Abstract
Space agriculture, pivotal for sustainable extraterrestrial missions, requires plants that can adapt to altered gravitational conditions. This study delves into the adaptive responses to altered gravity of Wolffia globosa, an aquatic plant known for its rapid growth and high nutritional value. The research aimed to analyse the effect of simulated microgravity and hypergravity on relative growth rate (RGR), morphological characteristics, protein content, and the correlation between plant size and growth rate of Wolffia globosa. The study highlighted the responses of the species to altered gravity, uncovering inherent variability among seven different clones of W. globosa. Results show a base variability among clones in terms of RGR, size and protein content. Furthermore, some clones are affected by simulated microgravity, showing a decrease in RGR. Differently, under hypergravity, clones showed RGR higher than in 1 g control, therefore revealing a novel plant response to hypergravity. Morphological adaptations to gravity alterations were also evident. Among the studied clones, significant morphological changes were observed, further underlining the peculiar adaptation to the hypergravity environment. Differently, under simulated microgravity, morphology was generally stable across clones. A key finding of the study was the significant negative correlation between RGR and the physical dimensions of the plants: the fastest growth was associated with the smallest dimensions of the plants. This correlation might have practical implications in selecting clones for space cultivation, that leads to compact yet highly productive clones. The analysis of the protein content of all the clones revealed mostly no significant changes under hypergravity. Otherwise, a general decrease in protein content was observed under simulated microgravity. Overall, the study confirms the suitability of W. globosa for space agriculture and provides new insights into the perspective of using W. globosa as an alternative crop species for protein production for manned Space missions. Furthermore, it underscores the need for focusing on the clones and the selection of the W. globosa plants that are best adapted to the environmental conditions of space; therefore, selecting those with the best combination of biomass production (by means of growth rate, size), and protein content.
Similar content being viewed by others
Introduction
We are at the verge of a new phase in space exploration. Projects like Artemis III are set to transform our endeavors outside Earth1. Stepping foot back on the Moon serves both as a pursuit of scientific knowledge and a fundamental step towards achieving the grand objective colonizing other planets1. However, these ambitious goals faces the reality of the limitations of current space technologies to carry cargo2. Overcoming this obstacle requires innovative approaches to sustainable living in space, where every resource is precious and must be regenerated in-situ3.
Fundamental in overcoming these challenges is the development of bioregenerative life support systems, as demonstrated by the MELiSSA loop initiative, which aims to develop self-sustaining man-made ecosystems4,5. Within these artificial ecosystems, plants play a crucial role not only as a source of nutrition for astronauts but also as key system in the recycling and purification of air and water, thus becoming crucial for the success of long-lasting interplanetary voyages6,7. The concept of cultivating crops in space significantly deviates from that on Earth8,9. The cultivation of crops in space must overcome severe constraints such as resource limitation thus highlighting the importance of efficiency and minimizing waste10,11. Contrary to Earth’s agriculture, space farming occurs within a sealed ecosystem where resources must be carefully preserved11,12.
In light of these challenges, our research contributes to the expanding knowledge on duckweeds, especially W. globosa, as an alternative space crop due to its high efficiency and low resource requirements3,13,14. This aquatic plant, part of the Lemnaceae family, is not only fast-growing and nutrient-rich but also has a global distribution, excluding the Arctic and sub-Arctic zones15,16. Its global distribution highlights its adaptability to various environments17. By analyzing the effects of differing gravity levels on seven clones of W. globosa, we aimed to provide insight into the species’ varying responses to gravity clues. In duckweed research, the term “clone” refers to the asexually reproduced individuals derived from a single frond or colony isolated in the wild18. This exploration into growing W. globosa under conditions that mimic space aims to evaluate its potential as a sustainable food source for space missions, meeting their strict requirments3,19. Our study includes a thorough investigation into the protein content, morphological features, and growth rates of W. globosa clones under varied gravity conditions, offering an in-depth understanding of the plant’s adaptability and resilience to the distinct challenges of space19. Clonal variability is a well known among plants of the family Lemnaceae, thus clonal20 selection being important to elucidate for specific applications such as the one provided by this investigation.
Materials and methods
Plant material and cultivation
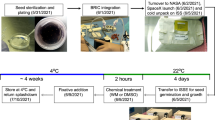
Seven different clones of W. globosa were utilised in this study. Plant material came from the Rutgers Duckweed Stock Cooperative (RDSC), an international network of duckweed collection centres21. More specifically, the plant material was obtained either from the Department of Plant Physiology, University of Jena, Germany (KJA), or from the Institute of Agriculture Biology and Biotechnology (IBBA), National Research Council of Italy (CNR). The clones from the IBBA collection and three from the KJA collection were originally from Asia, while the remaining two from KJA were from Europe (Table 1). All clones were identified maintaining the original four-digit code according to Landolt convention22. Taxonomical identification was confirmed at KJA and IBBA prior to sending. Upon receipt, the plant material was surface sterilised using a 0.3% bleach/water solution for 5 min23. After surface disinfection, plants were cultured in N-medium with 2% glucose. Fourteen days post-disinfection, plants were subcultured for 30 days in N-medium23 under axenic conditions. Subsequently, the plant material was transferred to a laminar flow hood and allocated into sets of 12-well plates. Each well was filled with 3 ml of N-medium solution and sealed with Micropore® tape. Before the experimental run, plants were acclimatised for 36 h at 30 °C. Post-acclimatization, plants were relocated to each well of a 12-multiwell plate, previously filled with 3 ml of N-medium and 0.8% Agar to create a semi-solid substrate. The experiment was conducted at an average temperature of 30.44 ± 1.24 °C, with a photoperiod of 16/8 hours light/dark and a total Photon Flux Density (PFD) of 130.60 ± 11.74 µmol·m−2·s−1 for 168 h24.
Light exposure was provided using six 40 cm 12 V LED bars, with each bar illuminating a multiwell plate (refer to Fig. 1). The light emission spectrum was characterised using a spectroradiometer (Model SS-110, Apogee Instruments Inc.). This measurement was conducted at eight different positions within the experimental setup (N = 8), covering a wavelength range of 340–820 nm. A comprehensive breakdown of the light spectrum’s composition is presented in Table 2; Fig. 1.
Gravity treatments
The study incorporated two gravity treatments: simulated microgravity (RPM) and hypergravity (4 g). For each treatment, a corresponding control group under Earth gravity conditions was included for comparative purposes.
Simulated microgravity was achieved using a Random Positioning Machine (RPM). The RPM was set to a maximum random speed of 60°/s, the samples were maximum 27 cm from the center of rotation resulting in a maximum residual gravity at the outer perimeters of the set-up of 0.03 g. The RPM’s operational parameters included random intervals and directions to enhance the simulation’s authenticity25.
Hypergravity conditions were simulated using a large-diameter centrifuge (LDC)26. The chosen hypergravity level was 4 g, representing four times Earth’s gravitational force. This setup enabled the investigation of W. globosa clones’ response to the increased gravitational forces.
Two control groups were established. The first control group (CO) involved plants grown under normal gravity conditions in a static gondola close to the LDC. The second control (CO-RPM) was set up in a Velp Scientifica FOC l 120 incubator set with the identical environmental parameters to the RPM treatment, and maintaining Earth gravity conditions. These control groups provided a baseline for comparing the growth, morphology, and soluble protein content of W. globosa clones under varying gravity treatments. Each experiment lasted 7 days (168 h).
Details of the experimental setup and the number of replicates for each treatment are summarised in Table 3.
On the final experimental day (t168), duckweed samples (n = 3) were collected from each clone and treatment group, including controls according to the method outlined by Walsh et al.20. These samples were then stored at -20 °C before protein extraction. A buffer solution consisting of 50 mM potassium phosphate (pH 7), with 0.1 mM polyvinylpyrrolidone (PVP, Mw 40,000), and 0.1 mM EDTA was used for the extraction. To prepare the samples, samples of 110 to 119 mg of plants were homogenised in this buffer, maintaining a ratio of 1 mL buffer for every 110 mg of plants. Following homogenisation, the samples were centrifuged at 20,000 x g for 30 min at a temperature of 4 °C27. The supernatant from this centrifugation was then utilised for protein concentration analysis using the Bradford assay, with bovine serum albumin as the reference standard28. For determining absorbance, a mixture of 50 µL of the extracted sample was added to 1.5 mL Bradford reagent (Pierce™ Bradford Protein Assay Kit, ThermoFisher, USA) was placed in a cuvette and left in the dark for ten minutes. The absorbance of this mixture was measured at 595 nm using the Onda V-11 SCAN (Giorgio Bormac, Italy) spectrophotometer. Protein concentration was obtained by correlating the unknown concentration with a BSA standard (Pierce™ Bradford Protein Assay Kit, ThermoFisher, USA) (Supplementary material Fig. 1).
Data collection and analysis
As outlined in Romano et al.14, the collection of data involved imaging multiwell plates. This was achieved using two specific imaging setups:
-
1.
Camera Setup: A Sony Alpha II camera with an 18–70 mm lens, mounted on a stand, was used to capture top-view images (6000*4000 pixel) of the multiwell plates (Supplementary Fig. 2 supplementary material). These plates were placed on a lighting table covered with a white semi-transparent Polyvinyl chloride panel and illuminated by an LED light panel. This setup was pivotal for growth assessment, particularly for calculating the Relative Growth Rate (RGR) by comparing the pixel area occupied by the plants at the beginning (t0) and at the end of the experiments (t168). Doubling Time (DT) was then derived from the RGR values.
-
2.
Stereo Microscope Setup: A Leica® MZ8 Stereo zoom Microscope was employed for detailed morphological analysis. This allowed to capture images (2592*1944 pixel) representative of each treatment’s replicates. The images were key in evaluating the growth and morphological characteristics of W. globosa. Using Fiji® software, the dimensions of the mother fronds’ long and short axes were measured, and the ratios of these axes were calculated to determine frond roundness.
Image analysis was conducted on each digital image, aiming at measuring growth parameters (RGR and DT) and at highlighting morphological variances. RGR was ascertained by comparing plant area in pixels at the beginning and end of the tests according to29, while DT was calculated based on RGR following Naumann30 both measurements were based on a total of 12 replicates. For morphological analysis, twenty-five mother fronds were randomly selected from different wells within each treatment to measure their dimensions and calculate ratios (N = 25)13,31, using Fiji® software. The measured dimensions were the length of the long side and length of the short side and the existing ratio between the long and short side. A more in-depth explanation can be found in Romano et al. 2024 Fig. 5.
Morphological, relative growth rate (RGR), doubling time (DT), and protein content data were then processed and analysed using SPSS Statistics ver. 29 (IBM Corp.). The aim was to examine the differences in morphological traits, relative growth rate, and soluble protein content among the different gravity treatments. Initially, all datasets have been tested for normal distribution using Shapiro-Wilk before performing two-way univariate Analysis of Variance (ywo-way ANOVA). The morphological ratios, relative growth rates and protein content were compared separating the two control groups and their respective treatments (4 g and RPM); Where appropriate differences were tested by Student-Newman-Keuls post-hoc tests. Subsequent in-depth analyses involved testing differences within each clone between the RPM treatment and its control group as well as 4 g and its control group (CO-RPM vs. RPM and CO vs. 4 g). These assessments were conducted using a one-way ANOVA at a significance level of p < 0.05. The analytical approach provided an in-depth understanding of how variations in gravity affected the growth, morphology, and protein content of W. globosa clones.
SPSS Statistics ver. 29 (IBM Corp.) has also been used to evaluate the possible correlation between the size and the growth rates of different clones. More specifically, RGR representative of each clone was obtained averaging the 48 replicates of the two treatments and their respective controls. Similarly, the Average Long Side axis of each clone was also calculated by averaging the 100 replicates. For both parameters a total of 28 data points (7 clones x 4 mean values per treatment) was obtained. Correlation was assessed by performing Pearson correlation. Additionally, to test if the presence of outliers (clone 9582) had a significant impact on the correlation analysis, an additional test was performed by excluding this clone. Additionally, correlation within each gravity treatment and controls has been evaluated, by merging data sets (RGR and mean length) from the 7 clones within each gravity treatment. Microsoft Excel has been used to prepare data for SPSS and to create all the graphs and tables used across the manuscript.
Results
Comparison of growth rates and doubling times of clones under different gravity levels
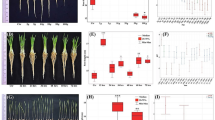
Independently from the gravity treatments, growth characteristics of W. globosa clones analysed in terms of the relative growth rate (RGR), revealed that the seven evaluated clones did not reproduce at the same rate. The two-way ANOVA results showed highly significant differences in clone vs. treatment by means of RGR (F = 4.39; P < 0.001). More specifically, clone 9498 exhibited the highest mean RGR (0.326), while clone 9582 displayed the lowest mean RGR (0.219). These findings were further substantiated by the Student-Newman-Keuls post-hoc tests, which identified several statistically distinct groups among the clones, thereby confirming the significant inter-clone variances in growth rates (Fig. 2).
Observed Relative growth rate (RGR) across all clones. The graph reports the RGR for all clones by merging RGR across all treatments. Data refer to means ± SD (N = 48). Significant differences between clones are expressed with different letters (P < 0.05) as result of the Student-Newman-Keuls post-hoc analysis.
Regarding the Doubling Time (DT), the two-way ANOVA also showed high significant differences in clone vs. treatment by means of RGR (F = 4.33; P < 0.001). Among the clones, the slowest (Clone 9582) took an average of three days (3.189 days) to double the surface occupied by the plants, whereas the fastest clone (9498) reached the DT in 2.151 days only, consistent with its faster growth rate (Fig. 3).
Observed Doubling time (DT) across all clones. The graph reports the DT for all clones by merging DT across all treatments. Data refer to means ± SD (N = 48). Significant differences between clones are expressed with different letters (P < 0.05) as result of the Student-Newman-Keuls post-hoc analysis.
Among clones Effect of Gravity Conditions on Relative Growth Rate (RGR) of W. globosa clones under varying gravitational conditions showed different responses among gravity treatments (CO-RPM vs. RPM and CO vs. 4 g). Results from the two-way ANOVA show that the two groups were both significant in comparison of treatment vs. clone effect on the RGR, when comparing the effect of simulated microgravity, with it’s control results show significance (F = 5.96; p < 0.001) and this was also true for the hypergravity vs. control samples (F = 3.60; p 0.002) More specifically, in the CO-RPM vs. RPM setting (Fig. 4a), three of the seven clones showed a significant decrease in RGR as an effect of the simulated microgravity. In more detail, clone 8356 displayed a significant alteration (F(1, 22) = 17.035, p < 0.001), and clone 9006 followed suit with a notable shift in RGR (F(1, 22) = 19.002, p < 0.001), and clone 9498 showed the least significant response (F(1, 22) = 5.565, p = 0.028) in the simulated microgravity versus control setting. The growth rate of the other clones was unaffected by the altered gravity conditions.
Turning to the effect of hypergravity (Fig. 4b), the scenario is different to what was previously observed under simulated microgravity. Although less than half of the samples showed significant differences, two showed an increase in RGR (8356 and 9910), while one showed a reduction in RGR (9582). To go in a more detailed observation of the results of the ANOVA, the most pronounced difference in this scenario was observed in Clone 9910, which showed a substantial increase in RGR (F(1, 22) = 19.446, p < 0.001) followed by clone 8356 (F(1, 22) = 4.459, p = 0.046). Lastly, clone 9582 demonstrated a marked decreased response (F(1, 22) = 7.300, p = 0.013).
Observed mean RGR in CO-RPM vs. RPM and 1 g CO vs. 4 g. a Shows the RGR displayed by different clones under simulated microgravity (RPM), against their control (CO-RPM). b Shows the RGR displayed by different clones under hypergravity (4 g), against their control (CO). Data refer to means ± SD (N = 12). Statistical differences are marked by *if p < 0.05, ** if p < 0.01, *** if p < 0.001 and n.s. (not significant) if p > 0.05.
Morphological responses (of clones) to altered gravity
The two-way ANOVA conducted on morphological data from W. globosa under the two distinct gravitational scenarios has provided insightful information about the plant’s morphological adaptations to different gravities. Results from the Treatment vs. clone interaction show a significant p 0.021 with an F value of 1.80. Comparison of clone vs. treatments shows that hypergravity affects the morphological ratios by means of long side ratios of W.globosa plants (F = 2.91; p 0.008), while simulated microgravity seems to be not affecting this ratio (F = 0.851; p 0.531).
The higher ratios observed under hypergravity conditions lead to a morphological shift towards less rounded plants. Under simulated microgravity, the Calculated Long Side Ratio was the highest in Clone 9299 (1.448), followed by Clone 9498 (1.437), while Clone 8356 exhibited the lowest ratio (1.219). Likewise, in hypergravity, the Calculated Long Side Ratio of Clone 9299 exhibited the highest ratio (1.479), followed by lone 9498 (1.445), and Clone 9006 with the lowest ratio (1.227).
In-depth analysis of the morphological reaction of single clones versus their controls under the two gravitational settings revealed that under the CO-RPM vs. RPM scenario (Fig. 5a), the responses among the tested clones were generally uniform, with no significant morphological changes observed in the Calculated Long Side Ratio. This consistency suggests a general morphological stability of W. globosa clones under simulated microgravity conditions.
In contrast, the CO vs. 4 g condition (Fig. 5b) revealed significant morphological changes in three of the seven clones. Worth noticing is that the significant differences were always towards a higher long-side/short side ratio. Clone 8356 showed the most significant difference to its control (F = 18.899, p < 0.001), followed by clone 9006 (F = 8.504, p = 0.005) and lastly, Clone KJA 0025 (F = 5.492, p = 0.023).
Observed mean long side ratio in CO-RPM vs. RPM and CO vs. 4 g. a Shows the Long side ratio displayed by different clones under simulated microgravity (RPM), against their control (CO-RPM). b Shows the Long side ratio displayed by different clones under hypergravity (4 g), against their control (CO). Data refer to means SD (N = 25). Statistical differences are marked by *if p < 0.05, ** if p < 0.01, *** if p < 0.001 and n.s. (not significant) if p > 0.05.
Correlation between relative growth rate and morphological traits
The study explored the connection between W. globosa’s growth rate and its biometrical traits. Specifically, it investigated how the plant’s average growth rate (RGR Mean) relates to the average length of its longest side among the clones (as depicted in Fig. 6). The data showed a notably strong inverse link between these factors. This was evidenced by a Pearson correlation coefficient of – 0.925, paired with a highly significant p-value 0.003, indicating a robust inverse relationship. Essentially, as the average growth rate of the plant increases, the average length of the clones tends to decrease. These insights shed light on the fundamental growth behaviours of W. globosa, emphasizing the inverse relationship between its growth rate and physical size. Additionally, to further test the strength of this correlation we have also excluded from the analysis the data from clone 9582, considering that this clone might have acted as outlier leading to a false positive result. However, even by excluding this clone, correlation resulted significant (p 0.018), Pearson correlation coefficient of – 0.889 (Table 4).
Correlation between Relative Growth Rate and size of the fronds. For each of the seven clones, four dots are reported representing the mean RGR and their mean long-side length (mm) for each of the gravity treatment. A significant negative correlation was verified and is represented by the trend line (R2 = 0.907). To further evaluate the effect of different gravity treatment on the existing correlation between RGR and mean length, we have analysed correlation within each gravity treatment and their controls. Results are summarized in the following table.
Analysis of the protein content across different gravity levels
In this study, we analysed the effects of different gravitational conditions on the protein content of various W. globosa clones. The results of the two-way ANOVA revealed a significant interaction between clone and treatment (F = 3.48, p < 0.001). When comparing control conditions (CO-RPM) with simulated microgravity (RPM), a significant interaction between clone and treatment was found (F = 2.69, p = 0.035). Similar significant differences were observed when comparing control (1 g) and 4 g conditions.
Moreover, while the effects between subjects (clones and treatments) were consistently significant in previous comparisons (p < 0.001), this was not the case for the hypergravity condition. In hypergravity, the treatment effect was not significant (F = 0.009, p = 0.95), indicating that the observed variability primarily stems from differences in protein content among clones (F = 16.79, p < 0.001).
Clone vs. treatments
To underline the variability in protein content between clones under simulated microgravity, we compared samples from the Control-Microgravity (CO-RPM) vs. simulated microgravity (RPM) condition (Fig. 7a). Results highlighted a significant variation in response among different clones. In general, a reduction in protein content was underlined among all clones. The lowest p-value was observed for clones 9299 (p = 0.001), followed by clones 9498 (p = 0.005), 9006 (p = 0.039) and KJA 0025 (p = 0.032).
Under the Control (CO) vs. 4 g condition (Fig. 7b), results revealed a notable reduction in protein content only in clone 9498 (p = 0.027), suggesting that this clone is susceptible to both altered gravity conditions in terms of protein content.
Percentage of the protein content of the fresh weight (FW) in CO-RPM vs. RPM and CO vs. 4 g. a Shows the % protein content of the FW displayed by different clones under simulated microgravity (RPM), against their control (CO-RPM). b Shows the % protein content of the FW displayed by different clones under hypergravity (4 g), against their control (CO). Data refer to means ± SD (N = 3). Statistical differences are marked by *if p < 0.05, ** if p < 0.01, *** if p < 0.001 and n.s. (not significant) if p > 0.05.
Discussion
This study provides insights into the adaptive responses of W. globosa to altered gravitational environments pertinent to space agriculture. Notably, our investigation focused on the effects of simulated microgravity and hypergravity on different clones of W. globosa by considering three main characteristics: relative growth rate, morphological traits and protein content. Furthermore, we investigated the correlation between the size of the plant (long axis length) and its relative growth rate.
It is particularly intriguing that while general trends in higher plant biology suggest a negative impact of microgravity on plant growth of most analysed species32,33,34, our findings reveal a more complex scenario for W. globosa. Some clones showed a decline in growth under simulated microgravity, aligning with these general trends14. Yet, others exhibited resilience or minimal impact, as was previously observed by Yuan and Xu13. These findings underscore the need for focusing on individual clone instead of a generic reference to the species to identify the effective cultivation strategies both in space and on Earth.
The reaction of Wolffia clones to hypergravity in terms of RGR was different from what was observed under simulated microgravity; in fact, significant differences underlined a higher growth rate among some clones. Previous studies on W. globosa report a growth increment under hypergravity, but statistical analysis resulted not significant14. The higher growth rates observed under the hypergravity condition open up new interesting scenarios for maximising cultivation efficiency for future terrestrial and in space applications. This novel result underlines the need for future investigation concerning plant resilience and peculiar reactions to the hypergravity environment35.
The peculiar adaptation to the hypergravity environment showed by Wolffia plants was also confirmed by the significance of the morphological results. Although no morphological changes were observed under simulated microgravity, higher long-side/short-side ratios were significant higher under the hypergravity treatment if compared to their controls. This finding can shed light on what might be a hint of how W. globosa perceives gravity. Although in its sister species Wolffia australiana the lack of gravity sensing mechanisms was verified by the recent genome sequencing36, previous studies on starch-deficient mutants of Arabidopsis thaliana have proven the restoration of gravitropic sensitivity under hypergravity conditions37. The possible gravitropic restoration under hypergravity conditions needs further confirmation, and we are currently investigating the genetic response of Wolffia australiana under different gravity levels.
Our data on W. globosa highlighted a strong inverse relationship between the mean relative growth rate (RGR) and the mean plant length of the clones. This negative correlation is crucial in understanding how these plants will adapt and thrive in space environments. In the realm of space cultivation, where efficiency and the optimization of scarce resources are paramount, it’s intriguing to note the capabilities of micro plants3,38.
Despite being among the smallest higher plants known, these diminutive species exhibit a remarkable ability to vary their size, potentially enhancing their growth rate. This adaptability presents a subtle yet significant advantage, particularly when resources are constrained. In such an environment, acknowledging and capitalizing on the nuanced relationship between a plant’s size and its growth rate becomes crucial. It allows for a more informed selection process, ensuring a balance is struck—a prudent trade-off between size and growth that optimizes both yield and resource utilization in the challenging confines of space cultivation.
The analysis of protein content in W. globosa under varying gravitational conditions has provided valuable insights into the plant’s physiological responses to environmental stressors. The study’s findings indicate a differential impact of gravity on protein synthesis and accumulation across various clones and different gravity treatments. In the comparison between control and 4 g conditions, there were no significant changes in protein content except for one clone (9498). This lack of variance suggests that increased gravitational level does not substantially affect W. globosa’s protein synthesis pathways. Differently, past proteomic analyses of rice seedlings grown in space have proven that the microgravity environment significantly affects plant protein production39. Likewise, in our study, we have observed that more than half of the W. globosa clones cultivated in simulated microgravity were subjected to a reduction in the soluble protein content. Notably, Clone 9498 uniquely exhibited significant changes in protein content under both gravitational conditions. This observation remarks once more the differences between clones and the relevance of clone selection, especially in the context of space cultivation. The overall estimation of the single clone suitability for space cultivation deserves careful consideration. For instance, the relative growth rate (RGR) of Clone 9498 is the highest among the clones tested, suggesting best suitability for space agriculture. However, a deeper analysis of its protein content reveals a significant decrease under altered gravity conditions. This reduction in protein content, particularly under simulated microgravity, challenges the assumption of its optimal suitability for space cultivation. Additionally, it is worth mentioning that in the framework of optimising the duckweed cultivation settings, factors like light quality and quantity, liquid substrate, and an optimised nutrient medium can enhance both protein content and biomass accumulation, as has been seen for other duckweeds or other plant species40,41,42.
Considering the estimation of the protein quality of duckweed from past research16,43 and aligning with the European Food and Safety Authority (EFSA) recommendation of a daily protein intake of 0.8 g/kg of weight per person (aged 25–51 years old), the application of W. globosa in space agriculture presents intriguing possibilities. The hypothetical scenario drawn from the observed protein content in Clone 8356 under simulated microgravity conditions suggests that about 600 g of fresh Wolffia might suffice for an adult’s daily protein needs. Additionally, thanks to the vegetative reproductive nature of duckweed, cultivation can be easily started from a few grams (e.g. 10 g) of fresh biomass and in a few weeks (approximately 20 days) it is possible to reach a stable production of 600 g a day (approximately 2000 g of existent biomass). This is unlike other crop species that require a high quantity of seeds (microgreens)43 or regeneration of seeds from the adult plant (seed to seed cycle)44.
In conclusion, this study sheds light on the promising prospects of cultivating W. globosa in future space agriculture by remarking the stable reactions of different clones both under simulated microgravity and hypergravity. Furthermore, this study emphasises the need to rethink the species W. globosa as a generic species, but it sheds light on the importance of further considering the genetic variability among its clones. Considering the broad geographical distribution of the species15,16, we envisage that the existing genetic variability can be a starting point to analyse the adaptability of single clones to space environments, resulting in a clone-to-environment choice rather than a generic overall species selection.
Plant material
The plant collection and use were in accordance with all the relevant guidelines.
Clones utilized in this study have been identified by staff members prior addition to the collection ‘s list. Clones of W. globosa have been received from Duckweed collection centres at:
-
Duckweed stock collection at Friedrich Schiller University of Jena, Jena, Germany.
-
Duckweed stock collection at National research council (CNR) IBBA, Milan, Italy.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
NASA Artemis. https://www.nasa.gov/specials/artemis/
Rosen, P. E. et al. Impact of Economic constraints on the projected timeframe for human-crewed Deep Space Exploration. Galaxies 2022. 10, Page 88 (10), 88 (2022).
Romano, L. E. & Aronne, G. The World Smallest plants (Wolffia Sp.) as potential species for Bioregenerative Life Support systems in Space. Plants 2021. 10, Page 1896 (10), 1896 (2021).
Gòdia, F. et al. MELISSA: a loop of interconnected bioreactors to develop life support in space. J. Biotechnol. 99, 319–330 (2002).
Santomartino, R. et al. Toward sustainable space exploration: a roadmap for harnessing the power of microorganisms. Nat. Commun. 14, 1–11 (2023).
Aronne, G. et al. ROADMAP #11: bio-regenerative life support systems in space: space biotechnology & space agriculture.
Lasseur, C. et al. MELiSSA: the European project of closed life support system. Gravitat. Space Biol. 23, 3–13 (2010).
Paradiso, R. et al. Soilless cultivation of soybean for Bioregenerative Life-Support systems: a literature review and the experience of the MELiSSA project – food characterisation phase I. Plant. Biol. 16, 69–78 (2014).
De Micco, V., Aronne, G., Colla, G., Fortezza, R. & De Pascale, S. Agro-biology for bioregenerative Life Support Systems in long-term Space missions: General constraints and the Italian efforts. J. Plant Inter. 4(4), 241–252. https://doi.org/10.1080/17429140903161348 (2009).
De Pascale, S. et al. Biology and crop production in Space environments: challenges and opportunities. Life Sci. Space Res. (Amst). 29, 30–37 (2021).
Liu, Y., Xie, G., Yang, Q. & Ren, M. Biotechnological development of plants for space agriculture. Nature Communications 12, 1–3 (2021). (2021).
McGreevy, S. R. et al. Sustainable agrifood systems for a post-growth world. Nature Sustainability 2022 5:12 5, 1011–1017 (2022).
Yuan, J. & Xu, K. Effects of simulated microgravity on the performance of the duckweeds Lemna aequinoctialis and Wolffia globosa. Aquat. Bot. 137, 65–71 (2017).
Romano, L. E., van Loon, J. J. W. A., Izzo, L. G., Iovane, M. & Aronne, G. Effects of altered gravity on growth and morphology in Wolffia globosa implications for bioregenerative life support systems and space-based agriculture. Scientific Reports 2024 14:1 14, 1–11 (2024).
Yang, J., Zhao, X., Li, G., Hu, S. & Hou, H. Frond architecture of the rootless duckweed Wolffia globosa. https://doi.org/10.1186/s12870-021-03165-5
Appenroth, K. J. et al. Nutritional value of the Duckweed species of the Genus Wolffia (Lemnaceae) as human food. Front. Chem. 0, 483 (2018).
Pietryka, M., Richter, D. & Podlaska, M. Distribution and ecology of Wolffia arrhiza (L.) Horkel ex Wimm. In the lowland part of Lower Silesia (Poland). Biol. (Bratisl). 78, 971–978 (2023).
Webber, H. J. New horticultural and agricultural terms. Science. 18, 501–503 (1903).
Herranz, R. et al. Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. (2013). https://home.liebertpub.com/ast 13, 1–17.
Walsh, É., Cialis, E., Dillane, E. & Jansen, M. A. K. Lemnaceae clones collected from a small geographic region display diverse traits relevant for the remediation of wastewater. Environ. Technol. Innov. 28, 102599 (2022).
Database - Rutgers Duckweed Stock Cooperative. http://www.ruduckweed.org/database.html
Braglia, L. et al. Duckweed species genotyping and interspecific hybrid Discovery by Tubulin-based polymorphism fingerprinting. Front. Plant. Sci. 12, 625670 (2021).
Appenroth, K. J., Teller, S. & Horn, M. Photophysiology of turion formation and germination in Spirodela polyrhiza. Biol. Plant. 38, 95–106 (1996).
Ziegler, P., Adelmann, K., Zimmer, S., Schmidt, C. & Appenroth, K. J. relative in vitro growth rates of duckweeds (Lemnaceae) – the most rapidly growing higher plants. Plant. Biol. 17, 33–41 (2015).
van Loon, J. J. W. A. Some history and use of the random positioning machine, RPM, in gravity related research. Adv. Space Res. 39, 1161–1165 (2007).
van Loon, J. J. W. A. et al. The large diameter centrifuge, LDC, for life and physical sciences and technology. Life Space Life Earth. 553, 92 (2008).
Balen, B. et al. Biochemical responses of Lemna minor experimentally exposed to cadmium and zinc. Ecotoxicology. 20, 815–826 (2011).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Romano, L. E., Iovane, M., Izzo, L. G. & Aronne, G. A Machine-Learning Method to Assess Growth Patterns in Plants of the Family Lemnaceae. Plants 11, 1910 (2022).
Naumann, B., Eberius, M. & Appenroth, K. Growth rate based dose-response relationships and EC-values of ten heavy metals using the duckweed growth inhibition test (ISO 20079) with Lemna minor L. clone St. J. Plant. Physiol. 164, 1656–1664 (2007).
Swamy, B. K. et al. Novel hypergravity treatment enhances root phenotype and positively influences physio-biochemical parameters in bread wheat (Triticum aestivum L). Sci. Rep. 11(1), 11–11 (2021). 2021.
Kiss, J. Z. Mechanisms of the Early Phases of Plant Gravitropism. Critical Reviews in Plant Sciences 19(6), 551–573. https://doi.org/10.1080/07352680091139295 (2000).
Medina, F. J., Manzano, A., Villacampa, A., Ciska, M. & Herranz, R. Understanding reduced gravity effects on early Plant Development before attempting life-support farming in the Moon and Mars. Front. Astronomy Space Sci. 8, 729154 (2021).
De Micco, V., Aronne, G., Joseleau, J. P. & Ruel, K. Xylem Development and Cell Wall changes of soybean seedlings grown in space. Ann. Bot. 101, 661 (2008).
Hosamani, R., Swamy, B. K., Dsouza, A. & Sathasivam, M. Plant responses to hypergravity: a comprehensive review. Planta. 257, 1–17 (2022).
Michael, T. P. et al. Genome and time-of-day transcriptome of Wolffia australiana link morphological minimization with gene loss and less growth control. Genome Res. 31, 225–238 (2021).
Fitzelle, K. J. & Kiss, J. Z. Restoration of gravitropic sensitivity in starch-deficient mutants of Arabidopsis by hypergravity. J. Exp. Bot. 52, 265–275 (2001).
Wheeler, R. M. Agriculture for space: people and places paving the way. Open. Agric. 2, 14–32 (2017).
Zeng, D. et al. Proteomic analysis in different development stages on SP0 generation of rice seeds after space flight. Life Sci. Space Res. (Amst). 26, 34–45 (2020).
Baek, G. Y., Saeed, M. & Choi, H. K. Duckweeds: their utilization, metabolites and cultivation. Appl. Biol. Chem. 64, 1–15 (2021).
Ullah, H. et al. Effect of growth medium nitrogen and phosphorus on nutritional composition of Lemna minor (an alternative fish and poultry feed). BMC Plant. Biol. 22, 1–7 (2022).
Monostori, I. et al. LED lighting – modification of growth, metabolism, yield and flour composition in wheat by spectral quality and intensity. Front. Plant. Sci. 9, 346801 (2018).
Parkes, M. G., Azevedo, D. L., Cavallo, A. C., Domingos, T. & Teixeira, R. F. M. Life cycle assessment of microgreen production: effects of indoor vertical farm management on yield and environmental performance. Sci. Reports. 13, 1–12 (2023).
Link, B. M., Busse, J. S. & Stankovic, B. Seed-to-seed-to-seed growth and development of Arabidopsis in Microgravity. Astrobiology. 14, 866 (2014).
Acknowledgements
Special thanks are extended to Alan Dowson for his support within the ESA-LIS laboratory.
Author information
Authors and Affiliations
Contributions
L.E.R. and G.A. conceived the experiment L.E.R. and J.J.W.A. planned and executed the experiment, LER wrote the manuscript. S.V.B, G.A., J.J.W.A. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Romano, L.E., van Loon, J.J.W.A., Vincent-Bonnieu, S. et al. Wolffia globosa, a novel crop species for protein production in space agriculture. Sci Rep 14, 27979 (2024). https://doi.org/10.1038/s41598-024-79109-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79109-4
Keywords
This article is cited by
-
Cultivation of Wolffia globosa and its application in functional food development
Scientific Reports (2025)