BD Biocoat™ T-cell activation assay plates (BD Biosciences, San Jose, CA, USA) for 72 h, and the supernatants were recovered for cytokine assays. The details of the procedures are provided in the online supplementary file.
Flow cytometry
Flow cytometry was used to examine the surface phenotypes of lung Treg and Breg cells using a three-color immunofluorescence test. The details of the antibodies, reagents, procedures and control stainings are provided in the online supplementary file.
Detection of total IgE and IgA, and Der f specific IgE, IgG, and IgA antibodies
To measure immunoglobulins in serum and BALF, we used commercially available enzyme-linked immunosorbent assay (ELISA) kits and ELISA systems at our facility30. The details of the procedures are provided in the online supplementary file.
Lung histology
After obtaining BAL fluid, the mice were euthanized by sectioning their abdominal aorta and vena cava to induce massive hemorrhage and terminal bleeding. The left lung was fixed in 10% formalin and immersed in paraffin. After deparaffinization, the samples were stained with hematoxylin and eosin and periodic acid-Schiff (PAS) stain for histological analysis. Histological analyses were performed as described previously44. Details of the procedures are provided in the online supplementary file.
Preparation of CD4 + CD25 + T cell and transfer
Lung Treg cells were purified (purity > 90%) using a mouse CD4+CD25+ Treg cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). These lung CD4+CD25+ T cells were then administered intravenously (5 × 105 cells/mouse) to ovalbumin (OVA)-sensitized mice, followed by an OVA challenge. In another experiment, purified CD4 + CD25 + T cells were used for scRNA-seq and TCR repertoire analysis.
Single-cell RNA-sequencing
The scRNA-seq libraries were prepared using the Chromium Next GEM Single Cell 5′ Kit v2 (10X Genomics, PN-1000263) following the 10X Genomics protocol. The final libraries were assessed using a LabChip GX Touch instrument (PerkinElmer, Waltham, MA, USA) to verify the complementary DNA (cDNA) fragment size.
Raw data were processed with CellRanger 6.0.0 (10X Genomics) using the mm10-2020-A mouse reference genome. We recovered 13,566 and 8,326 cells with 1,653 and 2,405 median unique molecular identifiers per cell in the prophylactic and therapeutic samples, respectively. Gene expression-based filtering and cell clustering were performed using Seurat (4.0.6)45.
The uniform manifold approximation and projection (UMAP) of the combined and normalized samples were generated using the first 10 principal components, the 10 nearest neighbors, and a minimum distance of 0.12 (dims, n.neighbors, and min.dist parameters for Seurat::RunUMAP, respectively). Clustering was performed with the default (Louvain) algorithm using the first 15 principal components to identify the nearest neighbors (Seurat::FindNeighbors) and a resolution of 1 (Seurat::FindClusters). The cell types of the clusters were manually determined using marker gene expression. Neighboring clusters with identical cell type labels were combined. Differentially expressed genes between the clusters and samples were determined using the Seurat:FindAllMarkers function (with only pos = TRUE, min.pct = 0.0, logfc.threshold = 0.2).
Single-cell TCR-sequencing
For the scTCR-seq libraries, TCR-specific transcripts were amplified using a chromium single-cell mouse TCR amplification kit (PN-100254). Raw data were processed using the CellRanger VDJ software (10X Genomics). The resulting CellRanger VDJ output files were processed using custom Python (3.7.7) scripts to filter for clonotypes containing both alpha and beta chains and assign one clonotype to each cell. For cells with multiple possible clonotypes, the V/J gene pair with the highest number of reads was selected (clonotypes were marked as ambiguous if more than one V/J pair had at least 50% maximum reads). Any V/J pair without a resolved CDR3 sequence was filtered from the dataset. TCR clonotypes were defined as a unique combination of the V gene, CDR3 amino acid sequence, and the J gene. Using these definitions, the number of cells per clonotype was calculated.
General plots demonstrating the distribution of clonotypes between the two samples and cell types were constructed in Python using the seaborn package (0.11.2) and in R (4.1.2) using the tidyverse (1.2.1), ggplot2 (3.3.5), ggvenn (0.1.9), and viridis (0.6.2) packages.
Statistical analyses
Data from three independent experiments (3–4 mice/group in each experiment [n = 9–12]) were pooled. The differences between dichotomous variables were analyzed by Chi- squared test or Mann–Whitney U test. All paired values were compared using the Kruskal–Wallis test. TCR diversity metrics were calculated using Python’s skbio.diversity.alpha (0.5.6) package (Shannon’s index, Simpson’s index, Pielou evenness, and Gini index). Diversity evenness 50 score (DE50) was calculated as the ratio of the number of the most frequent clonotypes (ranked by the number of cells) within 50% of the total cell count to the total cell count. The number of clonotypes that overlapped between the inducible Treg (iTreg) and effector T cell types within each sample was compared in R using the base stats package fisher.test function and ggstatsplot package (0.9.1) for visualization.
Statistical significance was set at p values < 0.05. The values for all measurements are expressed as mean ± standard error of the mean.
Data availability
Data is provided within the manuscript or supplementary information files. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Holgate, S. T. & Polosa, R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 8, 218–230 (2008).
Durham, S. R. et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J. Allergy Clin. Immunol. 125(131–138), e131-137 (2010).
Illi, S. et al. Perennial allergen sensitisation early in life and chronic asthma in children: A birth cohort study. Lancet 368, 763–770 (2006).
Holt, P. G. et al. Prophylactic use of sublingual allergen immunotherapy in high-risk children: A pilot study. J. Allergy Clin. Immunol. 132, 991-993 e991 (2013).
Zolkipli, Z. et al. Randomized controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. J. Allergy Clin. Immunol. 136, 1541-1547 e1511 (2015).
Szepfalusi, Z. et al. Preventive sublingual immunotherapy in preschool children: First evidence for safety and pro-tolerogenic effects. Pediatr. Allergy Immunol. 25, 788–795 (2014).
Du Toit, G. et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 372, 803–813 (2015).
Alviani, C. et al. Follow-up, 18 months off house dust mite immunotherapy, of a randomized controlled study on the primary prevention of atopy. Allergy 74, 1406–1408 (2019).
Alviani, C. et al. Primary prevention of asthma in high-risk children using HDM SLIT: Assessment at age 6 years. J. Allergy Clin. Immunol. 145, 1711–1713 (2020).
Winkler, B. et al. The role of Foxp3+ T cells in long-term efficacy of prophylactic and therapeutic mucosal tolerance induction in mice. Allergy 61, 173–180 (2006).
Haspeslagh, E. et al. Prophylactic allergen immunotherapy with Der p 2 prevents murine asthma by regulating lung GM-CSF. J. Allergy Clin. Immunol. 143, 2307-2311 e2305 (2019).
Kucuksezer, U. C. et al. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol. Int. 69, 549–560 (2020).
Shamji, M. H. et al. Diverse immune mechanisms of allergen immunotherapy for allergic rhinitis with and without asthma. J. Allergy Clin. Immunol. 149, 791–801 (2022).
Musiol, S. et al. TGF-beta1 drives inflammatory Th cell but not treg cell compartment upon allergen exposure. Front. Immunol. 12, 763243 (2021).
Bacher, P. & Scheffold, A. The effect of regulatory T cells on tolerance to airborne allergens and allergen immunotherapy. J. Allergy Clin. Immunol. 142, 1697–1709 (2018).
Boonpiyathad, T., Sozener, Z. C., Akdis, M. & Akdis, C. A. The role of Treg cell subsets in allergic disease. Asian Pac. J. Allergy Immunol. 38, 139–149 (2020).
Syed, A. et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J. Allergy Clin. Immunol. 133, 500–510 (2014).
Mondoulet, L. et al. Gata3 hypermethylation and Foxp3 hypomethylation are associated with sustained protection and bystander effect following epicutaneous immunotherapy in peanut-sensitized mice. Allergy 74, 152–164 (2019).
Marogna, M., Spadolini, I., Massolo, A., Canonica, G. W. & Passalacqua, G. Long-lasting effects of sublingual immunotherapy according to its duration: A 15-year prospective study. J. Allergy Clin. Immunol. 126, 969–975 (2010).
Yamanaka, K. et al. Immunological parameters in prophylactic sublingual immunotherapy in asymptomatic subjects Sensitized to Japanese cedar pollen. Allergol. Int. 64, 54–59 (2015).
Westman, M. et al. Early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. J. Allergy Clin. Immunol. 135(1199–1206), e1191–e1111 (2015).
Westman, M. et al. Sensitization to grass pollen allergen molecules in a birth cohort-natural Phl p 4 as an early indicator of grass pollen allergy. J. Allergy Clin. Immunol. 145, 1174–11811176 (2020).
Tulaeva, I. et al. Preventive allergen-specific vaccination against allergy: Mission possible?. Front. Immunol. 11, 1368 (2020).
Seumois, G. et al. Single-cell transcriptomic analysis of allergen-specific T cells in allergy and asthma. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aba6087 (2020).
Monian, B. et al. Peanut oral immunotherapy differentially suppresses clonally distinct subsets of T helper cells. J. Clin. Invest. https://doi.org/10.1172/JCI150634 (2022).
Muschaweck, M. et al. Cognate recognition of microbial antigens defines constricted CD4(+) T cell receptor repertoires in the inflamed colon. Immunity 54, 2565-2577 e2566 (2021).
Secrist, H., Chelen, C. J., Wen, Y., Marshall, J. D. & Umetsu, D. T. Allergen immunotherapy decreases interleukin 4 production in CD4+ T cells from allergic individuals. J. Exp. Med. 178, 2123–2130 (1993).
Akoum, H. et al. Venom immunotherapy modulates interleukin-4 and interferon-gamma messenger RNA expression of peripheral T lymphocytes. Immunology 87, 593–598 (1996).
Shamji, M. H. et al. Differential induction of allergen-specific IgA responses following timothy grass subcutaneous and sublingual immunotherapy. J. Allergy Clin. Immunol. 148, 1061-1071 e1011 (2021).
Shima, K. et al. Effects of sublingual immunotherapy in a murine asthma model sensitized by intranasal administration of house dust mite extracts. Allergol. Int. 66, 89–96 (2017).
Demoly, P. & Calderon, M. A. Dosing and efficacy in specific immunotherapy. Allergy 66(Suppl 95), 38–40 (2011).
Demoly, P., Passalacqua, G., Calderon, M. A. & Yalaoui, T. Choosing the optimal dose in sublingual immunotherapy: Rationale for the 300 index of reactivity dose. Clin. Transl. Allergy 5, 44 (2015).
Wongsa, C. et al. Efficacy and safety of house dust mite sublingual immunotherapy tablet in allergic asthma: A systematic review of randomized controlled trials. J. Allergy Clin. Immunol. Pract. 10, 1342–13551324 (2022).
Heldner, A. et al. Immunological effects of adjuvanted low-dose allergoid allergen-specific immunotherapy in experimental murine house dust mite allergy. Allergy 77, 907–919 (2022).
Miyanaga, N. et al. Essential role of submandibular lymph node dendritic cells in protective sublingual immunotherapy against murine allergy. Commun. Biol. 3, 742 (2020).
Hagner, S. et al. House dust mite-specific sublingual immunotherapy prevents the development of allergic inflammation in a mouse model of experimental asthma. Int. Arch. Allergy Immunol. 170, 22–34 (2016).
Wambre, E. Effect of allergen-specific immunotherapy on CD4+ T cells. Curr. Opin. Allergy Clin. Immunol. 15, 581–587 (2015).
Wang, S. H. et al. An exhausted phenotype of T(H) 2 cells is primed by allergen exposure, but not reinforced by allergen-specific immunotherapy. Allergy 76, 2827–2839 (2021).
Jie, X. et al. Increased inhibitory surface marker PD-1 expression in CD4(+)T cells and Th2(+)T cells in allergen-specific immunotherapy. Immunobiology 229, 152824 (2024).
Zissler, U. M. et al. Early IL-10 producing B-cells and coinciding Th/Tr17 shifts during three year grass-pollen AIT. EBioMedicine 36, 475–488 (2018).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Ueno, H. et al. Cysteinyl leukotriene synthesis via phospholipase A2 group IV mediates exercise-induced bronchoconstriction and airway remodeling. Am. J. Respir. Cell Mol. Biol. 63, 57–66 (2020).
Koya, T. et al. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J. Allergy Clin. Immunol. 119, 1241–1250 (2007).
Koya, T. et al. Importance of myeloid dendritic cells in persistent airway disease after repeated allergen exposure. Am. J. Respir. Crit. Care Med. 173, 42–55 (2006).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573-3587 e3529 (2021).
Acknowledgements
The authors are grateful for the assistance of KOTAI Biotechnologies (Osaka, Japan) in managing the scRNA-seq and scTCR-seq data. We also would like to thank Editage (www.editage.jp) for English language editing. This work was supported in part by research funding from Torii Pharmaceutical CO., Ltd and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 24591462).
Author information
Authors and Affiliations
Contributions
AS and TKo designed the study and wrote the manuscript. AS, SN, HU YN, KSh, and KSu contributed to data collection. AS, TKo, AA, YK, TH, SW, YO, KOD, and TKi performed the statistical analysis and interpretation of the results. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
TKo has received funding from Torii Pharmaceutical CO., Ltd. All other authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Saito, A., Koya, T., Aoki, A. et al. Mechanism differences in the start time of sublingual immunotherapy in a mouse allergic airway inflammation model. Sci Rep 14, 26334 (2024). https://doi.org/10.1038/s41598-024-78062-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78062-6