Abstract
This study assessed the effect of composite resins, aggregated or not with S-PRG particles, and the use of toothpaste in controlling demineralization and bacterial growth. Human molars were distributed into 3 groups: control (CT) – sound teeth, Beautifil Bulk Restorative System (aggregated with S-PRG) (BB), Filtek One Bulk Fill (without S-PRG) (FB). Teeth destined for groups BB and FB previously received Class I preparations (4 × 4 × 4 mm), followed by single-increment restorations. All teeth were sectioned mesiodistally, with all specimens subjected to cariogenic challenge for 5 days, including microcosm biofilm formation. Half of each tooth was exposed to toothpaste (CTF, BBF, FBF). The loss of microhardness was assessed considering the initial microhardness as 100% on enamel, dentin, and composite resin substrates. Colony Forming Units (CFU/mL) were counted in 3 media. Data analysis used one-way ANOVA, Tukey HSD test, and paired t-test (α = 0.05). Toothpaste significantly reduced CFU/mL for total bacteria and genus Streptococcus (p < 0.05), with no significant difference for Streptococcus mutans. Enamel microhardness was positively affected by toothpaste. Both restorative systems controlled enamel demineralization, with FB and FBF outperforming BB and BBF. There was minor degradation of both composite resins, between 10% and 22%. Toothpaste effectively reduced microorganisms, irrespective of the composite resin. Regarding demineralization control, both restorative systems, with and without S-PRG particles, were effective on enamel.
Similar content being viewed by others
Introduction
Direct restoration is a procedure that is part of the dentist’s daily routine. Among the materials used, composite resin is the first choice for the vast majority of clinicians. This is due to the fact that this restorative material allows for minimal intervention due to its ability to adhere to the tooth structure and also provides reinforcement in regions where the preparation is weakened, which avoids unnecessary wear and tear on healthy tissue1. Furthermore, aesthetic and mechanical properties meet patient satisfaction2. However, replacement rates for restorations are still very high1,3, being higher than those for primary restorations1.
The relative low longevity of dental restorations increases the health costs related to a new intervention2. Especially in posterior teeth, the most used composite resins are microhybrid, nanohybrid and nanoparticle, with no difference between them with regard to clinical performance4. In addition to the material, the bulk fill technique has been widely used in posterior teeth5, presenting itself as a simpler procedure without negatively impacting clinical behavior, when compared to the incremental technique5,6. Thus, regardless of the classification of composite resin and technique used, the main reason for restoration failure is secondary caries1,3.
Secondary caries is a new lesion that occurs adjacent to a pre-existing restoration7. Composite resin has the inherent characteristic of polymerization contraction, which, added to mechanical and thermal loads in the oral environment, in which the restoration is subjected, can lead to the emergence of gaps at the tooth/restoration interface, facilitating the accumulation of biofilm and the development of carious lesion2. The roughness of the composite resin together with other surface properties can also favor bacterial colonization8,9. However, among the different etiological factors of caries, the risk of caries presented by the patient directly impacts the longevity of the restoration, that is, patients with a high risk of caries are more likely to develop carious lesions. This is justified due to the fact that these patients have health problems that can reduce protective effects, such as saliva and difficulty in performing/adhering to adequate oral hygiene3,10.
Thus, the use of restorative materials with principles of controlling biofilm and/or stimulating remineralization are adjuvants to be considered in the control of secondary caries, facilitating the longevity of restorations11,12. Some companies have launched restorative materials on the market that interact with the tooth structure in order to prevent the development of carious lesions13, such as composite resin aggregated with Surface Pre particles Reacted Glass ionomer (S-PRG), also called gionomer14. S-PRG is a glass particle surrounded by a silica gel, which releases strontium (Sr+2), borate (BO3−3), fluoride (F−), sodium (Na+), silicate (SiO3−2) and aluminum (Al+3). The release of these ions is related to antimicrobial effects15,16,17,18, preventing demineralization of the tooth structure19,20. Among the commercially available resins with S-PRG particles is Beautifil Bulk Restorative (Shofu, Japan). However, most previous studies were designed with experimental resins and different concentrations of S-PRG16,17,20, short biofilm formation time18or single-species biofilm15,20. Despite all the complexity of the oral environment, in vitro studies should seek to mimic this as much as possible as, in this way, they allow a better understanding of the material’s mechanism11.
Therefore, this in vitro study involves commercially available resins, a human tooth substrate in enamel and dentin and a microcosm biofilm model from human saliva. The objective was to assess the effect of composite resins, aggregated or not with S-PRG particles, and the use of toothpaste in controlling demineralization and bacterial growth. The null hypotheses to be tested were: (1) there would be no difference between composite resins, with and without S-PRG particles, in the control of biofilm and demineralization of the tooth structure; (2) there will be no difference detected when using the toothpaste with the restorative systems.
Methods
Ethical considerations
Human teeth and human saliva were used, with approval from the Human Research Ethics Committee – Federal University of Mato Grosso do Sul (MS/Brazil) (protocol: 3.678.506, CAAE: 21527119.6.0000.0021), in accordance with the code of ethics of the World Medical Association - Declaration of Helsinki.
Sample preparation
Healthy human third molars (n = 30) were randomly divided into 3 groups – CT, BB and FB, as shown in Table 1. Previously, all teeth were kept in Chloramine T for a maximum of 3 months before carrying out the restorative procedure and underwent by standardization process with regularization of the cusps 1 mm from the central groove using a polisher metallographic (Teclago PL01 Lagoa Vargem Grande Paulista, SP, Brazil) and #600 silicon carbide sandpaper (3 M, Sumaré, SP, Brazil).
In teeth that used composite resin, Class I (O) cavity preparations were performed manually with a cylindrical diamond tip with a rounded end (n° 3145 and 3145FF, KG Sorensen, Cotia, SP, Brazil) at high speed (Kavo Kerr, Brea, CA, USA), with the dimensions of 4 × 4 × 4 mm (mesio-distal distance, bucco-lingual distance, depth) checked with a periodontal probe. The diamond tips were replaced every 5 preparations. The prepared teeth were placed in an ultrasonic bath for 10 min (Schuster, L100, Santa Maria, RS, Brazil). An adhesive system, as indicated by the specific commercial brand, for composite resin groups without S-PRG particles – Filtek One Bulk Fill (FB/FBF) was used with Scotchbond Universal (3 M Oral Care, St Paul, MN, USA) and for the Beautifil Bulk Restorative resin (with S-PRG particles), the FL Bond II self-etching system (Shofu Inc., Kyoto, Japan). Before application, selective conditioning on the enamel was carried out with 37% phosphoric acid (Condac, FGM, SC, Brazil) for 15 s, followed by rinsing with a dental syringe for 20 s and air-dried surface. Table 2 lists the materials used in the study, their chemical composition, and a complete description of the adhesion protocols. After the adhesive procedure, the composite resin was inserted in a single increment of 4 mm and photoactivated for 40 s (Bluephase Ivoclar Vivadent, Barueri, SP, Brazil) with an irradiance of 1000 mW/cm2. After every five restorations, the irradiance was checked with a radiometer (Ecel, Ribeirão Preto, SP, Brazil).
After 48 h of storage in distilled water at 37 °C, the restored teeth were sectioned in the mesio-distal direction using a precision metallographic cutter (Buehler, Lake Bluff, Illinois, USA) with a diamond disc (Buehler, Lake Bluff, Illinois, USA) under constant irrigation. Each half obtained was polished with a sequence of silicon carbide sandpaper (#1000, #1200 and #2000, 3 M, Sumaré, SP), 12 mm diameter felt disc (TDV dental Ltda, Pomerode, SC, Brazil) and 0.5 μm diamond paste (Ultradent Products Inc., Indaiatuba, SP, Brazil). After each sanding, the halves passed through an ultrasonic bath for 5 min and at the end of polishing for 10 min in order to remove debris. Half of each specimen was protected with nail polish with the aim of having a control area (baseline) and a test area in the same specimen (Fig. 1). The samples were sterilized using ethylene oxide (90% ETO/10% CO2) for 2 h under pressure − 15 ± 0.1KgF/cm3. All samples were exposed to the inoculum to form microcosm biofilm, with only one half of each tooth also being exposed to fluoride toothpaste, forming the groups as described in Table 1.
Saliva collection
The inclusion criteria for human saliva to be used as inoculum were: children between 9 and 12 years old with caries activity and without periodontal disease; exclusion: volunteers who use or have used antibiotics in the last 3 months prior to collection.
The collection was carried out in the morning, and on that day the volunteers did not brush their teeth and were left to eat food for at least 2 h. Saliva was stimulated by chewing a sterile rubber material with a standard size of 1 cm in length for 10 min. The volunteers’ saliva pool was homogenized, diluted in glycerol in a proportion of 70% saliva/30% glycerol21, fractionated into 1 mL aliquots and stored at − 20 °C. This saliva was used as a microcosm for biofilm formation.
Microcosm biofilm formation
To construct the microcosm biofilm formation model using human saliva, modifications were made to a previously described model21,22 and its feasibility was verified in a previous pilot study. Aliquots of saliva were thawed, and part was used for biofilm formation and another part was analyzed for cell viability by counting colony-forming units in the Brain Heart Infusion (BHI), Mitis Salivarius (MS) and Mitis Salivarius Bacitracin Sucrose (MSBS).
To form the composite medium, the saliva was sterilized by the filtration process with 0.25 M dithiothreitol (DTT), centrifugation, filtration with 0.22 μm filters and stored at − 20 °C23. On the first day of the experiment, this saliva was thawed and mixed with the Mueller- Hinton (MH) culture medium, in a proportion of 60:40%, respectively.
Each tooth sample (sound or restored) was aseptically inserted into a well of a 24-well plate and 1.5 mL of inoculum composed of saliva and compound medium (sterile saliva and MH) in a ratio of 1:50, added and kept in microaerophilia, 5% CO2 and 37 ◦C, for 8 h. Next, the medium was removed, the samples were washed with phosphate-buffered saline (PBS) for 5 s and a new medium (MH/saliva) composed of 0.2% sucrose was added to the well (1.5 mL/well). The plate was incubated at 5% CO 2and 37 °C for 16 h to complete the first day21.
After the cariogenic challenge, the samples were washed with PBS and the groups with fluoride supplementation (CTF, BBF and TBF) were exposed to 1 mL of a toothpaste solution (Colgate Total 12 Clear Mint - Colgate-Palmolive Industrial Ltda, São Bernardo do Campo, SP, Brazil), in a ratio of 1:3 (toothpaste: deionized water)22,24, for 2 min. The samples were washed again and the new medium was added to well. The application of the toothpaste as well as the change of the medium occurred daily. The cariogenic challenge lasted five days, including microcosm biofilm formation.
Figure 2 summarizes the experiment protocol.
Microbial biofilm quantification
After the fifth day of the experiment, total microbial quantification was carried out on BHI agar (Brain Heart Infusion) and differentiation of oral streptococcus colonies using MS media (Mitis Salivarius) and MSBS (Mitis Salivarius Bacitracina Sucrose)21. Each specimen was placed in 5 mL of PBS and agitated using a vortex (Biomixer QL-901, Biomex biotechnology, Ribeirão Preto, SP, Brazil) for 1 min and an ultrasonic bath for 8 min. This suspension was diluted in saline solution serially from 10−1 to 10−8 and the quantification of colony-forming units per 1 mL (CFU/mL) was performed using the drop plating technique. The different species of Streptococcus bacteria had their colonies morphologically differentiated using a binolucar stereoscope with 20x magnification (ST30 2 L, Coleman, Santo André, SP, Brazil). Bacteria were counted using a manual colony counter (CP602, SPlabor, Presidente Prudente, SP, Brazil).
Microhardness test
The microhardness meter (Shimadzu HMV, São Paulo, SP, Brazil) with Knoop tip used a static force of 25 g for 10 s in enamel and composite resin25and 10 g for 15 s in dentin26. The strength and application time were also established in a pilot study that verified the possibility of correct reading in both the control area and the test area (demineralized). Six measurements were taken, with distances of 100 μm between them, on each substrate - enamel, dentin and composite resin; 3 in each area/substrate – control area (baseline) and test area, as shown in Fig. 3. For each sample, the average of the three indentations in each substrate/area was used to calculate mineral loss. Thus, in the baseline area a value for initial surface microhardness (MDSi) was obtained and in the test area a value for final surface microhardness (MDSf). To analyze mineral loss, the relative surface microhardness (rMDS) was measured for each substrate (enamel, dentin and composite resin) using the formula: rMDS = ( MDSf / MDSi )x 100, where the MDSi value was considered 100%27.
Scanning Electron Microscopy (SEM)
Qualitative analysis of the samples was carried out with a low vacuum scanning electron microscope (SEM) (Hitachi TM 3000, São Paulo, SP, Brazil), at an acceleration voltage of 20 kV on one sample from each group (n = 1). Prior to SEM analysis, no sample preparation was undertaken. Images were obtained at 30x magnification to visualize the 3 substrates in the same area and 1500x magnifications on each substrate, enamel, dentin, and composite resin. For this analysis, 3 teeth were used (n = 1).
Micro-CT imaging and measurements
Micro-CT measurements were performed using a SkyScan-Bruker 1173 model scanner. The voltage used in the X-ray tube was 50 kV with a current of 160 µA, and a 1 mm aluminum filter was used. Each projection was obtained by averaging 3 projections with an exposure time of 800 ms for each. The sample was rotated 180° with an angular step of 0.35°. The image resolution was 6 micrometers. The projections were reconstructed using the NRecon software and analyzed in the Data Viewer software, both provided by the scanner manufacturer. One representative sample from each group was used.
Data analysis
The experiment was carried out in three distinct phases, biological triplicates (n = 3 each), with a final n = 9 (Table 1) to reduce possible systematic errors and ensure the reproducibility of the results. The data obtained were subjected to the normality (Kolmogorov-Smirnov) and homogeneity (Bartlett) test, and were subsequently analyzed by one-way ANOVA, followed by Tukey’s post-test. Data regarding the use of toothpaste were also analyzed using paired t-tests. All tests used a p value < 0.05, as statistically significant. Statistical analysis was conducted with GraphPad Prism 5.0 (GraphPad Software).
Results
Microbial biofilm quantification
In view of the restorative materials tested, bacterial growth was observed on all substrates evaluated, enamel, dentin and composite resin, regardless of the restorative system. However, the use of toothpaste had a significant impact on reducing the growth of total bacteria (BHI medium) and in the selective medium for oral bacteria of the genus Streptococcus, showing a difference compared to the groups without using toothpaste, as shown in Fig. 4a and b. When comparing the restorative materials used (BB and FB), no statistically significant differences were observed between the groups (p > 0.05) in terms of the growth of total microorganisms, oral Streptococcus, and Streptococcus mutans, as illustrated in Fig. 4a and b, and 4c.
Microbial quantification graphs in BHI (a Total microorganisms), MS (b Total Streptococcus) and MSBS media (cStreptococcus Mutans), according to the groups evaluated. Columns represent mean and standard deviation (n = 9), when connected with the asterisk (*) they are statistically different (paired t-test, p < 0.05) and (**) p < 0.01. Capital letters compare groups without using toothpaste, while lowercase letters compare groups using toothpaste (Tukey test). Groups identified with different letters show a statistical difference (p < 0.05).
Assessment of relative microhardness
Table 3 shows the results of the microhardness test, according to the groups and substrates analyzed. All groups demonstrated a reduction in microhardness under the action of the cariogenic biofilm, since relative microhardness is related to the percentage of initial microhardness in each substrate evaluated. The toothpaste was effective on the enamel substrate, without the use of restorative material, where CTF > CT, showing no difference between the BB and BBF groups, as well as FB and FBF. Still in enamel, the restorative material influenced the highest relative microhardness, since all groups with restoration differed from the control (CT), without restoration (p < 0.05). On the dentin substrate, the toothpaste alone was not effective in controlling demineralization, as no difference was detected between CT and CTF, BB and BBF, FB and FBF. However, when associated with the restorative material, it demonstrated greater microhardness in dentin, FBF > CT (p < 0.05). The composite resin suffered a small change in microhardness when subjected to cariogenic biofilm, with the final microhardness being 80–90% of the initial microhardness. The toothpaste also did not change the microhardness property of the composite resin (p > 0.05).
Scanning Electron Microscopy (SEM)
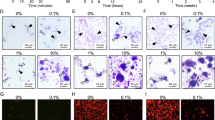
The SEM images in the overview (30X magnification - Fig. 5) demonstrate the difference, in the same tooth, between the area that was exposed to the biofilm (test) and the area that was protected (baseline), with the biofilm formed in the experiment being clear. A reduction in biofilm was observed in the groups that used the toothpaste in the experiment (CTF, BBF and FBF) when compared to the groups that did not use it (CT, BB and FB), which confirms the results obtained in the quantification of biofilm in the BHI medium.
On the enamel substrate, magnification of 1500X, a prismatic effect of the enamel is noted in the test area of all groups when compared to the baseline area, which indicates changes similar to those observed in carious lesions. It is also possible to observe biofilm adhered to the demineralized enamel (black arrows), mainly in the CT, BB and FB groups. Biofilm deposition is even clearer on the dentin substrate, where it is possible to see practically all of the dentin covered by coccus - shaped bacteria. In both enamel and dentin, in the groups that used fluoride toothpaste (CTF, BBF and FBF) a deposit of crystals suggestive of calcium fluoride (white stars) was noticed.
On the composite resin substrate, less biofilm adhesion is observed compared to the dentin substrate, especially. The difference in composition with types and formats of inorganic fillers is clear when comparing the groups that used the Beautifil Bulk Restorative composite resin and the groups using the Filtek composite resin One Bulk Fill.
Micrographs obtained by SEM with magnification of 30X (a-g) tooth / restoration) and 1500X (enamel (h-n), dentin (o-u) and composite resin (v-z)), in the protected (baseline) and unprotected (test) areas according to the groups evaluated: CT - control with healthy teeth, without the use of toothpaste; CTF - control with healthy teeth, using toothpaste; BB - S-PRG composite resin (Beautifil Bulk Restorative/FL Bond II adhesive system) without the use of toothpaste; BBF - S-PRG composite resin (Beautifil Bulk Restorative/FL Bond II adhesive system) using toothpaste; FB - composite resin (Filtek One Bulk Fill/ Scotchbond Universal) without using toothpaste; FBF - composite resin (Filtek One Bulk Fill/ Scotchbond Universal) with the use of toothpaste. Black arrows indicate biofilm on the substrate and white stars indicate the residual presence of toothpaste, which may be related to the formation of calcium fluoride
Micro-CT imaging and measurements
The representative micro-CT images (Fig. 6) demonstrate the differences between the area subjected to the cariogenic challenge and the control area (baseline), highlighting the different depths of the carious lesions formed in the enamel and dentin substrates. Table 4 presents the average depths of carious lesions in the tooth structure, suggesting a protective effect of the restorative material on the enamel and of the toothpaste on both substrates.
Discussion
Ion-releasing restorative materials and their interaction with tooth structure represent a growing topic in dental research and clinical practice. In this study, in addition to investigating composite resins, the use of a toothpaste containing fluorine and arginine on enamel, dentin and composite resin substrates was also evaluated. Our results detected a difference in the quantification of biofilm and demineralization in enamel between the restorative systems evaluated, as well as the effect of toothpaste on biofilm formation in BHI and MS media, which leads to the rejection of the first and second hypotheses. The detection of microorganisms of various species, the observed loss of microhardness in the analyzed substrates indicate the effectiveness of the experimental model, which aims to continuously expose the microcosm biofilm to sucrose for five days, as demonstrated in previous studies21,22.
Brain Heart Infusion medium, a significant reduction was observed in all groups that used the toothpaste (Figs. 4a and 6). The oral microbiota of caries-active individuals is diverse, with a predominance of firmicutes, such as Streptococcus and Lactobacillus. However, it is also important to highlight the considerable presence of other bacteria, with Actinomyces being the most abundant28,29. These bacteria, as demonstrated previously, are sensitive to fluoride30. In addition to fluorine, arginine stimulates the buffering capacity, which contributes to the reduction of total microorganisms and influences the functional profile of the biofilm29,31. A previous study on the composition and activity of oral biofilm revealed that oral Streptococcusare the species most affected by exposure to fluoride31. This explains the lower amount of total Streptococcus with the use of toothpaste in MS medium in the present study.
When the same restorative material is evaluated, without and with application of toothpaste in MS medium, a difference was observed between BB and BBF, with the latter showing a lower amount of oral Streptococcus.This can be attributed to the release of fluoride ions from the composite resin with S-PRG particles17, which, when combined with the toothpaste, increased the fluoride concentration, enhancing the reduction of microorganisms of this species. It is important to mention that Streptococcus mutans are more resistant to the action of fluoride, with their direct susceptibility depending on its concentration to reduce the effects of virulence. A previous study demonstrated that the volume and thickness of the S. mutans biofilm did not decrease, even under different fluoride concentrations32. This justifies the lack of action of the toothpaste on Streptococcus mutans, as represented in the middle MSBS graph (Fig. 4c). Furthermore, it is important to note that biofilm from caries-active individuals demonstrates greater resistance to the effects of toothpaste compared to biofilm from caries-free individuals29. In the present study, the biofilm was formed using saliva from individuals aged 9 to 12 years with mixed dentition and active caries. This approach more accurately reproduces the polymicrobial nature of dental caries33. Previous research indicates that mixed and permanent dentition have a greater abundance of cariogenic microorganisms in the biofilm of individuals with active caries than those without caries34. This increase in specific microbial load may have contributed to the observed resistance to the action of the toothpaste when the Streptococcus mutans medium was analyzed.
The images obtained by SEM revealed that the dental enamel in the area subjected to the cariogenic biofilm presented a prismatic appearance in contrast to the baseline area (Fig. 5 ). The lesion formed is visible in Fig. 6. The evaluation of microhardness showed significant differences between the groups in relation to this substrate. When analyzing the impact of the toothpaste, greater microhardness was observed in the control group that received fluoride + arginine (CTF) compared to the group that did not receive this treatment (CT). As mentioned previously, toothpaste played a role in reducing biofilm, which has a direct effect on reducing acidity and acid exposure time, which in turn is associated with less mineral loss. It is important to highlight that toothpaste that combines 1.5% arginine and fluoride has been shown to be more effective in preventing carious lesions compared to toothpastes that contain only fluoride35. This can be attributed to the prebiotic action of arginine, which neutralizes the pH of the biofilm, contributing to the reduction of demineralization29.
On the other hand, in the groups that used composite resin, the toothpaste did not have an effect on controlling demineralization in enamel. However, the restorative system, composed of the composite resin and the adhesive system, demonstrated effectiveness, as they differed from the control group (CT). The S-PRG filler itself and S-PRG filler-containing materials interact with the tooth structure, controlling caries lesions, due to the release of multiple ions, as previously reported14. Despite not preventing the penetration of bacteria into the adhesive interface20, this resin can modulate the metabolic activity of S. Mutans, especially in glycolysis, evidenced by the lower production of lactic and formic acid in the presence of ions F− and Bo3− 3 14,36. These ions also contribute to rapid pH neutralization36,37. These factors contribute to reducing demineralization in the tooth structure, according to the results found in the present study. Sr+2, synergistically with F−, contributes to the remineralization process37. A previous study indicated higher Ca and P content in enamel adjacent to the restoration with experimental resin containing 70% by weight of S-PRG particles, similar to commercial Beautifil Bulk Restorative composite resin, demonstrating the ability of this resin to induce remineralization20.
Products with S-PRG particles are considered bioactive by the manufacturer. Unlike the resinous system composed of Filtek resin, One Bulk Fill and Scotchbond Universal adhesive. The latter demonstrated a positive effect in controlling demineralization in enamel, superior to that shown by the BB and BBF groups. This result differs from a previous study, which investigated the combination of FL Bond II (FL) and Scotchbond Universal (SBU) adhesive systems with Beautifil II (BEF) and Estelite (EST) composite resins, with FL/BEF being the best combination to contain demineralization, as reported by the authors. Although SBU/BEF has shown similar efficacy to FL/BEF, at enamel lesion depth (µCT), under biofilm challenge and cyclic pH19. The Scotchbond Universal adhesive system contains the monomer 10- methacryloxydecyl phosphate(10-MDP) in its composition, which establishes chemical bonds with the calcium in the tooth structure, providing effective and stable bonding strength with enamel and dentin. Furthermore, it promotes the formation of an acid resistance zone at the adhesive interface38, thus substantiating the results obtained with this system.
In dentin, the higher organic content and complexity of the tissue make the control of the demineralization and remineralization process more complicated. In the dentin substrate, microhardness analysis revealed a significant difference only between the FBF and CT groups, in relation to demineralization. The 10-MDP monomer, present in the Scotchbond Universal adhesive system, chemically interacts with collagen fibrils in dentin39,40. In this process, there is a superficial dissolution of hydroxyapatite around the collagen fibrils, followed by the deposition of MDP-Ca salts, which are insoluble and contribute to the protection of these structures41. In addition to the salts, part of the 10-MDP monomer remains in the adhesive layer and contributes to reducing acidity and increasing hydrophobicity40. The formation of an acid-resistant layer provided by the 10-MDP monomer and the benefits of the previously discussed toothpaste contributed to the differential results obtained by the FBF group.
Previous studies have demonstrated the effect of composite resin with S-PRG particles in controlling carious lesions in dentin19,20, which contrasts with the results obtained in the present study. S-PRG particles exhibited a substantial reduction in metalloproteinase (MMP) activity, but did not influence cathepsins, demonstrating partial protection of enzymatic activity in the carious process42. These enzymes are related to the degradation of the dentin matrix in processes such as dental caries43. In this case, S-PRG particles were placed in direct contact with dentin and the concentration was not dependent on ion leaching from a resinous material. This result suggests that the inhibition of MMPs is correlated with the concentration of ions and also with their contact time with the dentin matrix. Furthermore, an acid resistance zone was not detected when the FL Bond II adhesive (with S-PRG particles) was used, while the groups that used Scotchbond Universal demonstrated a lower depth of carious lesion in dentin under cyclic pH19. Therefore, methodological differences between in vitro studies must be considered, with an emphasis on microcosm biofilm, since biofilms with multiple species are more resistant and challenging44. This suggests that the amount of ions released from the composite resin with S-PRG did not have an effective concentration to control demineralization in dentin. It is important to highlight that in the study by Zhou et al., 202120 the composite resins used were experimental and the S-PRG particles were not silanized, a relevant distinction from our study, which employed a commercial composite resin containing silanized S-PRG particles. Silanization of particles prolongs the time needed for ions to leach, as well as for recharge19.
Both composite resins evaluated, Beautifil Bulk Restorative and Filtek One Bulk Fill demonstrated degradation when subjected to cariogenic challenge, as observed in the relative surface microhardness, which comprises 80 to 90% of the initial microhardness. Although, biofilm formation on composite resin is not entirely avoidable45, in the SEM images from our study, it is possible to notice a smaller accumulation of biofilm on the composite resins when compared, for example, to the dentin substrate, regardless of the restorative material used. This similarity is mainly attributed to the surface smoothness obtained by polishing the specimens. Different material surface properties influence bacterial adhesion, such as wettability and surface energy. However, roughness can be considered the most relevant, as the smoother the surface, the lower the microbial adhesion will be8,9. The biodegradation of composite resin occurs through acid and enzymes produced in the oral microbiome8. Consequently, this degradation makes the composite resin rougher, providing greater bacterial adhesion. This circle between biofilm formation-degradation-increased roughness reduces the longevity of the restoration8.
The complex environment allows for a better understanding of the antibacterial effects of materials11. In this context, this in vitro study sought to simulate the situation of individuals who are at high risk of dental caries. Thus, the microcosm biofilm was formed from the saliva of individuals with active caries, the substrates used were obtained from extracted human teeth and the use of toothpaste was instituted. The results of our study ensured adequate biofilm formation, visible to the naked eye, including the formation of non- cavitated white spot. Although previous studies confirm the release of ions from products with S-PRG particles16,17,20,37,44, we did not detect superiority of the FL Bond II/ Beautifil Bulk Restorative restorative system over the Scotchbond Universal/ Filtek One Bulk Fill, when subjected to a challenging cariogenic environment. Although in vitro studies allow greater reproducibility and the ability to isolate factors to be observed, the design does not allow the oral environment to be copied with complete fidelity, which is a limitation of the study. Something to take into consideration is the removal/disintegration of biofilm that occurs during brushing, which was not reproduced in this in vitro study. Greater biofilm maturation tends to reduce the effects of antibacterial additives, as it allows microorganisms greater adaptation time11. On the other hand, only two clinical studies have been carried out comparing a composite resin with S-PRG particles and a conventional one46,47. While one evaluated resins composite in primary teeth, being favorable to conventional resin47. The other study evaluated resins composite in permanent teeth and the absence of secondary caries using Beautifil II LS composite resin46, both with two years of follow-up. However, no study included patients with high caries activity, not representing an inhospitable environment. Thus, the present study allows a better understanding of what happens in places where biofilm is difficult to remove, such as in Class II restorations of individuals with difficulty adhering to frequent and correct use of dental floss. Future studies involving different times of biofilm formation and more frequent use of toothpaste should be carried out.
In conclusion, the toothpaste helps to reduce microorganisms, regardless of the composite resin used. Regarding the control of demineralization of the tooth structure, both restorative systems, with and without S-PRG particles, were effective on human enamel, regardless of the use of toothpaste. Therefore, there is no superiority of the composite resin with S-PR G particles over a conventional bulk resin, when subjected to cariogenic challenge in biofilm microcosms.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Eltahlah, D., Lynch, C. D., Chadwick, B. L., Blum, I. R. & Wilson N.H.F. An update on the reasons for placement and replacement of direct restorations. J. Dent. 72 (5), 1–7. https://doi.org/10.1016/j.jdent.2018.03.001 (2018).
Aminoroaya, A. et al. A review of dental composites: challenges, chemistry aspects, filler influences, and future insights. Compos. Part. B. 216, 108852. https://doi.org/10.1016/j.compositesb.2021.108852 (2021).
Demarco, F. F. et al. N.J.M. Longevity of composite restorations is definitely not only about materials. Dent. Mater. 39 (1), 1–12. https://doi.org/10.1016/j.dental.2022.11.009 (2023).
Maran, B. M. et al. Nanofilled/nanohybrid and hybrid resin-based composite in patients with direct restorations in posterior teeth: a systematic review and meta-analysis. J. Dent. 99 (8), 103407. https://doi.org/10.1016/j.jdent.2020.103407 (2020).
Veloso, S. R. M. et al. Clinical performance of bulk-fill and conventional resin composite restorations in posterior teeth: a systematic review and meta-analysis. Clin. Oral Investig. 23 (1), 221–233. https://doi.org/10.1007/s00784-018-2429-7 (2019).
Kunz, P. V. M. et al. Is the clinical performance of composite resin restorations in posterior teeth similar if restored with incremental or bulk-filling techniques? A systematic review and meta-analysis. Clin. Oral Investig. 26 (3), 2281–2297. https://doi.org/10.1007/s00784-021-04337-1 (2022).
Machiulskiene, V. et al. Terminology of dental caries and dental caries management: consensus report of a workshop organized by ORCA and Cariology Research Group of IADR. Caries Res. 54 (1), 7–14. https://doi.org/10.1159/000503309 (2020).
Tu, Y., Ren, H., He, Y., Ying, J. & Chen, Y. Interaction between microorganisms and dental material surfaces: general concepts and research progress. J. Oral Microbiol. 15 (1), 2196897. https://doi.org/10.1080/20002297.2023.2196897 (2023).
Schmalz, G. & Cieplik, F. Biofilms on restorative materials. Monogr. Oral Sci. 29, 155–194. https://doi.org/10.1159/000510191 (2021).
Askar, H. et al. Secondary caries: what is it, and how it can be controlled, detected, and managed? Clin. Oral Investig. 24 (5), 1869–1876. https://doi.org/10.1007/s00784-020-03268-7 (2020).
Ibrahim, M. S. et al. How we are assessing the developing antibacterial resin-based dental materials? A scoping review. J. Dent. 99 (8), 103369. https://doi.org/10.1016/j.jdent.2020.103369 (2020).
Pinto, N. S. et al. Clinical efficacy of bioactive restorative materials in controlling secondary caries: a systematic review and network meta-analysis. BMC Oral Health. 23 (1), 394. https://doi.org/10.1186/s12903-023-03110-y (2023).
Lawson, N. C., Janyavula, S. & Price, R. B. Trends in Restorative Dentistry: composites, curing lights, and Matrix bands. Compend Contin Educ. Dent. 42 (2), 93–94 (2021). (2021).
Imazato, S. et al. Multiple-ion releasing bioactive surface pre-reacted glass-ionomer (S-PRG) filler: innovative technology for dental treatment and care. J. Funct. Biomater. 14 (4), 236. https://doi.org/10.3390/jfb14040236 (2023).
Nomura, R., Morita, Y., Matayoshi, S. & Nakano, K. Inhibitory effect of surface pre-reacted glass-ionomer (S-PRG) eluate against adhesion and colonization by Streptococcus mutans. Sci. Rep. 8 (1), 5056. https://doi.org/10.1038/s41598-018-23354-x (2018).
Miki, S. et al. Antibacterial activity of resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent. Mater. 32 (9), 1095–1102. https://doi.org/10.1016/j.dental.2016.06.018 (2016).
Hahnel, S. et al. Streptococcus mutans biofilm formation and release of fluoride from experimental resin-based composites depending on surface treatment and S-PRG filler particle fraction. J. Adhes. Dent. 16 (4), 313–321. https://doi.org/10.3290/j.jad.a31800 (2014).
Yoneda, M. et al. Effect of S-PRG eluate on biofilm formation and enzyme activity of oral bacteria. Int. J. Dent. 2012 (814913). https://doi.org/10.1155/2012/814913 (2012).
Lai, Y. J. et al. Anti-demineralization effects of dental adhesive-composites on enamel-root dentin junction. Polym. (Basel). 13 (19), 3327. https://doi.org/10.3390/polym13193327 (2021).
Zhou, Y. et al. Evaluation of tooth demineralization and interfacial bacterial penetration around resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent. Mater. 37 (5), 849–862. https://doi.org/10.1016/j.dental.2021.02.009 (2021).
Augustinho do Nascimento, C. et al. Effect of sweetener containing Stevia on the development of dental caries in enamel and dentin under a microcosm biofilm model. J. Dent. 115, 103835. https://doi.org/10.1016/j.jdent.2021.103835 (2021).
Braga, A. S. et al. Effect of commercial herbal toothpastes and mouth rinses on the prevention of enamel demineralization using a microcosm biofilm model. Biofouling. 35 (7), 796804. https://doi.org/10.1080/08927014.2019.1662897 (2019).
Cavalcanti, I. M., Del Bel Cury, A. A., Jenkinson, H. F. & Nobbs, A. H. Interactions between Streptococcus oralis, Actinomyces oris, and Candida albicans in the development of multispecies oral microbial biofilms on salivary pellicle. Mol. Oral Microbiol. 32 (1), 60–73. https://doi.org/10.1111/omi.12154 (2017).
Martinez-Mier, E. A. et al. ORCA Fluoride in Toothpaste Analysis Work Group. European Organization for Caries Research Workshop: methodology for determination of potentially available fluoride in toothpastes. Caries Res. 53 (2), 119–136. https://doi.org/10.1159/000490196 (2019).
Rechmann, P., Le, C. Q., Chaffee, B. W. & Rechmann, B. M. T. Demineralization prevention with a new antibacterial restorative composite containing QASi nanoparticles: an in situ study. Clin. Oral Investig. 25 (9), 5293–5305. https://doi.org/10.1007/s00784-021-03837-4 (2021).
Kirsten, G. A., Rached, R. N., Mazur, R. F., Vieira, S. & Souza, E. M. Effect of open-sandwich vs. adhesive restorative techniques on enamel and dentine demineralization: an in situ study. J. Dent. 41 (10). https://doi.org/10.1016/j.jdent.2013.07.003 (2013). 872 – 80.
Moecke, S. E., Silva, A. G. C. S., Andrade, A. C. M., Borges, A. B. & Torres, C. R. G. Efficacy of S-PRG filler varnishes on enamel caries remineralization. J. Dent. 119, 104074. https://doi.org/10.1016/j.jdent.2022.104074 (2022).
Su, S., Qi, T., Wang, W., Salama, E. S. & Li, Y. Investigation of the oral microbiome of children associated with dental caries: a systematic study. Arch. Oral Biol. 154, 105776. https://doi.org/10.1016/j.archoralbio.2023.105776 (2023).
Carda-Diéguez, M., Moazzez, R. & Mira, A. Functional changes in the oral microbiome after use of fluoride and arginine containing dentifrices: a metagenomic and metatranscriptomic study. Microbiome. 10(1), 159, https://doi.org/10.1186/s40168‐022‐01338‐4 (2022).
Phan, T. N., Reidmiller, J. S. & Marquis, R. E. Sensitization of Actinomyces naeslundii and Streptococcus sanguis in biofilms and suspensions to acid damage by fluoride and other weak acids. Arch. Microbiol. 174 (4). https://doi.org/10.1007/s002030000202 (2000). 248 – 55.
López-López, A. & Mira, A. Shifts in composition and activity of oral biofilms after fluoride exposure. Microb. Ecol. 80 (3), 729–738. https://doi.org/10.1007/s00248-020-01531-8 (2020).
Pandit, S., Kim, H. J., Song, K. Y. & Jeon, J. G. Relationship between fluoride concentration and activity against virulence factors and viability of a cariogenic biofilm: in vitro study. Caries Res. 47 (6), 539–547. https://doi.org/10.1159/000348519 (2013).
Veenman, F. et al. Oral microbiota of adolescents with dental caries: a systematic review. Arch. Oral Biol. 161, 105933. https://doi.org/10.1016/j.archoralbio.2024.105933 (2024).
Bhaumik, D. et al. Plaque Microbiome in caries-active and caries-Free Teeth by Dentition. JDR Clin. Translational Res. 9 (1), 61–71. https://doi.org/10.1177/23800844221121260 (2024).
Wolff, M. S. & Schenkel, A. B. The anticaries efficacy of a 1.5% arginine and fluoride toothpaste. Adv. Dent. Res. 29 (1), 93–97. https://doi.org/10.1177/0022034517735298 (2018).
Kitagawa, H. et al. Inhibitory effect of resin composite containing S-PRG filler on Streptococcus mutans glucose metabolism. J. Dent. 70, 92–96. https://doi.org/10.1016/j.jdent.2017.12.017 (2018).
Kaga, M. et al. Inhibition of enamel demineralization by buffering effect of S-PRG filler-containing dental sealant. Eur. J. Oral Sci. 122 (1), 78–83. https://doi.org/10.1111/eos.12107 (2014).
Carrilho, E. et al. 10-MDP based dental adhesives: adhesive interface characterization and adhesive stability-a systematic review. Mater. (Basel). 12 (5), 790. https://doi.org/10.3390/ma12050790 (2019).
Oliveira, B. et al. Chemical interaction and interface analysis of self-etch adhesives containing 10-MDP and methacrylamide with the dentin in noncarious cervical lesions. Oper. Dent. 43 (5), E253–E265. https://doi.org/10.2341/17-366-L (2018).
Motoyama, Y. et al. Hydroxyapatite affects the physicochemical properties of contemporary one-step self-etch adhesives. Mater. (Basel). 15 (22), 8255. https://doi.org/10.3390/ma15228255 (2022).
Fukegawa, D. et al. Chemical interaction of phosphoric acid ester with hydroxyapatite. J. Dent. Res. 85 (10), 941–944. https://doi.org/10.1177/154405910608501014 (2006).
Salim, I., Seseogullari-Dirihan, R., Imazato, S. & Tezvergil-Mutluay, A. The inhibitory effects of various ions released from S-PRG fillers on dentin protease activity. Dent. Mater. J. 42 (1), 99–104. https://doi.org/10.4012/dmj.2022-141 (2023).
Mazzoni, A. et al. Role of dentin MMPs in caries progression and bond stability. J. Dent. Res. 94 (2), 241–251. https://doi.org/10.1177/0022034514562833 (2015).
Kim, H. J., Cho, M. Y., Lee, E. S., Jung, H. I. & Kim, B. I. Effects of short-time exposure of surface pre-reacted glass-ionomer eluate on dental microcosm biofilm. Sci. Rep. 10 (1), 14425. https://doi.org/10.1038/s41598-020-71363-6 (2020).
Kusuma Yulianto, H. D. et al. Biofilm composition and composite degradation during intra-oral wear. Dent. Mater. 35 (5), 740–750. https://doi.org/10.1016/j.dental.2019.02.024 (2019).
Toz-Akalin, T. et al. Clinical evaluation of low-shrinkage bioactive material giomer versus nanohybrid resin composite restorations: a two-year prospective controlled clinical trial. Oper. Dent. 48 (1), 10–20. https://doi.org/10.2341/21-155-C (2023).
Sengul, F. & Gurbuz, T. Clinical evaluation of restorative materials in primary Teeth Class II lesions. J. Clin. Pediatr. Dent. 39 (4), 315–321. https://doi.org/10.17796/1053-4628-39.4.315 (2015).
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: A.F. & M.C.S.M. Conducted the study: A.F., M.C.S.M. & V.A.A.B. Analyzed the data: M.C.S.M., E.I.J., A.C.A. Interpreted the data: all authors. Wrote the manuscript: A.F. Read, revised, and agreed to be accountable for the manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures performed in this study involving human teeth were in accordance with the ethical standards of the institutional and national human research committee (CEP/UFMS - Federal University of Mato Grosso do Sul (MS/Brazil); protocol: 3.678.506, CAAE: 21527119.6.0000.0021) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Freire, A., Bento, V.A.A., Jussiani, E.I. et al. Resin composite aggregated S-PRG particles are not superior to non-S-PRG under microcosm biofilm. Sci Rep 15, 2173 (2025). https://doi.org/10.1038/s41598-024-78396-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78396-1