Abstract
This article provides a comprehensive hemodynamic assessment of two endovascular techniques for treating cerebral aneurysms. Through finite volume simulations of pulsatile blood flow in saccular aneurysms, we evaluated hemodynamic factors under two coiling conditions with varying porosities and deformation stages to determine the most effective treatment for each case. Our analysis shows that stent and coiling treatments are more effective for aneurysms with smaller sac volumes. In particular, stent placement is found to be more effective than coiling for managing saccular aneurysms. The results indicate that stent-induced deformation effectively redirects the main blood flow, significantly lowering the risk of aneurysm rupture near the ostium. Obtained results emphasize the role of deformation and porosity in controlling wall shear stress, which is essential for understanding aneurysm progression and potential rupture risk.
Similar content being viewed by others
Introduction
Cardiovascular system is crucial for human life and extensive researches have been done for the evaluation of this system in different conditions1,2,3. Cerebral aneurysms present a significant health risk due to the potential for rupture, which can lead to life-threatening hemorrhagic strokes4,5,6. The two most common endovascular techniques used to treat aneurysms are stenting and coiling7. Both methods aim to reduce the risk of rupture by altering the hemodynamics within the aneurysm and decreasing the stress on the aneurysm wall8,9. However, these techniques differ in their approach, efficacy, and applicability to various types of aneurysms10,11. This comparison will explore the strengths and limitations of stent and coiling techniques, with a focus on how each method influences hemodynamic changes and contributes to rupture risk reduction in different types of cerebral aneurysms12,13,14.
Comprehensive research has been conducted to improve the treatment of the biological systems15,16,17. Stents, particularly flow-diverting stents (FDS), have become a popular treatment for cerebral aneurysms. Stents are mesh-like structures deployed inside the parent blood vessel, covering the aneurysm neck. They work by redirecting blood flow away from the aneurysm sac, promoting clot formation within the aneurysm and stabilizing the aneurysm wall18,19,20. By reducing the blood flow into the aneurysm, stents aim to lower the wall shear stress (WSS) on the aneurysm wall, which is a key factor in rupture risk21,22.
Stents, especially flow diverters, significantly alter the hemodynamics of cerebral aneurysms. By acting as a barrier between the parent vessel and the aneurysm, stents reduce the velocity of blood entering the aneurysm sac23,24. This reduction in inflow velocity encourages the formation of thrombus (clot) inside the aneurysm, gradually reducing the pressure on the aneurysm wall and lowering the risk of rupture. Flow-diverting stents create a more uniform distribution of WSS along the parent vessel and aneurysm wall, contributing to vascular stabilization and brain injury25,26,27.
The hemodynamic changes induced by stenting have been extensively studied using Computational Fluid Dynamics (CFD)28,29. Studies have shown that flow-diverting stents can lead to a significant reduction in WSS and energy dissipation inside the aneurysm sac. These changes are particularly effective in reducing the risk of rupture in aneurysms with large necks and those located at bifurcations where flow patterns are more complex30,31.
Stenting is most effective for large, wide-necked aneurysms that are difficult to treat with other methods, such as coiling32,33,34. These aneurysms often have more complex flow patterns, and the wide neck allows for significant blood flow into the aneurysm sac. Stents are also beneficial in treating fusiform aneurysms, which are characterized by the dilation of the entire circumference of the vessel. Flow diverters can stabilize these aneurysms by reconstructing the blood vessel and promoting flow redirection18,19,35.
However, stenting may not be suitable for small aneurysms or aneurysms located in distal vessels where deploying a stent can be technically challenging36. Additionally, stents require patients to take long-term antiplatelet therapy to prevent clot formation on the stent itself, which may not be ideal for certain patients37.
This study examines critical hemodynamic factors associated with aneurysm rupture in stented Middle Cerebral Artery (MCA) aneurysms. Using computational fluid dynamics, the researchers analyzed pulsatile blood flow in patient-specific 3D MCA aneurysm models, comparing hemodynamics before and after simulated stent deployment. By comparing these factors in the original and stented aneurysm geometries, the study aimed to evaluate how effectively stent placement reduces rupture risk. The finite volume method was employed to model pulsatile blood flow within the aneurysms. This computational approach allowed for detailed examination of flow patterns and stresses acting on the aneurysm wall. Through this comparative analysis of pre- and post-stent hemodynamics, the researchers sought to quantify the impact of stent deployment on key risk factors for aneurysm rupture. The findings provide insights into the mechanisms by which stents may help stabilize aneurysms and prevent catastrophic bleeding events.
Modeling
Geometry
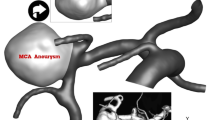
The selected MCA aneurysm is shown in Fig. 1, with the geometrical details of the model provided in Table 1. The original geometry was sourced from the Aneurisk webpage38. Figure 2 illustrates the shape of the selected case in two stages of deformation. The primary concept behind the deformed aneurysms relates to the alignment of the parent vessel due to stent placement. Specifically, the first deformation model represents an intermediate stage of the final deformation, labeled as the second deformation in the figure. As depicted, the parent vessel is considered fully aligned, allowing only limited blood flow to enter the sac region.
Coiling
The use of coiling in conjunction with stent treatment is also examined in this study. The main approach to coiling implementation involves filling the entire saccular region with porous materials, each with varying permeability factors, K, representing fluid conductivity within the porous medium. This factor indicates the surface area to volume ratio of the porous domain39,40. The study employed the Kozeny model to calculate permeability. Table 2 provides the details of the applied permeability factors and their corresponding porosity values41.
The Kozeny model, also known as the Kozeny-Carman equation, is a widely used empirical model for estimating the permeability of porous media. It relates the permeability to the geometric characteristics of the porous structure, such as porosity and specific surface area. The model assumes that the porous medium can be represented as a bundle of capillary tubes, where fluid flows through these channels, and it accounts for both the porosity and the complexity of the pore structure.
The permeability K in the Kozeny model is given by the following equation:
Where K is the permeability, \({\epsilon }\) is the porosity of the medium (the fraction of the total volume that is void space), S is the specific surface area (the surface area of the solid material per unit volume), c is the Kozeny constant, a dimensionless factor that accounts for the geometry and tortuosity of the flow paths through the porous material.
The model assumes laminar flow through the porous medium and is often applied in cases where the pore structure is relatively uniform. In this study, the Kozeny model is used to estimate the permeability of the coiled aneurysm, which is filled with a porous material to simulate the effect of the coiling treatment. By varying the permeability factors, the model allows for the evaluation of how different degrees of porosity and fluid conductivity within the coiled sac affect the hemodynamic performance of the treatment.
Governing equations
The simulation of pulsatile flow within the cerebral vessel containing the aneurysm is conducted using the Navier-Stokes equations, while the energy equation is excluded due to the minimal temperature variation in real blood flow. Since the flow is pulsatile, a transient simulation is required, and the Casson model, a widely used non-Newtonian model, is applied to estimate blood viscosity. The simulations are carried out using ANSYS FLUENT software42.
Applied boundary condition and grid production
Pulsatile blood flow is applied at both the inlet and outlet of the domain (Fig. 3). The boundary conditions used are a mass flow rate at the inlet and a pressure outlet at the outlet for the selected models. The blood flow cycle represents that of a normal body, with four stages of the cycle considered. The peak systolic stage corresponds to the maximum blood flow rate.
Boundary conditions at inlet and outlet43.
Figure 4 shows the generated grid for the selected aneurysm. The cells are uniformly distributed along the surface, while the resolution of the grid varies across the cross-section, with a finer grid near the sac wall compared to the vessel’s center. This refinement is necessary to optimize the grid for capturing critical hemodynamic factors near the wall. A grid study was conducted for both models by generating five grids. The quality of the grid was verified by comparing blood velocity at the ostium section across these grids, and the final grid was selected when increasing the grid size and number resulted in minimal changes in blood velocity at the neck area. As a result, the final grid for the selected model consists of 568,522 cells.
Results and discussion
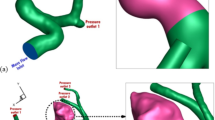
Figure 5 shows the variation of wall shear stress (WSS) on the sac surface for chosen case, across three porosity conditions and two deformation states, at peak systolic. As shown in Fig. 5, the WSS distribution on the sac surface, without coiling but after two stages of deformation treatment, reveals that the critical high wall shear stress is concentrated near the ostium region. The aneurysm’s deformation leads to a reduction in blood flow entering the sac surface region. In the model with low porosity (high permeability), blood entry is further restricted, resulting in lower WSS at the ostium section. The primary reason for rupture in this case is the high tension at the ostium. Coiling treatment has a limited effect on the WSS in this model. Figure 6 also illustrates and compares the effects of coiling and deformation on the average wall shear stress (AWSS) on the sac surface. The AWSS analysis further underscores the significance of deformation in this case.
Figure 7 illustrates the pressure variations on the sac surface for selected model at two stages of deformation and two porosity conditions during stage II (peak systolic). The pressure contour on the aneurysm and vessel wall shows that coiling treatment reduces the extent of the high-pressure region, though it has minimal effect on the pressure gradient across the sac surface. However, the aneurysm’s deformation significantly lowers the high-pressure values near the ostium. Indeed, the effect of coiling is minimal due to the limited blood flow entering the sac region. On the other hand, the deformation caused by the stent significantly reduces the pressure on the sac surface.
Figure 8 shows the blood flow structure within the sac region and parent vessel, using iso-velocity surfaces to compare the effects of coiling and stenting on blood hemodynamics. The stent proves to be a more effective treatment method for chosen case, which features a low sac volume. Additionally, the stent-induced deformation plays a crucial role in obstructing blood flow into the sac region, making it a more impactful factor in reducing flow into the aneurysm.
The evaluation of the Oscillatory Shear Index (OSI) at the end of the cardiac cycle was conducted to identify regions with a high risk of aneurysm rupture. Figure 9 presents a comprehensive OSI contour for the models. The OSI contour reveals that the maximum OSI is located at the top dome and the ostium region. Deformation of the aneurysm reduces the extent of the high OSI region, while coiling alone has little effect on decreasing OSI on the aneurysm wall. However, the combination of stenting and coiling effectively reduces OSI on the sac surface.
Figure 10 compares the quantitative evaluation of a chosen case under the impacts of coiling and stent treatments. The image shows a 3D bar plot illustrating the maximum Oscillatory Shear Index (OSI) on the sac surface under various conditions. The x-axis represents different deformation states (without deformation, deformation 1, and deformation 2), while the z-axis indicates different porosity levels (without porosity, porosity = 0.85, and porosity = 0.65). The y-axis represents the maximum OSI values. It is observed that, in the absence of porosity and deformation, the OSI reaches its highest value. Besides, deformation significantly reduces the maximum OSI, especially in the second deformation state. This plot effectively demonstrates how porosity and deformation influence the OSI, which is critical for understanding the potential rupture risk of aneurysms. Similar results also observed for the mean OSI.
Figure 10c and d display a 3D bar plot that illustrates the max Average Wall Shear Stress (AWSS) and mean AWSS, respectively, on the aneurysm sac surface under different porosity and deformation conditions. The x-axis indicates various deformation states (without deformation, deformation 1, and deformation 2), while the z-axis represents porosity levels (without porosity, porosity = 0.85, and porosity = 0.65). The highest AWSS occurs without porosity or deformation, indicating the largest wall shear stress is present in this scenario. The introduction of deformation (particularly deformation 2) significantly reduces AWSS. This plot emphasizes the role of deformation and porosity in controlling wall shear stress, which is essential for understanding aneurysm progression and potential rupture risk.
Conclusion
In this study, an inclusive assessment of hemodynamic features was conducted on single cerebral saccular MCA aneurysms with different endovascular treatment. computational fluid dynamics (CFD) is used to compare stent and coiling treatments to assess their effectiveness in reducing aneurysm rupture risk by analyzing hemodynamic factors. Coiling was modeled as a porous condition, while the effects of the stent were represented by aneurysm deformation. The contours of wall shear stress (WSS), pressure, and oscillatory shear index (OSI) were compared across two stages of aneurysm deformation and varying coiling porosities. The results indicate that stent and coiling treatments have a lesser impact on hemodynamic factors in large aneurysms, but they can effectively reduce rupture risk in aneurysms with smaller sac volumes. Introducing porosity (at values of 0.85 and 0.65) further reduces the OSI, with porosity = 0.65 showing a greater reduction. The combined effect of deformation and porosity leads to the lowest OSI values, highlighting the importance of these factors in minimizing the OSI on the aneurysm sac surface. Adding porosity (values of 0.85 and 0.65) further decreases the AWSS, with the lower porosity value (0.65) showing a more pronounced reduction in shear stress. The combined effects of deformation and porosity lead to the lowest AWSS values, showing that both factors are critical for minimizing shear stress on the aneurysm sac.
Data availability
All data generated or analysed during this study are included in this published article.
References
Mousavi, S. et al. Impacts of the aneurysm deformation induced by stent on hemodynamic of blood flow in saccular internal carotid artery aneurysms. AIP Adv. 14, 9 (2024).
Hu, R. & Feng, H. Lenticulostriate artery and lenticulostriate-artery neural complex: New concept for intracerebral hemorrhage. Curr. Pharm. Des. 23(15), 2206–2211. https://doi.org/10.2174/1381612823666170220163750 (2017).
Zhang, C. et al. Hematoma evacuation via image-guided para-corticospinal tract approach in patients with spontaneous intracerebral hemorrhage. Neurol. Ther. 10(2), 1001–1013. https://doi.org/10.1007/s40120-021-00279-8 (2021).
Huang, Y. et al. Lumican promotes calcific aortic valve disease through H3 histone lactylation. Eur. Heart J. 45(37), 3871–3885. https://doi.org/10.1093/eurheartj/ehae407 (2024).
Shen, B. et al. Cerebral hemodynamics underlying ankle force sense modulated by high-definition transcranial direct current stimulation. Cereb. Cortex 34(6), bhae226. https://doi.org/10.1093/cercor/bhae226 (2024).
Zhao, Y. et al. Peripheral nerve injury repair by electrical stimulation combined with graphene-based scaffolds. Front. Bioeng. Biotechnol. 12, 1345163. https://doi.org/10.3389/fbioe.2024.1345163 (2024).
Rostamian, A., Fallah, K., Rostamiyan, Y. & Alinejad, J. Computational study of the blood hemodynamic inside the cerebral double dome aneurysm filling with endovascular coiling. Sci. Rep. 13(1), 2909 (2023).
Zhu, X. et al. Neuregulin-1, a member of the epidermal growth factor family, mitigates STING-mediated pyroptosis and necroptosis in ischaemic flaps. Burns Trauma 12, tkae035. https://doi.org/10.1093/burnst/tkae035 (2024).
Hao, S. et al. Group identity modulates bidding behavior in repeated lottery contest: neural signatures from event-related potentials and electroencephalography oscillations. Front. NeuroSci. 17 https://doi.org/10.3389/fnins.2023.1184601 (2023).
Zhao, Y. et al. Hypoxia-induced signaling in the cardiovascular system: Pathogenesis and therapeutic targets. Signal Transduct. Target. Ther. 8(1), 431. https://doi.org/10.1038/s41392-023-01652-9 (2023).
Zhao, Y. et al. Loss of m6A demethylase ALKBH5 promotes post-ischemic angiogenesis via post-transcriptional stabilization of WNT5A. Clin. Transl. Med. 11(5), e402. https://doi.org/10.1002/ctm2.402 (2021).
Chen, L. et al. HPDA/Zn as a CREB inhibitor for ultrasound imaging and stabilization of atherosclerosis plaque. Chin. J. Chem. 41(2), 199–206. https://doi.org/10.1002/cjoc.202200406 (2023).
Shao, F. et al. Causal association of plasma circulating metabolites with nephritis: A mendelian randomization study. Front. Nutr. 11, 1364841. https://doi.org/10.3389/fnut.2024.1364841 (2024).
Bing, P. et al. A novel approach for denoising electrocardiogram signals to detect cardiovascular diseases using an efficient hybrid scheme. Front. Cardiovasc. Med. 11, 1277123. https://doi.org/10.3389/fcvm.2024.1277123 (2024).
Bao, M. et al. Long noncoding RNA LINC00657 acting as a mir-590-3p sponge to facilitate low concentration oxidized low-density lipoprotein–induced angiogenesis. Mol. Pharmacol. 93(4), 368–375. https://doi.org/10.1124/mol.117.110650 (2018).
Li, H. et al. The effects of ferulic acid on the pharmacokinetics of warfarin in rats after biliary drainage. Drug Des. Dev. Ther. 10, 2173–2180. https://doi.org/10.2147/DDDT.S107917 (2016).
Gao, X., Tang, J., Liu, H., Liu, L. & Liu, Y. Structure–activity study of fluorine or chlorine-substituted cinnamic acid derivatives with tertiary amine side chain in acetylcholinesterase and butyrylcholinesterase inhibition. Drug Dev. Res. 80(4), 438–445. https://doi.org/10.1002/ddr.21515 (2019).
Shiryanpoor, I., Valipour, P., Barzegar Gerdroodbary, M., Abazari, A. M. & Moradi, R. Using computational fluid dynamic for evaluation of rupture risk of micro cerebral aneurysms in the growth process: Hemodynamic analysis. Int. J. Mod. Phys. C (2024).
Sajad Salavatidezfouli, A. et al. Investigation of the stent induced deformation on hemodynamic of internal carotid aneurysms by computational fluid dynamics. Sci. Rep. 13(1), 7155 (2023).
Sadeh, A. et al. Computational study of blood flow inside MCA aneurysm with/without endovascular coiling. Sci. Rep. 13, 4560. https://doi.org/10.1038/s41598-023-31522-x (2023).
Hu, E. et al. A novel microbial and hepatic biotransformation-integrated network pharmacology strategy explores the therapeutic mechanisms of bioactive herbal products in neurological diseases: the effects of Astragaloside IV on intracerebral hemorrhage as an example. Chin. Med. 18(1), 40. https://doi.org/10.1186/s13020-023-00745-5 (2023).
Cheng, M. et al. A novel strategy of integrating network pharmacology and transcriptome reveals antiapoptotic mechanisms of Buyang Huanwu Decoction in treating intracerebral hemorrhage. J. Ethnopharmacol. 319, 117123. https://doi.org/10.1016/j.jep.2023.117123 (2024).
Wang, Y., Zhai, W., Zhang, H., Cheng, S. & Li, J. Injectable polyzwitterionic lubricant for complete prevention of cardiac adhesion. Macromol. Biosci. https://doi.org/10.1002/mabi.202200554 (2023).
Shepherd, N. M. & Carle, C. Should all critical care patients with a central venous catheter in situ be screened for central catheter related thrombosis? Br. J. Hosp. Med. 85(5), 1–3. https://doi.org/10.12968/hmed.2024.0017 (2024).
Lu, Q. et al. Nitrogen-containing flavonoid and their analogs with diverse B-ring in acetylcholinesterase and butyrylcholinesterase inhibition. Drug Dev. Res. 81(8), 1037–1047. https://doi.org/10.1002/ddr.21726 (2020).
Feng, D. et al. N6-methyladenosine profiling reveals that Xuefu Zhuyu decoction upregulates METTL14 and BDNF in a rat model of traumatic brain injury. J. Ethnopharmacol. 317, 116823. https://doi.org/10.1016/j.jep.2023.116823 (2023).
Wang, Y. et al. Establishments and evaluations of post-operative adhesion animal models. Adv. Ther. https://doi.org/10.1002/adtp.202200297 (2023).
Poueinak, M. M. Abdollahi, S. A., Alizadeh, A. A., Youshanlui, M. A., Zekri, H. & Gerdroodbary, B. Computational study of blood hemodynamic in ICA aneurysm with coiling embolism. Int. J. Mod. Phys. C 34(6), 2350138. https://doi.org/10.1142/S0129183123501383 (2023).
Sabernaeemi, A. et al. Influence of stent-induced vessel deformation on hemodynamic feature of bloodstream inside ICA aneurysms. Biomech. Model. Mechanobiol. https://doi.org/10.1007/s10237-023-01710-9 (2023).
Hariri, S., Poueinak, M. M., Hassanvand, A., Barzegar Gerdroodbary, M. & Faraji, M. Effects of blood hematocrit on performance of endovascular coiling for treatment of middle cerebral artery (MCA) aneurysms: Computational study. Interdiscip. Neurosurg. 32, 101729 (2023).
Zan-Hui Jin, M., Barzegar Gerdroodbary, P., Valipour, M., Faraji, Nidal, H. & Abu-Hamdeh CFD investigations of the blood hemodynamic inside internal cerebral aneurysm (ICA) in the existence of coiling embolism. Alex. Eng. J. https://doi.org/10.1016/j.aej.2022.10.070 (2023).
Armin Sheidani, M., Barzegar Gerdroodbary, A., Poozesh, A., Sabernaeemi, S. & Salavatidezfouli Arash Hajisharifi, influence of the coiling porosity on the risk reduction of the cerebral aneurysm rupture: Computational study. Sci. Rep. 12, 19082 (2022).
Shen, Xiao-Yong, M., Barzegar Gerdroodbary, A. M., Abazari & Moradi, R. Computational study of blood flow characteristics on formation of the aneurysm in internal carotid artery. Eur. Phys. J. Plus 136(5), 541 (2021).
Poueinak, M. M. Abdollahi, S. A., Alizadeh, A. A., Youshanlui, M. A., Zekri, H. & Gerdroodbary, B. Computational study of blood hemodynamic in ICA aneurysm with coiling embolism. Int. J. Mod. Phys. C 34(10), 2350138 (2023).
Sadeh, A., Kazemi, A., Bahramkhoo, M. & Barzegar Gerdroodbary, M. Computational study of blood flow inside MCA aneurysm with/without endovascular coiling. Sci. Rep. 13(1), 4560 (2023).
Valipour, P. Effects of coiling embolism on blood hemodynamic of the MCA aneurysm: A numerical study. Sci. Rep. 12(1), 22029 (2022).
Rostamian, A., Fallah, K. & Rostamiyan, Y. Reduction of rupture risk in ICA aneurysms by endovascular techniques of coiling and stent: Numerical study. Sci. Rep. 13(1), 7216 (2023).
AneuriskWeb project website.http://ecm2.mathcs.emory.edu/aneuriskweb (Emory University, Department of Math&CS, 2012).
Wu, W., Alizadeh, A. A. & Hekmatifar, M. The effect of initial temperature on the mechanical interaction of 3DN5/5OTF protein-based structures using molecular dynamics simulation. Int. J. Biol. Macromol. 123235 (2023).
Wang, H. et al. Investigation of the effects of porosity and volume fraction on the atomic behavior of cancer cells and microvascular cells of 3DN5 and 5OTF macromolecular structures during hematogenous metastasis using the molecular dynamics method. Comput. Biol. Med. 158, 106832 (2023).
Jiang, H., Lu, Z., Barzegar Gerdroodbary, M., Sabernaeemi, A. & Sajad Salavatidezfouli. The influence of sac centreline on saccular aneurysm rupture: Computational study. Sci. Rep. 13(1), 11288 (2023).
Ansys, I. ANSYS® Fluent User’s Guide, Release 2020 R2 (ANSYS, Canonsburg, 2020).
Dian, W. et al. Evaluation of coiling endovascular technique on treatment of Middle cerebral artery cerebral aneurysms in different blood haematocrits: numerical study. Int. J. Mod. Phys. C https://doi.org/10.1142/S0129183124502528 (2024).
Author information
Authors and Affiliations
Contributions
P.Z. and X.L. wrote the main manuscript text and Y.F. and X.L. prepared figures and A.A.K. analysed data. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, P., Liu, X., Fan, Y. et al. Enhancing understanding of stent-induced deformation in MCA aneurysms: a hemodynamic study. Sci Rep 14, 28036 (2024). https://doi.org/10.1038/s41598-024-78664-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78664-0