Abstract
Pembrolizumab-containing regimens are the standard first-line treatment for recurrent/metastatic squamous cell carcinoma of the head and neck (R/M HNSCC). The neutrophil-to-lymphocyte ratio (NLR) and C-reactive protein-to-albumin ratio (CAR) have been reported to be important prognostic factors in a variety of carcinomas, but none have been investigated in combination with pembrolizumab and chemotherapy or in first-line treatment. Seventy-four patients with R/M HNSCC received pembrolizumab-containing regimens at Tohoku University Hospital, Sendai, Japan, from April 2020 to March 2023. Patient characteristics, tumor response, overall survival (OS), progression-free survival (PFS), and laboratory findings were reviewed. Associations between NLR, CAR, and survival outcomes were analyzed. The 1-year OS and 1-year PFS rates were 60.4% and 18.1%, respectively. The disease control rate was 66.2%. In multivariate analysis, low NLR (< 5) was significantly associated with better OS and PFS. NLR may be a predictive factor for OS and PFS in patients with R/M HNSCC treated with a pembrolizumab-containing regimen.
Similar content being viewed by others
Introduction
Treatment strategies for recurrent and metastatic head and neck cancer (R/M HNSCC) are evolving rapidly. Following the results of the CheckMate 141 trial, nivolumab, an anti-programmed death-1 (PD-1) antibody, was approved for the treatment of R/M HNSCC1. Moreover, the subsequent results of the KEYNOTE-048 trial led to the approval of pembrolizumab, another anti-PD-1 antibody, either alone or in combination with chemotherapy. In the KEYNOTE-048 trial, as in previous trials, PD-L1 expression (CPS) was investigated as a biomarker and was used as an indicator for patient selection. However, even in the population with a CPS > 20, the 1-year survival rate was only approximately 57%, which was only 11% higher than that in the control group2. Therefore, various studies have been conducted to predict the treatment outcomes of immune checkpoint inhibitors (ICI)3,4,5,6,7,8,9,10,11. These include serological and tissue-derived markers such as microsatellite instability and tumor mutational burden12,13. Hematological markers, which can be measured using peripheral blood, are easy to test and can provide immediate results14,15. Notably, recent reports indicate that tumor inflammation and low nutritional status are important prognostic factors in various carcinomas16,17,18. Moreover, the neutrophil-to-lymphocyte ratio (NLR) has been reported to be an independent prognostic factor for various tumors19,20,21. The C-reactive protein (CRP)-to-albumin ratio (CAR) has also been found to be an independent prognostic factor for various tumors22,23,24. Importantly, NLR and CAR values are negatively correlated with prognosis. However, most of these studies were conducted with chemotherapy alone or ICI alone, and few reports investigate the combination of ICI and chemotherapy. Similarly, few studies have examined the efficacy of NLR and CAR in patients with head R/M HNSCC treated with nivolumab4, and none have investigated the efficacy of CAR and NLR in combination with ICI and chemotherapy. In addition, no report has been published on the association between NLR and the efficacy of first-line pembrolizumab-containing regimens. Therefore, we performed a retrospective analysis of patients treated with pembrolizumab alone or in combination with chemotherapy to evaluate the usefulness of the CAR and NLR.
Methods
Patients and study design
We retrospectively reviewed the medical records of patients with R/M HNSCC who received a pembrolizumab-containing regimen (pembrolizumab with or without chemotherapy) as a first-line therapy at Tohoku University Hospital, Sendai, Japan, from April 2020 to March 2023. This study was performed in line with the principles of the Declaration of Helsinki. Because of the retrospective nature of the present study, informed consent was not obtained from each patient. The consent was waived by the Ethics Committee of the Faculty of Medicine of the Tohoku University School of Medicine (Approval No.2021-1-351). Patients were eligible for inclusion in this study if they met the following criteria: histologically confirmed recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) and age over 20 years. Patients were excluded if they had adenocarcinoma or adenoid cystic carcinoma, or if they had received prior immunotherapy. A total of 81 patients were screened, and 7 patients were excluded based on these criteria. The collected patient data included age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), primary and metastatic sites, CPS, progression-free survival (PFS), overall survival (OS), tumor response, body mass index, and laboratory findings.
OS was defined as the time from diagnosis to death from any cause. PFS was defined as the time from the initiation of treatment to the first occurrence of disease progression or death.
Statistical analysis
The response was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1. The adverse events (AEs) were evaluated and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. The NLR was calculated by dividing the absolute neutrophil counts by the absolute lymphocyte counts of a full blood count. The CAR was calculated by dividing the serum CRP by the serum albumin. The values calculated using statistical methods (e.g., receiver operating characteristic curves) varied for each cohort. Based on previous reports, we chose a fixed threshold value (NLR, 5; CAR, 0.3). Survival curves were analyzed using the Gehan-Breslow-Wilcoxon test. Additionally, univariate and multivariate analyses were performed using the Cox proportional hazards model to evaluate prognostic factors.
Statistical analyses of clinical outcomes were performed using the JMP Pro17 software (SAS Institute Inc., Cary, NC, USA).
Treatment exposure
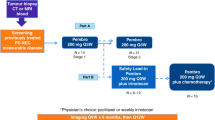
Pembrolizumab was administered at a dose of 200 mg/body every 3 weeks. The chemotherapy regimen combined with pembrolizumab consisted of 5-fluorouracil (continuous infusion of 1000 mg/m2 per day for 4 days) and either cisplatin (100 mg/m2 on day 1) or carboplatin (area under the curve of 5 mg/m2 on day 1) every 3 weeks. In the combination regimen, sequential maintenance therapy with pembrolizumab (200 mg/body) every 3 weeks was planned after up to six courses of pembrolizumab plus chemotherapy.
Results
Patient characteristics
Patient characteristics are presented in Table 1. A total of 74 patients with R/M HNSCC (59 males and 15 females) were enrolled in this study. The median age of the patients was 69 years (range: 21–88 years). The number of patients with ECOG PS 0, 1, and 2 or more was 21 (28.4%), 43 (58.1%), and 10 (13.5%), respectively. The primary tumor sites were the oral cavity in 27 patients (36.5%), nasopharynx in five (6.8%), oropharynx in seven (9.5%), hypopharynx in 24 (32.4%), larynx in three (4.1%), unknown primary origin in three (4.1%), and other locations in five (6.8%). In most oropharyngeal cancers, four of seven cases were positive for p16. CPS scores were ≥ 20 in 38 (51.4%) patients, 1–19 in 22 (29.7%), < 1 in 6 (8.1%), and not evaluated in 8 (10.8%). Metastatic sites included the lungs in 27 (36.5%), the liver in 3 (4.1%), lymph nodes in 38 (51.4%), and bones in 11 (14.9%) patients; 17 (23.0%) patients reported local recurrence. For the treatment regimen, 46 patients received pembrolizumab with chemotherapy, and 28 received pembrolizumab alone.
Treatment response and survival outcome
The best overall response was observed in 49 patients (66.2%) with measurable lesions. Partial response and stable disease were observed in 25 (33.8%) and 24 (32.4%) patients, respectively. The disease control rate was 66.2% (Table 2). The median OS was 17.4 months (95% confidence interval [CI], 10.3–20.0), and the median PFS was 5.5 months (95% CI 4.5–6.4) (Fig. 1). The 1-year survival and PFS rates were 60.4% and 18.1%, respectively.
Impact of the NLR
The OS and PFS curves stratified by NLR cutoff are shown in Fig. 2. Patients with a low NLR experienced significantly longer OS compared to those with a high NLR (median not reached vs. 10.4 months, respectively; p = 0.0140). Similarly, those with low NLR had significantly longer PFS than did patients with high NLR (median, 7.2 vs. 3.5 months, respectively; p = 0.0003). In patients treated with pembrolizumab plus chemotherapy, a low NLR was associated with a longer OS (p = 0.0171). In those treated with pembrolizumab alone, a low NLR was associated with a longer PFS (p = 0.0004). (Fig. 2C–F) In terms of tumor response, patients with a low NLR had a higher disease control rate than did those with a high NLR (83.8% vs. 47.2%; p = 0.0010).
Kaplan–Meier survival curves for OS and PFS according to the NLR. (A, B) Kaplan–Meier curves for all regimens: OS (A) and PFS (B). (C, D) Kaplan–Meier curves for pembrolizumab plus chemotherapy: OS (C) and PFS (D). (E, F) Kaplan–Meier curves for pembrolizumab monotherapy: OS (E) and PFS (F). OS: overall survival; PFS: progression-free survival; NLR: neutrophil–lymphocyte ratio.
Impact of the CAR
The OS and PFS curves stratified by the CAR cutoff are shown in Fig. 3. Patients with low CAR had significantly longer OS than did those with high CAR (median, 18.8 vs. 10.3 months, respectively; p = 0.0241). Those with low CAR had significantly longer PFS than did patients with high CAR (median, 6.3 vs. 3.8 months, respectively; p = 0.0027). In patients treated with pembrolizumab alone, a low CAR was associated with longer PFS (p = 0.0055) (Fig. 3C–F). In terms of tumor response, patients with a low CAR had a higher disease control rate than did those with a high CAR (84.9% vs. 50.0%; p = 0.0018).
Kaplan–Meier survival curves for OS and PFS according to the CAR. (A, B) Kaplan–Meier Curves for All Regimens: OS (A) and PFS (B). (C, D) Kaplan–Meier Curves for Pembrolizumab Plus Chemotherapy: OS (C) and PFS (D). (E, F) Kaplan–Meier Curves for Pembrolizumab Monotherapy: OS (E) and PFS (F). OS: overall survival; PFS: progression-free survival; CAR: C-reactive protein-to-albumin ratio.
Univariate and multivariate analysis
In the univariate analysis of OS, ECOG PS (0–1 vs. ≥ 2, HR, 0.35; 95% CI 0.15–0.80; p = 0.0126) and NLR (< 5 vs. ≥ 5, HR, 0.38; 95% CI 0.20–0.73; p = 0.0179) were significantly associated with OS. When multivariate analysis was performed with these two factors, both better ECOG PS (HR, 0.37; 95% CI 0.15–0.86; p = 0.0207) and low NLR (HR, 0.39; 95% CI 0.21–0.75; p = 0.049) were associated with better OS. (Table 3) In the univariate analysis of PFS, ECOG PS (0–1 vs. ≧2, HR, 0.47; 95% CI 0.24–0.94; p = 0.0323), CAR (< 0.3 vs. ≥ 0.3, HR, 0.53; 95% CI 0.31–0.91; p = 0.0199) and NLR (< 5 vs. ≥ 5, HR, 0.42; 95% CI 0.25–0.72; p = 0.0015) were significantly associated with PFS. When multivariate analysis was performed with these factors, a low NLR (HR, 0.48; 95% CI 0.26–0.89; p = 0.0216) was independently associated with better PFS (Table 3).
Discussion
In this report, we found that pretreatment NLR was a significant prognostic factor in patients with R/M HNSCC treated with a pembrolizumab-containing regimen as a first-line therapy. Pretreatment NLR and ECOG PS have both been previously reported to be significant prognostic factors for patients with R/M HNSCC treated with nivolumab monotherapy6,21,25. To the best of our knowledge, this is the first report to investigate the prognostic impact of NLR in combination with pembrolizumab and chemotherapy in patients with R/M HNSCC. We found a correlation between NLR and CRP (r = 0.47; p < 0.0001), albumin (r = − 0.40; p = 0.0004), and CAR (r = 0.50; p < 0.0001) (Supplementary Fig. 1). Nutrition status scores, such as CAR or Glasgow Prognostic Score (GPS), have also been reported as prognostic factors for survival in HNSCC26,27,28. This suggests that nutritional and immune status are closely related in patients with R/M HNSCC. However, there was no significant association between CAR and survival (OS and PFS) in multivariate analysis, and GPS did not predict survival in this study. In a previous report, the use of immune-modified GPS (imGPS) was demonstrated in patients with HNSCC, including all clinical stages11. The imGPS is a novel score created by combining the mGPS with the peripheral lymphocyte count and is a more useful prognostic marker. However, in our cohort, including only stage IV patients, imGPS was not associated with survival. The clinical stage differences between these cohorts may be a contributing factor. This discrepancy indicates that different prognostic tools are effective for different stages and treatment types. We observed a favorable survival benefit of the pembrolizumab-containing regimen with a 1-year OS of 60.4% and 1-year PFS of 18.1% (Fig. 1), which were comparable to those of KEYNOTE-048 (53.0% and 16.7%, respectively) and previously reported real-world data in Japanese patients (64.5% and 54.9%, 58.8% and 20.2%, respectively)29,30. In particular, patients with a low NLR showed a 1-year OS of 72.1% and a 1-year PFS of 48.2%. This indicates that NLR is a predictor of a good prognosis. Patients with a low NRL showed significantly higher disease control rates (83.8% vs. 48.7%; p = 0.0014). Moreover, low CAR patients had a significantly higher rate (85.3% vs. 50.0%; p = 0.0014) (Supplementary Table 1). Neutrophils produce cytokines and growth factors, such as interleukin (IL)-6, IL-8, vascular endothelial growth factor, and epidermal growth factor, as well as proteases that promote tumor cell invasion and metastasis31. In contrast, lymphocytes release cytokines that promote tumor cell apoptosis and are involved in monitoring the host immune response32,33,34. Previous studies have also shown that inflammation promotes tumor cell development, proliferation, hematogenous metastasis, and survival. In our analysis, the NLR was correlated with CRP and albumin levels, suggesting that NLR reflects not only immune status but also inflammation and nutritional status. NLR has been reported to correlate with prognosis in various carcinomas treated with ICIs, as well as with the therapeutic efficacy of cytotoxic anticancer agents19,20,22,35. Therefore, NLR may be a useful prognostic factor not only in patients treated with ICIs alone but also in patients treated with ICI in combination with chemotherapy. Several studies have reported that the kinetics of on-treatment CRP and fibrinogen may be useful in predicting the efficacy of ICI36,37. Given that NLR correlates with CRP and albumin levels, it is also plausible that the kinetics of NLR could provide valuable prognostic information. Further investigation into the potential of NLR kinetics as a predictive marker for ICI treatment response is warranted. This study had several limitations. First, this was a retrospective study conducted at a single institution with a small sample size. Second, there were several of censored cases due to insufficient observation periods. The results from this study are significant because the further expansion of indication is expected to increase the opportunities for combination therapy with ICI and chemotherapy.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
Saijo, K. et al. Depth of response may predict clinical outcome in patients with recurrent/metastatic head and neck cancer treated with pembrolizumab-containing regimens. Front. Oncol. 13, 1230731 (2023).
Tanoue, K. et al. Predictive impact of C-reactive protein to albumin ratio for recurrent or metastatic head and neck squamous cell carcinoma receiving nivolumab. Sci. Rep. 11, 2741 (2021).
Matsuo, M. et al. Inflammation-based prognostic score as a prognostic biomarker in patients with recurrent and/or metastatic head and neck squamous cell carcinoma treated with nivolumab therapy. In Vivo 36, 907–917 (2022).
Takenaka, Y., Oya, R., Takemoto, N. & Inohara, H. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck squamous cell carcinoma treated with immune checkpoint inhibitors: Meta-analysis. Head Neck 44, 1237–1245 (2022).
Ueta, R. et al. Antibiotics may interfere with nivolumab efficacy in patients with head and neck squamous cell carcinoma. Oncology (2023).
Pan, C. et al. Neutrophil to lymphocyte ratio and peripheral blood biomarkers correlate with survival outcomes but not response among head and neck and salivary cancer treated with pembrolizumab and vorinostat. Head Neck 45, 391–397 (2023).
Suzuki, S. et al. Association of pretreatment neutrophil-to-eosinophil ratio with clinical outcomes in patients with recurrent or metastatic head and neck squamous cell carcinoma treated with nivolumab. Cancer Manag. Res. 14, 3293–3302 (2022).
Rosculet, N. et al. Neutrophil-to-lymphocyte ratio: Prognostic indicator for head and neck squamous cell carcinoma. Head Neck 39, 662–667 (2017).
Terazawa, K., Ohashi, T., Shibata, H., Ishihara, T. & Ogawa, T. Immune-modified Glasgow prognostic score: A new prognostic marker for head and neck cancer. Head Neck 44, 2555–2563 (2022).
Lenz, H. J. et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: The phase II CheckMate 142 study. J. Clin. Oncol. 40, 161–170 (2022).
Valero, C. et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 12, 729 (2021).
Voutsadakis, I. A. Prediction of Immune checkpoint inhibitors benefit from routinely measurable peripheral blood parameters. Chin. Clin. Oncol. 9, 19 (2020).
Minohara, K. et al. Novel prognostic score for recurrent or metastatic head and neck cancer patients treated with Nivolumab. Sci. Rep. 11, 16992 (2021).
Crusz, S. M. & Balkwill, F. R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 12, 584–596 (2015).
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 4, 17105 (2018).
Kao, H. K. et al. Nomogram based on albumin and neutrophil-to-lymphocyte ratio for predicting the prognosis of patients with oral cavity squamous cell carcinoma. Sci. Rep. 8, 13081 (2018).
Lino-Silva, L. S., Salcedo-Hernandez, R. A., Garcia-Perez, L., Meneses-Garcia, A. & Zepeda-Najar, C. Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res. 27, 140–144 (2017).
Diem, S. et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 111, 176–181 (2017).
Yasumatsu, R. et al. Monitoring the neutrophil-to-lymphocyte ratio may be useful for predicting the anticancer effect of nivolumab in recurrent or metastatic head and neck cancer. Head Neck 41, 2610–2618 (2019).
Bilen, M. A. et al. Association between pretreatment neutrophil-to-lymphocyte ratio and outcome of patients with metastatic renal-cell carcinoma treated with nivolumab. Clin. Genitourin. Cancer 16, e563–e575 (2018).
Ishizuka, M. et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann. Surg. Oncol. 23, 900–907 (2016).
Wei, X. L. et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: The C-reactive protein/albumin ratio. BMC Cancer 15, 350 (2015).
Ueda, T. et al. Baseline neutrophil-to-lymphocyte ratio (NLR) is associated with clinical outcome in recurrent or metastatic head and neck cancer patients treated with nivolumab. Acta Otolaryngol. 140, 181–187 (2020).
Luan, C. W. et al. Prognostic value of C-reactive protein-to-albumin ratio in head and neck cancer: A meta-analysis. Diagnostics 11 (2021).
Ueki, Y. et al. Predicting the treatment outcome of nivolumab in recurrent or metastatic head and neck squamous cell carcinoma: Prognostic value of combined performance status and modified Glasgow prognostic score. Eur. Arch. Otorhinolaryngol. 277, 2341–2347 (2020).
Chikuie, N. et al. Baseline neutrophil-to-lymphocyte ratio and glasgow prognostic score are associated with clinical outcome in patients with recurrent or metastatic head and neck squamous cell carcinoma treated with nivolumab. Acta Med. Okayama 75, 335–343 (2021).
Nakano, T. et al. Real-world experience with pembrolizumab for advanced-stage head and neck cancer patients: A retrospective, multicenter study. Anticancer Res. 42, 3653–3664 (2022).
Sano, D. et al. Real-world therapeutic outcomes of the pembrolizumab regimen as first-line therapy for recurrent/metastatic squamous cell carcinoma of the head and neck: A single-center retrospective cohort study in Japan. Anticancer Res. 42, 4477–4484 (2022).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell. 140, 883–899 (2010).
Carnevale, S., Ghasemi, S., Rigatelli, A. & Jaillon, S. The complexity of neutrophils in health and disease: Focus on cancer. Semin. Immunol. 48, 101409 (2020).
Lippitz, B. E. & Harris, R. A. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology 5, e1093722 (2016).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011).
Bagley, S. J. et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 106, 1–7 (2017).
Haas, M. et al. Early on-treatment C-reactive protein and its kinetics predict survival and response in recurrent and/or metastatic head and neck cancer patients receiving first-line pembrolizumab. Invest. New Drugs 41, 727–736 (2023).
Nenclares, P. et al. On-treatment immune prognostic score for patients with relapsed and/or metastatic head and neck squamous cell carcinoma treated with immunotherapy. J. Immunother. Cancer 9 (2021).
Acknowledgements
The authors thank the patients and their families for participating in this study, and the medical staff for their respective contributions to the treatment of patients.
Author information
Authors and Affiliations
Contributions
Y.K. and K.S. designed the study. Y.K., K.S., and H.I. carried out the statistical analysis and discussed it with. R.U., R.N., K.S, Y.Y., S.T., K.O., K.K., H.S., and M.T. carried out the clinical study. Y.K., K.S., and C.I. wrote the paper, and all authors critically read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Ethics Committee of the Faculty of Medicine of the Tohoku University School of Medicine (Approval No.2021-1-351) and performed in line with the ethical standards described in the Declaration of Helsinki.
Patient consent statement
Patient consent for this study was obtained with the opt-out method.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kasahara, Y., Saijo, K., Ueta, R. et al. Pretreatment neutrophil–lymphocyte ratio as a prognostic factor in recurrent/metastatic head and neck cancer treated with pembrolizumab. Sci Rep 14, 28255 (2024). https://doi.org/10.1038/s41598-024-79130-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79130-7
Keywords
This article is cited by
-
Prognostic significance of the CALLY index for cancer risk and survival: evidence from NHANES 2001–2018
World Journal of Surgical Oncology (2025)
-
Relationship between the effectiveness of pembrolizumab monotherapy and ABO blood type in recurrent and metastatic squamous cell carcinoma of the head and neck
Discover Oncology (2025)