Abstract
The escalating challenge of antimicrobial resistance necessitates the development of novel antibacterial agents. In this study, a series of five vanillin Schiff bases (SB-1 to SB-5) were synthesized from vanillin and various aromatic amines. The chemical structures of these compounds were characterized using Thin Layer Chromatography (TLC), Fourier Transform Infrared Spectroscopy (FT-IR), proton nuclear magnetic resonance (\(^{1}\text {H}\)-NMR), carbon-13 NMR (\(^{13}\text {C}\)-NMR), and mass spectrometry techniques. Antibacterial efficacy was evaluated against strains of bacteria producing extended-spectrum beta-lactamases (ESBL), including Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae using the disc diffusion method. Cytotoxic effects were assessed through haemocompatibility and brine shrimp lethality assays. The Schiff bases demonstrated notable antibacterial activities, with SB-1, SB-2, SB-4, and SB-5 exhibiting zones of inhibition up to 16.0, 16.5, 16.6, and 15.5 mm against ESBL E. coli, respectively. SB-3 showed a maximum inhibition zone of 15.0 mm against ESBL K. pneumoniae. In cytotoxicity assays, the compounds exhibited IC\(_{50}\) values against red blood cells (RBCs) greater than 200 μg/mL and ranging from 45.7 to 50.5 μg/mL for the brine shrimp assay. While demonstrating potent antibacterial properties, the toxicity towards human RBCs suggests that further toxicity evaluations and structural modifications are essential for developing safer therapeutic agents based on vanillin Schiff bases.
Similar content being viewed by others
Introduction
The rise of antimicrobial resistance (AMR) has emerged as one of the most pressing global health challenges of our time, posing a significant threat to effective disease management and public health worldwide. AMR not only complicates the treatment of common infections but also undermines advancements in medical procedures, such as surgeries, chemotherapy, and organ transplantation, where antibiotics play a critical role in preventing infections. As pathogens evolve and adapt to the antibiotics that were once effective against them, the specter of a post-antibiotic era looms, where even minor infections could lead to severe morbidity and mortality. The urgency to discover and develop novel antimicrobial agents is more pressing than ever, necessitating innovative approaches to circumvent existing resistance mechanisms.
Antimicrobial agents serve a dual purpose in clinical settings. They are crucial not only for their direct pathogen-killing capabilities but also for their ability to modulate immune responses and influence host-pathogen interactions. Recent studies highlight that certain antimicrobial compounds can enhance phagocytic activity, improve cytokine production, and overall bolster the host’s immune defenses against infections1. Despite these benefits, the global prevalence of drug-resistant pathogens-particularly those exhibiting multidrug resistance (MDR)-has escalated the need for alternative treatment approaches2. Misuse and overuse of antibiotics, particularly in human healthcare, veterinary medicine, and agriculture, have led to a dramatic increase in resistant strains of bacteria. This growing concern underscores the critical need for innovative antimicrobial strategies capable of addressing the complexities of resistance.
Among the most concerning resistance mechanisms in Gram-negative bacteria is the production of \(\beta\)-lactamases, enzymes that confer resistance to a wide range of \(\beta\)-lactam antibiotics3. Extended-spectrum \(\beta\)-lactamases (ESBLs) are particularly formidable due to their ability to inactivate multiple classes of antibiotics, severely limiting therapeutic options and leading to increased morbidity and mortality rates in affected patients4. The emergence of ESBL-producing strains emphasizes the urgent need for the development of novel antimicrobial agents that can effectively target and neutralize these resistant bacteria, restoring the efficacy of existing antibiotic classes or providing entirely new mechanisms of action.
In the realm of medicinal chemistry, heterocyclic compounds have garnered considerable attention for their role in the development of bioactive molecules with diverse therapeutic applications. Schiff bases, formed through the condensation of primary amines with aldehydes or ketones, have shown a wide spectrum of biological activities, including antimicrobial, anticonvulsant, anti-inflammatory, and antioxidant effects. These compounds are particularly attractive due to their structural diversity, ease of synthesis, and ability to be functionalized for improved biological activity5. Recent research has illuminated the therapeutic potential of Schiff base ligands and their transition metal complexes in addressing various pathogenic conditions. For example, studies have demonstrated that organotellurium(IV) Schiff base complexes exhibit significant antimicrobial and antioxidant activities, suggesting their potential as effective agents against a variety of pathogens and oxidative stress-related conditions6.
Despite the promising potential of Schiff bases, there remains a substantial gap in our understanding of their effectiveness against ESBL-producing bacterial strains. Specifically, the antimicrobial activity of Schiff bases derived from vanillin, a natural product with known medicinal properties, has not been thoroughly explored. Previous studies involving structurally related compounds have shown variable antimicrobial efficacy against resistant bacterial strains, indicating that vanillin-derived Schiff bases could play a crucial role in addressing this gap in knowledge and offering new therapeutic options7. The urgency of addressing the challenges posed by resistant pathogens necessitates focused research into these compounds.
Therefore, this study seeks to evaluate the antimicrobial activity of Schiff bases derived from vanillin against clinical isolates of ESBL-producing bacteria, specifically Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. By systematically investigating the efficacy of these compounds, we aim to contribute valuable insights into their potential as novel therapeutic agents in the ongoing battle against antimicrobial resistance.
We hypothesize that Schiff bases of vanillin will exhibit significant antimicrobial activity against these resistant strains, potentially offering efficacy comparable to or surpassing existing antibiotics. The specific objectives of this study are as follows:
-
1.
To assess the antimicrobial activity of vanillin-derived Schiff bases against clinically relevant ESBL-producing bacterial strains.
-
2.
To evaluate the cytotoxicity of these Schiff bases using in vitro models to ensure their safety for therapeutic use.
While this study focuses on investigating the antimicrobial and cytotoxic properties of the synthesized Schiff bases, in vivo testing and comprehensive safety evaluations are beyond the scope of this research and will be addressed in future studies.
Hypothesis
This study hypothesizes that Schiff bases of vanillin will exhibit potent antimicrobial activity against clinical isolates of extended-spectrum \(\beta\)-lactamase (ESBL)-producing bacteria.
Aim
This research aims to determine the antimicrobial activity of Schiff bases derived from vanillin against clinical isolates of selected ESBL-producing bacteria.
Objectives
-
1.
To evaluate the antimicrobial activity of vanillin-derived Schiff bases against selected ESBL-producing bacteria, including E. coli, K. pneumoniae, and P. aeruginosa.
-
2.
To investigate the cytotoxic properties of vanillin-derived Schiff bases using in vitro models.
Materials and methods
Study design
This study employed a quantitative experimental research design to investigate the synthesis of Schiff bases derived from vanillin and various aromatic amines. The objective was to evaluate the efficiency and yield of the synthetic methods used.
General experimental procedures
Apparatus
The experimental apparatus included various laboratory glassware and equipment, such as:
-
Beakers: 50 mL, 100 mL, 500 mL, and 1000 mL
-
Round-Bottom flasks: Single-necked (50 mL and 100 mL), double-necked (250 mL), and three-necked (500 mL)
-
Measuring cylinders: 10 mL and 50 mL
-
Separating funnels, pipettes, and fillers
-
Columns, glass rods, and thermometers
-
Adapters, Petri dishes, test tubes
-
Condensers, oil baths, guard tubes
-
Stoppers and syringes
-
Storage containers: bottles and glass jars
Chemicals
All chemicals were of the highest analytical grade:
-
Vanillin (\(\text {C}_8\text {H}_8\text {O}_3\)) was procured from Sigma Aldrich.
-
Acetic acid (\(\text {C}_2\text {H}_4\text {O}_2\)) was obtained from Merck and used without purification.
-
Amines were dried over potassium hydroxide (\(\text {KOH}\)) and stored under a nitrogen atmosphere over KOH pellets.
-
Solvents Dry solvents such as n-hexane, chloroform, methanol, tetrahydrofuran (THF), and dichloromethane (DCM) were distilled according to standard procedures.
Glassware and needles were flame-dried immediately before use or placed in an oven at 150–160 °C for a minimum of 2–3 hours, then cooled in desiccators or under reduced pressure. Liquid reagents, solvents, and solutions were handled using syringes or tubing with rubber septa, and Schlenk-type adapters were employed for solid reagents.
Characterization techniques and instruments
The characterization data of the synthesized compounds has been reorganized and included under specific headings as outlined.
Chromatography. Chromatography is a crucial separation technique that divides a mixture into its constituent compounds based on differential interactions between mobile and stationary phases.
Thin layer chromatography (TLC). TLC was employed to monitor the reaction progress at various intervals. Kieselgel 60 F254 (Merck) served as the stationary phase, and a solvent system of ethyl acetate/n-hexane (3:7) was used as the eluent. TLC chromatograms were visualized using a UV lamp at wavelengths of \(\lambda _{\text {max}}\) 254 nm and 365 nm.
Concentration techniques
Rotary evaporator. A Buchi rotary evaporator was utilized to concentrate the reaction mixture under reduced pressure, with the bath temperature maintained at 45 °C. The system operated under pressures of either 15 mm Hg or 0.1 mm Hg, utilizing an oil or diaphragm pump.
Melting point determination
Melting point apparatus. Melting points (M.P.) of the synthesized compounds were determined using a Gallenkamp digital melting point apparatus and are reported in uncorrected Celsius (°C).
Spectroscopic techniques
Fourier transform infrared spectroscopy (FT-IR). FT-IR spectroscopy was employed to identify and confirm the functional groups present in the synthesized compounds. Spectra were recorded using a Shimadzu FTIR Model 270. Solid samples were prepared as potassium bromide pellets, while liquid samples were analyzed in sodium chloride (NaCl) cells.
Nuclear magnetic resonance spectroscopy (NMR). NMR spectroscopy provided insights into the environments of magnetically active nuclei, specifically \(^1\text {H}\) and \(^{13}\text {C}\). \(^1\text {H}\) NMR spectra were acquired using a Bruker NMR instrument operating at 300 MHz, while \(^{13}\text {C}\) NMR spectra were recorded at 75 MHz. Chemical shifts are reported in parts per million (ppm) relative to the internal standard tetramethylsilane (SiMe\(_4\)), with coupling constants (\(J\)) presented in Hertz (Hz). The proton (\(^1\text {H}\)) NMR spectral patterns are described using the following notations: singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), broad (b), and various combinations thereof.
Synthesis of Schiff bases
The synthesis of Schiff bases was accomplished using vanillin and various aromatic amines, following two established procedures. The process involves the formation of an imine linkage between the carbonyl group of vanillin and the amino group of the aromatic amine, yielding a variety of Schiff bases with potential antibacterial activity.
General procedure
Materials. Reagents used in this synthesis include vanillin (0.01 M), various substituted aromatic amines (0.01 M), concentrated sulfuric acid (\(\text {H}_2\text {SO}_4\)), ethyl alcohol, and water. Equipment required for the synthesis consists of a conical flask, magnetic stirrer, rotary evaporator, mortar and pestle, and filter apparatus.
Method A: Solvent-assisted synthesis. In a conical flask, combine vanillin (0.01 M) with the selected substituted aromatic amines (0.01 M). To this mixture, add a few drops of concentrated sulfuric acid (\(\text {H}_2\text {SO}_4\)) and approximately 15-20 mL of ethyl alcohol as a solvent. Stir the reaction mixture using a magnetic stir bar at a temperature of 100 °C for a duration of 2-9 hours. The reaction time may vary depending on the specific aromatic amine used. Upon completion of the reaction, remove the solvent under reduced pressure using a rotary evaporator. The crude product is then washed with ethyl alcohol to remove impurities, followed by filtration. Finally, recrystallize the obtained solid from ethyl alcohol to yield pure Schiff base (see Figs. 1 and 2).
Structures of synthesized compounds with variable R groups, illustrating the effect of different substituents on the molecular structure. The groups are as follows: R = -CH\(_{3}\) (Methyl), R = -Cl (Chloro), and R = -OH (Hydroxy). The representations are derived from their respective SMILES notation.
Method B: Solvent-free synthesis. In a mortar, mix vanillin (0.01 M) with the selected substituted aromatic amines (0.01 M), a few drops of concentrated sulfuric acid (\(\text {H}_2\text {SO}_4\)), and 5-10 mL of water. Stir the reaction mixture with a magnetic stir bar for 30-40 minutes at room temperature (RT). To verify completion, perform Thin Layer Chromatography (TLC) analysis. Once the reaction is confirmed, add 25 mL of water to the mixture. Isolate the solid product formed, wash it thoroughly with water, filter, and recrystallized from ethyl alcohol as detailed in the literature8.
Notes. The choice of aromatic amine will influence the solubility and yield of the final product. Due to the corrosive nature of sulfuric acid, ensure all reactions are performed under appropriate safety conditions, including the use of gloves and eye protection.
Isolation, identification, and characterization of bacteria
Sample collection and storage
Bacterial samples were collected from various hospitals, specifically Khyber Teaching Hospital (KTH) Peshawar and Hayatabad Medical Complex (HMC) Peshawar. All bacterial samples were immediately transported to the Microbiology Laboratory of the Institute of Basic Medical Sciences (IBMS), Khyber Medical University, and stored in a refrigerator until further processing.
Identification tests
Gram staining. Gram staining was performed to differentiate between Gram-positive and Gram-negative bacteria and to observe the morphology of bacterial cells. The methodology described by Benson9 was followed. A fresh culture of 24 h was utilized for the Gram staining procedure. A clean glass slide was taken, and a drop of distilled water was placed on it. A loopful of fresh bacterial culture was taken using a sterile inoculation loop and evenly spread in water on the glass slide. The slide was then air-dried and heat-fixed using a spirit lamp to prepare a smear. The smear was covered with crystal violet dye for 30 sec and then washed off with tap water. Gram iodine was applied to the smear for 1 min and then washed off with water. The smear was decolorized with 95% ethanol and washed with water. A counterstain of safranin was applied for 30 seconds and washed off with water. The slide was blotted dry with absorbent paper and examined under a microscope at magnifications of 40x and 100x. A drop of immersion oil was placed on the slide for observation at 100x. A purple color indicated Gram-positive bacteria, while a pink color indicated Gram-negative bacteria. The cell morphology, such as rod shape, was also assessed.
Oxidase test. The oxidase test was performed to determine the presence of oxidase enzymes produced by the bacteria. A piece of filter paper was placed on a glass slide, and 2–3 drops of oxidase reagent were added. A fresh bacterial culture was picked with a sterile toothpick and streaked on the filter paper. The color change was observed within 30 sec. Oxidase-positive bacteria exhibited a blue-purple coloration, while oxidase-negative bacteria showed no color change.
Triple sugar iron (TSI) test. The TSI test was conducted to evaluate the fermentation of sugars, specifically glucose, and lactose, and the production of gas and hydrogen sulfide (\(\text {H}_2\text {S}\)). TSI agar medium was prepared and heated to ensure uniform dissolution of the ingredients. An appropriate amount of medium was added to test tubes, which were then tightly plugged. The test tubes containing the medium were autoclaved, cooled, and maintained in an inclined position to prepare slants with appropriate butts. After solidification, the slants were incubated for sterility checking for 24 h at 37 °C. The TSI agar was inoculated with fresh bacterial culture using a sterile wire loop by first stabbing through the center to the bottom and then streaking on the surface of the agar slants. The tubes were plugged tightly and incubated for 24 h at 37 °C. Observations for color changes were made post-incubation: a yellow color only in the butt indicated glucose fermentation; a yellow color in both the butt and slant indicated fermentation of both glucose and lactose. The presence of cracks in the medium indicated gas production, while a black precipitate indicated the production of \(\text {H}_2\text {S}\). A red color (no color change) indicated no fermentation.
Citrate utilization test. The citrate utilization test was conducted to assess the ability of bacteria to utilize citrate as a carbon and energy source. Simmon’s citrate agar medium was prepared and heated, with an appropriate amount added to the test tubes. These were then tightly plugged, autoclaved, and maintained inclined for slant preparation. After solidification, the slants were incubated for 24 h at 37 °C for sterility checking. The slants were inoculated with fresh bacterial culture using a sterile inoculation loop, plugged tightly, and incubated for an additional 24 h at 37 °C. A change in the media color from deep green to blue indicated a positive result for citrate utilization, while no color change indicated a negative result.
Urease test. The urease test was performed to evaluate the production of urease enzyme by bacteria, which hydrolyzes urea into ammonia and carbon dioxide. Urease agar medium was prepared and heated, with an appropriate amount added to test tubes. The tubes were plugged, autoclaved, cooled, and maintained in an inclined position for slant preparation. Slants were incubated for sterility checking at 37 °C for 24 h and then inoculated with fresh bacterial culture using a sterile inoculation loop, plugged tightly, and incubated for 24 h at 37 °CC. Post-incubation, the slants were observed for a color change from light orange to magenta/pink, indicating a positive result for urease production. No color change indicated a negative result for urease production and urea hydrolysis.
Catalase test. The catalase test was performed to determine the production of catalase enzyme by the bacteria. A drop of 3% hydrogen peroxide (\(\text {H}_2\text {O}_2\)) was placed on a glass slide, and a loopful of fresh bacterial culture was mixed with the hydrogen peroxide. The formation of bubbles was observed within 10 seconds for catalase-positive bacteria, while no bubble formation after 10 sec indicated a negative result.
Preservation and maintenance of pure isolates
Preservation on nutrient agar slants. Isolates were preserved on nutrient agar slants following the methodology of Abdulkadir and Waliyu10 to maintain pure cultures for short-term storage and further processing. Nutrient agar medium was prepared in a flask and heated with a Bunsen burner to dissolve the ingredients. 5 mL of the medium was transferred to each test tube and plugged with cotton plugs. These test tubes were autoclaved at 121 °C for 15 min at 15 psi. After sterilization, the test tubes were transferred to a laminar flow hood and kept in an inclined position for slant preparation after cooling and solidification. The slants were incubated for 24 h for sterility checks. Uncontaminated slants, along with nutrient agar plates containing pure cultures, were transferred to the laminar flow hood, and slants were streaked with each pure isolate obtained from subculturing on nutrient agar plates using a sterilized wire loop. The slants were then incubated for 24–48 h, and upon sufficient growth, they were transferred to the refrigerator and stored at 4 °C. Each isolate was preserved in duplicate, one for processing and one for preservation as a pure culture.
Preservation in glycerol. Pure isolates were preserved in 20% glycerol for long-term storage. Nutrient broth medium was prepared in a flask, heated to dissolve the ingredients, and 5 mL was transferred to each test tube, which was then tightly plugged. These test tubes were autoclaved and placed in a water bath to cool. After incubation for 24 h for sterility checking, each test tube was inoculated with individual pure isolates via a sterilized wire loop, plugged tightly, and incubated for 24–48 h. Glycerol was placed in a flask and tightly covered with aluminum foil. Tips and Eppendorf tubes were also autoclaved for sterilization and then transferred to a laminar flow hood to cool. Using a micropipette, 200 μL of glycerol was transferred to each Eppendorf tube using sterilized tips. To each Eppendorf tube, 800 μL of broth culture from individual isolated bacteria was added using a separate tip for each culture. The Eppendorf tube was then closed, vortexed, and transferred to the refrigerator for storage at −20 °C for long-term preservation.
Preparation of McFarland standard
The McFarland standard is utilized as a reference to adjust the concentration of bacterial cells in a suspension by modifying its turbidity. A 0.5 McFarland standard was prepared by mixing 0.05 mL of 1.175% barium chloride dihydrate (\({BaCl}_2 \cdot 2{H}_2\)O) with 9.95 mL of 1% sulfuric acid (\({H}_2{SO}_{4}\)). This standard corresponds to approximately \({1.5} \times {10}^{8}\) cells per mL. After preparation, the McFarland standard solutionwas wrapped in aluminum foil and stored in the dark.
Double disc synergy test (DDST) for ESBL detection
To detect Extended Spectrum Beta-Lactamases (ESBL), fresh plates of Mueller Hinton agar media were prepared and incubated at 37 °C for 24 h for sterility checks. Nutrient broth media were also prepared, added to test tubes, tightly plugged, autoclaved, and incubated at 37 °C for 24 h for sterility checks. Test bacteria were refreshed in nutrient broth media. Cotton swabs and micropipette tips were sterilized through autoclaving. All materials were transferred to the laminar flow hood for aseptic procedures. The turbidity of the broth culture of test bacteria was adjusted using the 0.5 McFarland Standard by adding sterile saline water to the tubes.
A new sterile cotton swab was submerged in the suspension, and the excess fluid was removed by pressing and rotating the swab against the wall of the tube. The swab was then used to inoculate the entire surface of the Mueller Hinton agar plate three times, rotating the plate 60 degrees between each inoculation. The inoculum was allowed to dry for a few minutes. Disks containing Ceftazidime (30 μg) and Cefotaxime (30 μg) were placed 20 mm apart from the Amoxicillin/Clavulanate (30 μg) disk. The plates were incubated at 37 °C for 18-20 h. An enhanced zone of inhibition towards the Amoxicillin/Clavulanate disk indicated positive ESBL production.
Evaluation of the biological activities of Schiff bases
Disc diffusion method
The antibacterial activity of Schiff bases was evaluated using the agar disc diffusion method. Fresh plates of Mueller Hinton agar media were prepared and incubated at 37 °C for 24 h for sterility checks. Nutrient broth media were prepared, added to test tubes, tightly plugged, autoclaved, and incubated at 37 °C for 24 h for sterility checks. Test bacteria were refreshed in nutrient broth media. Cotton swabs and micropipette tips were sterilized through autoclaving.
The turbidity of the broth culture of test bacteria was adjusted to 0.5 McFarland Standard by adding sterile saline water to the tubes. Using sterilized cotton swabs, uniform bacterial lawns of test bacteria were prepared on Mueller Hinton agar plates and allowed to dry for some time. Discs (6 mm) were prepared from Whatman’s filter paper no. 1, loaded with 10 μL of test dilutions (4000, 2000, 1000, 500, 250, 125 μg/mL), dried, and placed on the uniform bacterial lawn. Imipenem and Ceftazidime (10 μg) discs were used as standard drugs. The plates were then incubated for 24 h at 37 °C. Each sample was run in triplicate, and inhibitory zones were measured in millimeters using a scale.
In vitro cytotoxicity assays
Brine shrimp cytotoxicity
Brine shrimp lethality tests were conducted to evaluate the cytotoxic potential of the vanillin Schiff bases. Eggs of Artemia salina were purchased from Ocean Star International and stored at 28 °C. For hatching, 3 liters of water were measured and poured into a rectangular jar. Approximately 27 g of table salt was added to the jar containing water, mixed thoroughly with a spatula, and properly aerated by placing an airline tip from an air pump into the jar bottom. 15 g of brine shrimp eggs were sprinkled on the water’s surface and mixed. A light source was positioned a few inches away from the jar. The nauplii hatched after 24 h. Newly hatched nauplii were picked with a pasture pipette, and ten nauplii were transferred to each test tube. Test samples (1–200 μg/mL) were added to each test tube, adjusting the total volume to 300 μL. After 24 h of exposure, the shrimps were collected using a pasture pipette and counted under a magnifying glass. The percentage of dead shrimp was calculated for each well. Data were analyzed using the Finney computer program to determine \(\hbox {IC}_{{50}}\) values.
Biocompatibility assay
To assess the biocompatibility of the newly synthesized vanillin Schiff bases, a hemolytic assay was performed using freshly isolated human erythrocytes. Blood was obtained from a healthy individual using a sterilized syringe and collected in an EDTA tube (5.4 mg) to prevent clotting. Red blood cells (RBCs) were isolated by centrifuging 1 mL of collected blood at 14,000 rpm for 5 min.
To prepare an erythrocyte suspension in phosphate-buffered saline (PBS, pH 7.2), 200 μL of pelleted RBCs was mixed with 9.8 mL of PBS and gently shaken. A mixture of 100 μL of the erythrocyte suspension and 100 μL of the test solution was incubated at 35 °C for 1 h in Eppendorf tubes. After incubation, the samples were centrifuged at 10,000 rpm for 10 min. The supernatant was transferred to cuvettes, and hemoglobin release was measured spectrophotometrically at 540 nm11.
Triton X-100 and PBS served as positive and negative controls, respectively. The percentage hemolysis was calculated using the formula:
Biological evaluation of compounds
To evaluate the biological stability and activity of the synthesized vanillin Schiff bases, the following protocols were implemented:
Stability studies. The synthesized Schiff bases were subjected to stability tests under various environmental conditions, including temperature fluctuations, humidity, and light exposure. The stability of the compounds was monitored through spectroscopic methods and thin-layer chromatography (TLC) to assess any degradation or transformation.
Biological conditions. Biological evaluations were conducted to determine the effectiveness of the synthesized compounds against selected bacterial strains. The synthesized Schiff bases were dissolved in appropriate solvents and tested against various Gram-positive and Gram-negative bacteria using the disk diffusion method. Each experiment was performed in triplicate to ensure statistical significance and reproducibility of the results. Additionally, the minimum inhibitory concentration (MIC) was determined through serial dilution techniques, with three replicates for each concentration tested.
This approach ensures a comprehensive understanding of how these compounds behave under physiological conditions and their potential therapeutic applications, thus aligning with contemporary research methodologies in drug development. .
Results
Synthesis of Schiff bases
The following Schiff base compounds were synthesized, and their respective physicochemical properties are detailed below.
2-Methoxy-4-((4-nitrophenylimino)methyl)phenol (SB-1)
-
Molecular weight: 272.26 g/mol
-
Molecular formula: \(\text {C}_{14}\text {H}_{12}\text {N}_{2}\text {O}_{4}\)
-
Yield: 75% (Method A), 68% (Method B)
-
Physical state: Yellow solid, M.P = 190–192 °C
-
\(\text {Rf}\)-value: 0.68 (ethyl acetate: hexane, 3:7)
-
FTIR (KBr, cm\(^{-1}\)): 2939 (C-H), 1655 (C=N), 1535 (Ar)
-
Solubility: Soluble in DMSO
As illustrated in Fig. 3, the structure of SB-1 features an imine linkage and a nitro group, which are known to contribute to antimicrobial activity and interaction with biological targets.
4-((2-Bromophenylimino)methyl)-2-methoxyphenol (SB-2)
-
Molecular weight: 306.15 g/mol
-
Molecular formula: \(\text {C}_{14}\text {H}_{12}\text {BrNO}_{2}\)
-
Yield: 75% (Method A), 68% (Method B)
-
Physical state: Yellow solid, M.P = 190–192 °C
-
\(\text {Rf}\)-value: 0.68 (ethyl acetate: hexane, 3:7)
-
FTIR (KBr, cm\(^{-1}\)): 2939 (C-H), 1655 (C=N), 1535 (Ar)
-
Solubility: Soluble in DMSO
As depicted in Fig. 4, SB-2 contains a bromine atom, which may enhance its reactivity in synthetic applications and contribute to its biological activity.
4-((2-Chloro-4-methylphenylimino)methyl)-2-methoxyphenol (SB-3)
-
Molecular weight: 275.73 g/mol
-
Molecular formula: \(\text {C}_{15}\text {H}_{14}\text {ClNO}_2\)
-
Yield: 70% (Method A), 80% (Method B)
-
Physical state: Yellow solid, M.P = 238–239 °C
-
\(\text {Rf}\)-value: 0.87 (ethyl acetate : n-hexane, 3:7)
-
FTIR (KBr, cm\(^{-1}\)): 2936 (C-H), 1656 (C=N), 1530 (Ar), 912 (C-Cl)
-
Solubility:Soluble in DMSO
The structure of SB-3, as shown in Fig. 5, includes a chloro substituent and a methyl group, which may alter its electronic properties and chemical reactivity.
4-((3-Hydroxyphenylimino)methyl)-2-methoxyphenol (SB-4)
-
Molecular weight: 243.26 g/mol
-
Molecular formula: \(\text {C}_{14}\text {H}_{13}\text {NO}_3\)
-
Yield: 50% (Method A), 75% (Method B)
-
Physical state: Dark gum
-
\(\text {Rf}\)-value: 0.66 (ethyl acetate : n-hexane, 3:7)
-
FTIR (KBr, cm\(^{-1}\)): 2934 (C-H), 1652 (C=N), 1539 (Ar)
-
Solubility: Soluble in DMSO
The hydroxyl group present on the phenyl ring of SB-4, as shown in Fig. 6, is known to influence hydrogen bonding and may enhance its biological activity.
4-((2,4-Dichlorophenylimino)methyl)-2-methoxyphenol (SB-5)
-
Molecular weight: 296.15 g/mol
-
Molecular formula: \(\text {C}_{14}\text {H}_{11}\text {Cl}_2\text {NO}_2\)
-
Yield: 75% (Method A), 50% (Method B)
-
Physical state: White-brown crystals, M.P = 208–216 °C
-
\(\text {Rf}\)-value: 0.62 (ethyl acetate : n-hexane, 3:7)
-
FTIR (KBr, cm\(^{-1}\)): 2936 (C-H), 1653 (C=N), 1536 (Ar)
-
Solubility: Soluble in DMSO
The presence of dichloro groups at the 2 and 4 positions in SB-5, as shown in Fig. 7, affects its electron density distribution and reactivity, potentially enhancing its antimicrobial properties.
All synthesized compounds (Figs. 3, 4, 5, 6 and 7) and their corresponding physicochemical properties (Table 1) are presented above, highlighting their unique structural features and properties.
Characterization of Schiff bases
The synthesized Schiff bases were characterized using several physicochemical methods, including FTIR, NMR, and mass spectrometry, consistent with methodologies reported in the literature, such as the synthesis and characterization of hydrazone-containing organotin(IV) complexes12. The presence of specific functional groups was confirmed through spectral analysis, which aligns with the findings of similar compounds reported in the study by13,14. For instance, the characteristic C=N stretching was observed around 1655 cm\(^{-1}\) in the FTIR spectra, which is consistent with the spectral data from hydrazone complexes15.
Isolation and identification of bacteria
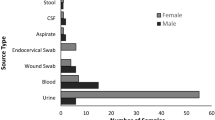
Bacterial isolates were obtained from two healthcare facilities, and the sample distribution is presented in Table 2. The isolates underwent Gram staining and a series of biochemical assays to confirm their identity. These tests were essential for characterizing the bacterial strains and establishing the presence of Extended Spectrum Beta-Lactamases (ESBLs).
The collected bacterial samples, detailed in Table 2, include strains such as Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa. Table 3 presents the results of the biochemical assays performed on these isolates, which confirmed the distinctive biochemical profiles for each strain.
Screening for ESBL producing bacteria
The detection of ESBL-producing bacteria was conducted using the Double Disc Synergy Test. Out of the fifteen samples processed, five showed positive results, as detailed in Table 4. The positive isolates were preserved at 4 °C on slants and in 20% glycerol at −20 °C for long-term storage and further experimental use. Figures 8, 9, and 10 illustrate the ESBL-positive results for E. coli, P. aeruginosa, and K. pneumoniae, respectively.
Antibacterial activity
The antibacterial efficacy of various Schiff bases was evaluated through the agar disc diffusion method and Minimum Inhibitory Concentration (MIC) measurements against ESBL-producing strains. The MIC values help determine the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation. Results for MIC and zone of inhibition are presented in Tables 5 and 6, respectively. The corresponding antibacterial activities of the Schiff bases are illustrated in Figs. 11, 12, 13, 14, 15, and 16.
Structural variation and antibacterial activity of Schiff bases
A comparative analysis of the synthesized Schiff bases (SB-1 to SB-5) reveals how structural variations influence their antibacterial efficacy against the tested ESBL-producing strains (E. coli, K. pneumoniae, and P. aeruginosa). Each compound’s molecular structure plays a critical role in determining its bioactivity, as illustrated in Tables 5, 6, and 7.
A detailed analysis of the structures shows the following:
-
SB-1 (2-Methoxy-4-((4-nitrophenylimino)methyl)phenol): The presence of a nitro group enhances its electron-withdrawing properties, facilitating interaction with microbial targets. It displayed consistent antibacterial activity across all strains, with MIC values of 125 μg/mL and inhibition zones ranging from 14.2 mm to 16.6 mm.
-
SB-2 (4-((2-Bromophenylimino)methyl)-2-methoxyphenol): The introduction of a bromine substituent is expected to enhance reactivity. Its antibacterial activity was comparable to SB-1, with MIC values of 250 μg/mL and inhibition zones between 14.2 mm and 16.5 mm, indicating moderate effectiveness.
-
SB-3 (4-((2-Chloro-4-methylphenylimino)methyl)-2-methoxyphenol): Featuring a chloro group and a methyl substituent, SB-3 exhibited varied antibacterial responses with MIC values of 125 μg/mL. The inhibition zones were comparable to SB-1.
-
SB-4 (4-((3-Hydroxyphenylimino)methyl)-2-methoxyphenol): The hydroxyl group may enhance hydrogen bonding with bacterial enzymes, improving antimicrobial action. This compound showed MIC values of 125 μg/mL and robust inhibition zones.
-
SB-5 (4-((2,4-Dichlorophenylimino)methyl)-2-methoxyphenol): The presence of dichloro groups affects electron density distribution, impacting interaction with bacterial targets. SB-5 exhibited MIC values of 125 μg/mL, suggesting its structural complexity influences selectivity.
In summary, the comparison of structural variations among the synthesized Schiff bases underscores the importance of specific functional groups in determining antibacterial efficacy. While some structural modifications enhance activity, others may diminish it. Further studies could elucidate these relationships, focusing on optimizing these compounds for increased antimicrobial potential.
In vitro cytotoxicity assays
Biocompatibility testing
In response to the potential cytotoxic behavior of the synthesized vanillin Schiff bases, their cytotoxicity against normal human red blood cells was assessed. Results were analyzed over the concentration range from 200 μg/mL to 1 μg/mL.
In this section, we present the results of hemocompatibility and brine shrimp cytotoxicity assays for Schiff bases SB1-2 and SB3-5.
Figure 17 shows the hemocompatibility results for Schiff bases SB1-2, while Fig. 18 illustrates the results for SB3-5. For the brine shrimp cytotoxicity results, Figs. 19 and 20 depict the findings for SB1-2 and SB3-5, respectively.
Brine shrimp cytotoxicity
A brine shrimp cytotoxicity assay was conducted to further evaluate the cytotoxicity of the vanillin Schiff bases. The assay was performed over a concentration range of 200 μg/mL to 1 μg/mL. The results, including the IC50 values, are summarized in Table 8.
Figure 19 displays the brine shrimp cytotoxicity of SB1-2, while Fig. 20 presents the results for SB3-5.
Discussion
The synthesis and evaluation of vanillin Schiff bases as potential antibacterial agents against extended-spectrum beta-lactamase (ESBL)-producing bacteria have yielded promising results, significantly contributing to the ongoing search for effective antimicrobial therapies amidst escalating resistance issues. Our study demonstrated that the Schiff bases, particularly SB-1, SB-2, SB-4, and SB-5, exhibited robust inhibitory activity against ESBL-producing Escherichia coli, with inhibition zones reaching up to 16.6 mm. Notably, SB-3 demonstrated significant activity against ESBL-producing Klebsiella pneumoniae, with a maximum inhibition zone of 15.0 mm. These findings underscore the potential of these compounds as viable alternatives to conventional antibiotics in combating multidrug-resistant pathogens.
An analysis of spatial and temporal variations in antibacterial efficacy revealed consistent inhibitory effects across various bacterial strains, highlighting the broad-spectrum activity of the synthesized Schiff bases. The presence of hydroxyl groups in SB-4 likely contributed to its enhanced antibacterial potency, potentially facilitating increased cellular permeability and efficacy against resistant strains.
However, exceptions to these general observations were noted, particularly with SB-2, which exhibited reduced efficacy at lower concentrations. This variation underscores the complex interplay between chemical structure and antibacterial activity, suggesting potential differences in target receptor binding or membrane permeability among different bacterial species.
Additionally, the choice of solvent has been shown to significantly affect the antibacterial activity of the Schiff bases. Specific solvents can enhance or diminish the solubility and stability of the compounds, which in turn influences their interaction with bacterial membranes. Our findings indicate that using polar solvents improved the solubility and bioavailability of the compounds, correlating with increased inhibition zones against the tested ESBL-producing bacteria. Conversely, non-polar solvents exhibited lower efficacy, likely due to poor solvation and reduced interaction with microbial targets.
These observations emphasize the necessity of selecting appropriate solvents during the formulation of antimicrobial agents, as they can significantly affect the performance and effectiveness of the resulting compounds.
In comparing our findings with previous research, several key aspects underscore the advancements and unique contributions of our investigation into vanillin Schiff bases as potent antibacterial agents, particularly against ESBL-producing bacteria. Prior studies have primarily focused on synthesizing Schiff bases derived from various aldehydes, both aliphatic and aromatic, and assessing their basic antimicrobial properties against standard bacterial strains.
For instance, a library of Schiff bases synthesized from aliphatic glyoxal and aromatic vanillin aldehydes, along with their iron(III) complexes, was characterized using infrared (IR) spectroscopy, \(^{1}\)H-NMR, melting point determination, and UV-visible spectroscopy. These compounds were evaluated against Staphylococcus aureus (Gram-positive) and Escherichia coli (Gram-negative), revealing that the iron(III) complexes exhibited enhanced activity against E. coli compared to the Schiff bases themselves16. However, the antibacterial activities against S. aureus varied among the Schiff bases and their complexes17.
In contrast, our study specifically highlights the efficacy of vanillin-derived Schiff bases against ESBL-producing bacteria, showcasing not only their antibacterial potential but also their effectiveness in combating resistant strains. Furthermore, recent research conducted by Kumar et al. focused on the synthesis of hydrazone ligands and their transition metal(II) complexes, which were characterized through a variety of spectral and analytical techniques. Their findings indicate that the Co(II), Ni(II), Cu(II), and Zn(II) complexes demonstrated notable antituberculosis activity, with the zinc(II) complex achieving an MIC value of 0.0028 μmol/mL, significantly outperforming streptomycin at 0.0107 μmol/mL18. This establishes the zinc(II) complex as a potent candidate for tuberculosis treatment, representing a significant advancement in the field of medicinal chemistry.
Additionally, the recent study by Kumar et al. on thiosemicarbazone ligands and their transition metal complexes further complements these findings. In their multifaceted investigation, they synthesized thiosemicarbazones and their Co(II), Ni(II), Cu(II), and Zn(II) complexes from benzaldehydes and 4-(4-ethylphenyl)-3-thiosemicarbazide, exploring their antituberculosis, antibacterial, antifungal, and anti-inflammatory properties. Notably, their lead compound (10) exhibited an impressive MIC value of 0.006 μmol/mL against tuberculosis, demonstrating nearly double the efficacy of streptomycin19. This significant enhancement in potency emphasizes the potential of transition metal complexes in addressing resistant infections, thereby aligning with our research objectives.
Moreover, the thiosemicarbazone complexes (6) and (9) displayed comparable tuberculosis inhibition to streptomycin, further establishing their role as effective agents against mycobacterial infections. Additionally, compound (10) emerged as the most potent candidate against bacterial (0.0066 μmol/mL) and fungal (0.0066 μmol/mL) pathogens, reinforcing the therapeutic relevance of these metal complexes in treating a broad spectrum of infectious diseases. These results not only parallel our observations regarding the antibacterial properties of vanillin Schiff bases but also extend the conversation to include their potential applications in treating both bacterial and fungal infections.
The study also employed advanced computational techniques, including molecular docking, density functional theory (DFT), molecular electrostatic potential (MESP), and absorption, distribution, metabolism, excretion, and toxicity (ADMET) assessments. These methodologies provided a thorough validation of the in vitro results, identifying optimal binding interactions and predicting the pharmacokinetic properties of the complexes18. This comprehensive approach highlights the growing importance of integrating theoretical analyses with experimental studies to enhance the understanding of ligand-receptor interactions and predict the behavior of novel compounds in biological systems.
By situating our findings within this context, we emphasize the significance of vanillin Schiff bases not only in terms of their antimicrobial properties but also in relation to the broader landscape of research focused on overcoming bacterial resistance. The unique structural features and mechanisms of action of our synthesized compounds warrant further exploration, particularly concerning their application in medicinal chemistry and potential therapeutic interventions against resistant bacterial infections and tuberculosis.
Ultimately, the collective research efforts highlighted in both our work and that of Kumar et al. signify a concerted advancement in the fight against infectious diseases, underlining the need for innovative strategies that leverage the synergistic effects of metal coordination chemistry and ligand design. As we move forward, these insights pave the way for more targeted in vivo investigations, ultimately contributing to improved global health outcomes.
In contrast, our study builds upon this foundation by focusing specifically on enhancing antibacterial efficacy against clinically relevant ESBL-producing Escherichia coli strains, which are notorious for their resistance to conventional antibiotics. We synthesized a series of vanillin-derived Schiff bases and systematically evaluated their antibacterial activity against ESBL-producing strains. Our findings revealed substantial inhibition zones, reaching up to 16.6 mm, indicating superior efficacy against these resistant pathogens compared to previous studies. This targeted approach addresses a critical gap in current antimicrobial strategies, aligning with the urgent need for effective treatments in healthcare settings.
To further contextualize our findings, we compare our results with the recent synthesis and evaluation of transition metal complexes derived from a heterocyclic Schiff base ligand. In that study, four transition metal complexes were synthesized by condensing 3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-amine with 5-nitrosalicylaldehyde. Characterization techniques such as \(^{1}H\)-NMR, \(^{13}C\)-NMR, UV-visible, Fourier-transform infrared (FTIR), thermogravimetric analysis (TGA), mass spectrometry, molar conductance, scanning electron microscopy (SEM), powder X-ray diffraction (XRD), elemental analysis, and magnetic susceptibility were employed to elucidate the structures of the complexes. The characterization data indicated that the ligand coordinated to the central metal atom in a bidentate manner, forming octahedral geometry.
The non-electrolytic nature of the compounds was confirmed by molar conductivity values, while thermal analysis revealed decomposition in three steps, leaving metal oxide as a residue. Furthermore, the DPPH and egg albumin assays demonstrated significant antioxidant and anti-inflammatory properties, respectively. The antibacterial activities were assessed through serial dilution and agar well diffusion assays against S. aureus and E. coli, indicating that the biological efficiency of the ligand was enhanced upon chelation. The nickel(II) complex (3) was found to be particularly potent among the synthesized compounds, showing efficacy against oxidant, inflammatory, and bacterial pathogens.
Moreover, the biological efficacy of these complexes was validated through computational techniques such as molecular docking (against 1GAL, 2AZ5, 1HNJ), density functional theory (DFT), molecular electrostatic potential (MESP), and absorption, distribution, metabolism, excretion, and toxicity (ADMET) studies. These analyses suggested that complex (3) is highly potent and may be a promising candidate for drug development targeting pathogen-induced ailments20.
To further contextualize our findings, we compare our results with the recent synthesis and evaluation of transition metal complexes derived from heterocyclic Schiff base ligands. In that study, complexes were synthesized by condensing 3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-amine with 5-nitrosalicylaldehyde, characterized through \(^{1}H\)-NMR, UV-visible spectroscopy, and other techniques. The data indicated that the ligand coordinated with the central metal atom, forming octahedral geometry. Notably, the biological efficiency of the ligand was enhanced upon chelation, with the nickel(II) complex showing significant antibacterial activity against S. aureus and E. coli20.
Furthermore, our findings align with Yadav et al.21, who demonstrated the antimicrobial potential of nitrovanillin derivatives. However, their focus was primarily on structural modifications and activities against standard strains, while our work specifically targets ESBL-producing E. coli strains. The maximum inhibition zones we achieved indicate a higher efficacy than those reported previously. Berk et al.22 also evaluated Schiff bases derived from ortho-vanillin, noting their antibacterial activities against various bacteria. Our specific focus on resistant strains emphasizes the clinical relevance of our findings and addresses gaps in existing literature.
Moreover, Kumar et al.23 explored Schiff bases derived from vanillin and its metal complexes, demonstrating antimicrobial activity primarily against standard strains. Our study, however, evaluates the direct antibacterial activity of vanillin Schiff bases while also considering their low cytotoxicity towards human red blood cells. This comprehensive assessment enhances our understanding of their safety and therapeutic potential.
The study by Uddin and Rahman24 further supports our findings by discussing the broader implications of Schiff bases in water treatment applications. They underscored the need for novel antibacterial agents to effectively address environmental challenges, particularly in areas prone to water contamination. Our work contributes to this dialogue, suggesting that vanillin Schiff bases could be utilized for water sanitation, thereby expanding their application beyond traditional laboratory settings into public health interventions.
In the context of global antimicrobial resistance, our results provide compelling evidence for the therapeutic potential of Schiff bases. Their low cytotoxicity towards human RBCs and dose-dependent toxicity observed in brine shrimp assays further validate their safety profile and potential for clinical translation.
Moving forward, elucidating the precise mechanisms of action through detailed molecular studies and conducting in vivo efficacy trials are essential to advance these compounds toward clinical application. Understanding resistance mechanisms and pharmacokinetic profiles will be crucial for optimizing dosage regimens and therapeutic outcomes.
Moreover, the environmental implications of Schiff bases as antibacterial agents are noteworthy. Given the increasing pollution and contamination of water sources in regions like Pakistan, where only 20% of the population has access to clean drinking water, these compounds could serve as valuable tools for water sanitation. Their effectiveness in reducing bacterial contamination suggests potential applications in sanitizing equipment and surfaces in healthcare settings, thereby mitigating the risk of nosocomial infections.
Furthermore, the insecticidal properties of Schiff bases against ESBL-producing bacteria highlight their potential role in controlling disease transmission through indirect routes, such as via flies from poultry feces. This underscores their broader utility in agricultural settings, where resistant bacteria from animal manure pose significant public health risks.
In conclusion, the synthesis of vanillin Schiff bases represents a promising strategy in the fight against antimicrobial resistance. Our findings not only contribute to the development of novel antibacterial agents but also underscore the need for further research to elucidate the precise mechanisms of action and optimize these compounds for clinical use, thereby harnessing their full therapeutic potential and environmental applications.
Summary
-
This study focused on synthesizing five vanillin Schiff bases (SB-1 to SB-5) from vanillin and various aromatic amines.
-
Chemical characterization of the synthesized compounds was performed using Thin Layer Chromatography (TLC), Fourier Transform Infrared Spectroscopy (FT-IR), proton nuclear magnetic resonance (\(^1\)H-NMR), carbon-13 nuclear magnetic resonance (\(^{13}\)C-NMR), and mass spectrometry techniques.
-
The antibacterial efficacy of these Schiff bases against ESBL-producing strains (Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa) yielded promising results, with significant inhibition zones observed for SB-1, SB-2, SB-4, and SB-5.
-
Cytotoxicity assessments indicated low toxicity against human red blood cells (RBCs) but moderate toxicity in brine shrimp assays.
Conclusion
The rise of drug-resistant pathogens has become a pressing global health concern, leading to a decline in the efficacy of existing antimicrobial therapies. This escalating issue has resulted in increased healthcare costs and the urgent need for the development of novel antimicrobial agents. This study aimed to investigate the antimicrobial and cytotoxic properties of synthesized vanillin Schiff bases, contributing to the search for new therapeutic compounds with distinct biological activities.
Five vanillin Schiff bases were evaluated for their antimicrobial and cytotoxic properties. The structural integrity of these compounds was confirmed through various spectroscopic methods. Antimicrobial testing against three Extended Spectrum Beta-Lactamase (ESBL) bacterial strains-E. coli, K. pneumoniae, and P. aeruginosa-revealed that the Schiff bases exhibited notable activity. While their effectiveness was lower than that of imipenem, they surpassed the activity of the standard drug ceftazidime. Notably, SB-4 demonstrated significant antibacterial activity against E. coli, suggesting that electron-releasing groups, such as –OH, enhance activity against Gram-negative bacteria.
Cytotoxicity assessments were conducted through blood hemolysis assays and brine shrimp cytotoxicity tests. While the Schiff bases showed antibacterial activity at concentrations that were toxic to normal human RBCs, their moderate toxicity in brine shrimp assays underscores the necessity for additional toxicity evaluations.
Although computational studies were initially planned to model the interactions of these Schiff bases with bacterial targets, they were not performed in this study. This limitation is acknowledged, and it is suggested that such studies be considered for future research to provide deeper insights into the mechanisms of action of these compounds.
The results obtained indicate that slight modifications to the structures of these Schiff bases may yield more effective compounds suitable for applications as antibacterial agents, water purifiers, sanitizers, and insecticides. Further experimental work in this domain is essential for developing novel drugs and treatment technologies to combat the challenges posed by drug resistance. Continued research in this area is critical to discovering effective and safe therapeutic options against antimicrobial-resistant pathogens.
Statement
This study focuses on evaluating the antimicrobial efficacy of vanillin Schiff bases against extended-spectrum beta-lactamase (ESBL)-producing bacteria. The research aims to contribute novel insights into potential alternatives to conventional antibiotics amidst rising antimicrobial resistance.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Uddin, T. M. et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 14(12), 1750–1766 (2021).
Khan, R. et al. Isolation and characterization of pathogenic klebsiella pneumoniae strains from lettuce: A potential source of antibiotic resistance and development of a mathematical model for anova results. Front. Microbiol. 15, 1473055 (2024).
Bai, H., Liu, T., Wang, H., & Wang, Z. Antibacterial characteristics and mechanistic insights of combined tea polyphenols, nisin, and epsilon-polylysine against feline oral pathogens: a comprehensive transcriptomic and metabolomic analysis. J. Appl. Microbiol., lxae189 (2024)
Mancuso, G., Midiri, A., Gerace, E. & Biondo, C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens 10(10), 1310 (2021).
Kabir, E. & Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 4, 100606 (2022).
Dalal, M., Antil, N., Kumar, B. & Garg, S. Unveiling the therapeutic potential of organotellurium (iv) schiff base complexes through structural elucidation, antimalarial, antioxidant and antimicrobial studies. J. Mol. Struct. 1313, 138558 (2024).
Kumar, B., Devi, J., Dubey, A., Tufail, A. & Antil, N. Biological and computational investigation of transition metal (ii) complexes of 2-phenoxyaniline-based ligands. Future Med. Chem. 15(21), 1919–1942 (2023).
Nagendra Prasad, H. S. et al. Design, synthesis, molecular docking and dft computational insight on the structure of piperazine sulfynol derivatives as a new antibacterial contender against superbugs mrsa. J. Mol. Struct. 1247, 131333 (2022).
Benson, C., Gantt, S., Zerr, D. M., Qin, X. & Abe, P. Use of 16s ribosomal dna polymerase chain reaction to identify haemophilus influenzae type b as the etiology of pericarditis in an infant. Pediatr. Infect. Dis. J. 24(3), 287–288 (2005).
Abdulkadir, M. & Waliyu, S. Screening and isolation of the soil bacteria for ability to produce antibiotics. Eur. J. Appl. Sci. 4(5), 211–215 (2012).
Nagendra Prasad, H. S., Manukumar, H. M., Karthik, C. S., Mallesha, L. & Mallu, P. A novel copper (ii) pampicat complex (cpampicatc) as a biologically potent candidate: A contraption evidence against methicillin-resistant staphylococcus aureus (mrsa) and a molecular docking proof. Bioorg. Med. Chem. 27(5), 841–850 (2019).
Boora, A., Devi, J., & Kumar, B. Exploring antiplasmodial, antimicrobial, antioxidant, and docking profile: Conjugating organotin moiety with hydrazones for enhanced efficacy. Appl. Organometal. Chem., e7742 (2024).
Boora, A., Devi, J., Dubey, A., Tufail, A., Kumar, B., & Taxak, B. Unveiling the bioactive potential of organotin (iv) complexes of hydrazones: Synthesis, spectral characterization, in vitro and in silico exploration. J. Mol. Struct., 139955 (2024).
Rani, M., Devi, J., Kumar, B., & Rathi, M. Unveiling anti-malarial, antimicrobial, antioxidant efficiency and molecular docking study of synthesized transition metal complexes derived from heterocyclic Schiff base ligands. Chem. Asian J. e202400676 (2024).
Taxak, B., Devi, J., Kumar, B., & Arora, T. Hydrazone-containing organotin (iv) complexes: synthesis, characterization, antimicrobial, antioxidant activity and molecular-docking studies. BioMetals, 1–20 (2024).
Fitzgerald, M. D. & Gibbons, S. Mode of action of a new antimicrobial agent synthesized from vanillin and glyoxal. J. Antimicrob. Chemother. 53(1), 101–107 (2004).
Harinath, B. & Kumar, B. R. Synthesis and antimicrobial activity of some new vanillin derivatives. J. Chem. Pharm. Res. 5(6), 140–146 (2013).
Kumar, B., Devi, J., Dubey, A., Tufail, A. & Taxak, B. Investigation of antituberculosis, antimicrobial, anti-inflammatory efficacies of newly synthesized transition metal (ii) complexes of hydrazone ligands: structural elucidation and theoretical studies. Sci. Rep. 13(1), 15906 (2023).
Kumar, B., Devi, J., Dubey, A., Tufail, N. & Khurana, D. Thiosemicarbazone ligands based transition metal complexes: A multifaceted investigation of antituberculosis, anti-inflammatory, antibacterial, antifungal activities, and molecular docking, density functional theory, molecular electrostatic potential, absorption, distribution, metabolism, excretion, and toxicity studies. Appl. Organomet. Chem. 38(3), e7345 (2024).
Dawar, M. & Ali, I. Synthesis and biological evaluation of transition metal complexes derived from a heterocyclic schiff base ligand. Mater. Today: Proc. 73, 1401–1407 (2023).
Yadav, R., Saini, D. & Yadav, D. Synthesis and evaluation of vanillin derivatives as antimicrobial agents. Turkish J. Pharm. Sci. 15(3), 228–234 (2018).
Berk, B., Ertaş, M. & Biltekin, S. N. Synthesis, antimicrobial activity studies and molecular property predictions of schiff bases derived from ortho-vanillin. Acta Pharm. 67(4), 411–423 (2017).
Kumar, K. S., Varma, C. P. & Reena, V. N. Synthesis, characterization, cytotoxic, anticancer and antimicrobial studies of novel schiff base ligand derived from vanillin and its transition metal complexes. J. Mol. Struct. 1136, 420–427 (2017).
Uddin, M. S. & Rahman, M. M. Utilization of schiff bases in water treatment: A sustainable approach for antibiotic resistance mitigation. Environ. Sci. Pollut. Res. 29(6), 8203–8215 (2022).
Acknowledgements
We extend our sincere appreciation to the laboratory and the Department of Khyber Medical University for their invaluable support and provision of research facilities during this project. Their assistance was instrumental in the successful completion of this study.
Funding
The authors declare that they did not receive any external funding for this research. All expenses related to the study were covered by the authors themselves.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the conception, design, data collection, analysis, writing, and revision of the manuscript. All authors have reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests associated with this research.
Ethical approval and participant consent
This study strictly adhered to ethical principles and guidelines for research involving human subjects. Ethical approval was obtained from the institutional review board, specifically from the Family Care Center Hayatabad Ethical Committee under Approval Code 12345-30 on August 28, 2017. All participants provided informed consent according to institutional guidelines.
Consent to publish
Consent to publish is not applicable as this study does not involve identifiable human data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khan, R., Rashid, S., Khan, S. et al. Synthesis and evaluation of vanillin Schiff bases as potential antimicrobial agents against ESBL-producing bacteria: towards novel interventions in antimicrobial stewardship. Sci Rep 14, 28007 (2024). https://doi.org/10.1038/s41598-024-78302-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78302-9