Abstract
Cyclooxygenase (COX) and lipoxygenase (LOX) enzymes play a pivotal role in producing pro-inflammatory eicosanoids, including prostaglandins (PGs) and leukotrienes (LTs), in the inflammation process. Mitragynine is a primary alkaloid contained in the kratom’s leaves and has been reported to show anti-inflammatory activity by suppressing COX-2 mRNA translation to lowering PGs synthesis. In this study, the Kratom’s alkaloid extract containing ~ 46% mitragynine was found to exhibit dual inhibition activity towards COX-2/5-LOX enzymes at concentrations below 25 ppm in the LPS-induced RAW 264.7 macrophage cells. At these levels, no cell toxicity was observed while the cells became death (e.g., 10–46% viability at 50–100 ppm) and only COX-2 inhibition activity was observed after exposed with more than 25 ppm of alkaloid extract. In contrast, the methanolic-crude extract of Kratom’s leaf containing ~ 5% mitragynine showed no inhibition toward COX-2/5-LOX enzymes and did not toxic onto the cells, even after treated at 100 ppm. The alkaloid extract suppressed several antiinflammation parameters, including ROS (64% reduction at 25 ppm), NO (30% reduction at 25 ppm), TNF-α (~ 50% reduction at 25 ppm), and IL-6 production (60% reduction at 6.25 ppm). In silico molecular studies indicated strong binding affinity of Kratom alkaloids to COX-2 and 5-LOX active sites, supporting the Kratom’s alkaloids to have great potential dual inhibition activity towards COX-2/5-LOX enzymes and to be developed as a safer NSAIDs with fewer side effects.
Similar content being viewed by others
Introduction
Inflammation is a cellular defense response against toxic or foreign substances and involves the elimination of damaged cells to heal injured tissues or organs1. It is characterized by activating signaling pathways that regulate the amounts of inflammatory mediators, including cytokines, chemokines, and lipid mediators in the local tissue cells2. The process results in a pain response, in which people often use non-steroidal anti-inflammatory drugs (NSAIDs) to treat the pain3. These drugs commonly act to alleviate inflammatory symptoms by targeting cyclooxygenase enzymes (COX-1 and COX-2) in the arachidonic acid (AA) metabolic pathway. However, the long-term use of NSAIDs often give side effects, including gastrointestinal issues4, hepatotoxicity as well as injury5, widespread edema6, and an increased propensity to bleed7. For example, rofecoxib (Vioxx™) and valdecoxib (Bextra™) are two COX-2 inhibitors that were removed from the market due to their severe adverse effects, including cardiovascular disease, risk of stroke and cardiac arrest8.
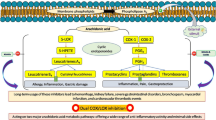
AA is the primary precursor for producing a cascade of pro-inflammatory metabolites in the inflammatory pathways. This process involves the enzymatic cleavage of membrane-bound AA (arachidonyl phospholipid) by phospholipase-A2, releasing free AA that is accessible to COX and Lipoxygenase (LOX) enzymes9. COX and LOX are widely recognized as pro-inflammatory enzymes that play a crucial role in producing pro-inflammatory eicosanoids, including prostaglandins (PGs) and leukotrienes (LTs). COX plays a vital role in inducing the formation of lipid mediators10. Particularly, COX-2 catalyzes the oxidation of arachidonate into prostaglandins G2 (PGE2) during inflammation11. The released arachidonate can also be further oxidized to produce a precursor for other prostaglandins, such as prostaglandin H2 (PGH2) and thromboxane. The overexpression of COX-2 is reported to implicate human cancers12.
In addition, the AA pathway produces LTs via the LOX pathway, mediated by the 5-LOX enzyme. LTs are a distinct group of AA derivatives that also have a notable impact on the process of inflammation. They are synthesized by LOX enzymes via hydroperoxy eicosatetraenoic acids (HPETEs)13. Among several LOX enzymes, 5-LOX plays a crucial role in the biosynthetic pathway, releasing 5-hydroperoxy eicosatetraenoic acid and leukotriene A4 (LTA4). Thereafter, 5-LOX enzyme converts LTA4 to lipoxins (LXs); i.e., LXA4 and LXB414. LXs and other specialized pro-resolving mediators are synthesized and released to cease and overcome inflammation, resulting in tissue repair and regeneration15. The 5-LOX route terminates upon the release of Leukotriene B4 (LTB4), which is a by-product that has a role in various inflammatory and allergic diseases, including atherosclerosis, cancer, and cardiovascular conditions13.
During the onset of inflammation, LTs and PGs are abundant14,15. The inhibition of COX enzymes (COX-1, COX-2), as typically performed by NSAIDs, results in an upregulation of the AA pathway. An inherent limitation of COX-2 inhibitors is their concurrent inhibition of COX-1, as selective COX-2 inhibitors typically also suppress COX-19. As a result, this condition increases the availability of AA, stimulating LOX enzymes to increase LT production16. In this respect, the decreased production of PGs by COX inhibitors shifts AA metabolism to the alternative LOXs pathway. The AA-LOXs pathway produces LTs, and consequently, the use of NSAIDs is often linked to asthma and allergic reactions8. This condition is reportedly associated with undesirable side effects and the severity of its disorders17. Furthermore, the long-term inhibition of COX-1 and COX-2 by non-selective NSAIDs leads to a decrease in the synthesis of PGs, which in turn impairs the function of the mucosa and causes damage to the gastrointestinal tract. Moreover, COX-2 is crucial for controlling renal function, as such, the use of COX-2 inhibitors can lead to significant adverse effects to individuals who are at risk of renal ischemia, liver cirrhosis, renal insufficiency, cardiovascular diseases, and congestive heart failure. Therefore, suppressing both COX-2 and 5-LOX to inhibit the production of PGs and LTs simultaneously may improve the efficacy of anti-inflammatory agents with fewer side effects. This approach has been considered an appealing choice for creating less risky NSAIDs18.
Currently, much effort is being invested in exploring anti-inflammatory compounds with dual inhibition activity targeting both COX-2 and 5-LOX enzymes, i.e. hybrid anti-inflammatory function. This strategy has been targeted to provide safer NSAIDS, wherein the active drugs exhibit good efficacy as anti-inflammatories with minimal side effects16. For instance, a novel anti-arthritic γ-sultam S-2474 was reported to display dual inhibition of COX-2/5-LOX without ulcerogenic effects in rats19. Flavocoxid showed anti-inflammatory benefits by reducing the number of neuronal losses in Alzheimer’s mice model due to its ability to inhibit COX-2 and 5-LOX enzymes20. Quercetin has recently been reported to demonstrate the ability to hinder the LDL-induced inflammatory process that leads to atherosclerosis by reducing the activity of the Toll-like Receptor or the nuclear factor kappa B (TLR/NF-κB) pathway, including COX-2 and 5-LOX21.
Medicinal plants have been used for decades and are to date considered to be the origin of modern medicines. It is regarded as a source of active compounds discovery, which has been proven to elicit significant scientific output22. Furthermore, medicinal plants are considered safe therapy and support the concept of medicinal plant uses for diseases, such as anti-inflammation, antioxidant, and antiviral agents, due to their long history of use and tradition (Dal23). According to a recent report24, more than 12 plant-derived natural products approved for therapeutic use in the last thirty years (1984–2014), including artemisinin for malaria treatment, originally derived from Artemisia annua L.25, masoprocol for cancer chemotherapy, isolated from Larrea tridentata26, and ingenol mebutate to treat actinic keratosis, isolated from Euphorbia peplus L.27. Moreover, one of medicinal plant extracts that have the potential to cure diseases is Mitragyna speciosa Korth.
Mitragyna speciosa Korth (family Rubiaceae); commonly known as ‘Kratom’, ‘Ithang’, or ‘Thom’ in Thailand, and ‘Ketum’ or ‘biak-biak’ in Malaysia; is a native tropical tree of Southeast Asia. It can be found in the northern part of Peninsular Malaysia, as well as Central and South Thailand28, and Indonesia. This plant has traditionally been used for its aqueous leaf extract to treat minor illnesses such as fever, diabetes, diarrhea, pain relief, wound healing, and opioid withdrawal symptoms29,30,31,32. To date, kratom or its ingredients, mytragynine or 7-OH mytragynine, has not been approved by the US Food and Drug Administration (FDA) due to its abuse potentials, such as intravenous self-administration (IV SA) and intracranial self-stimulation (ICSS). However, a recent cross-sectional human study reported that kratom extracts exerts low abuse liability29. On the other hand, kratom has attracted much attention in the United States of America and Europe due to its recreational effect and potential medical applications in relieving chronic pain, opioid use disorder symptoms, alcohol withdrawal, anxiety, and depression30,33. The majority of kratom users consume the natural product orally by chewing fresh leaves, preparing tea using powdered leaves through decoction, or utilizing organic extract in the form of capsules, tablets, and energy beverages34. However, the information about those kratom-based products’ effects on health, is still limited.
Kratom is well known for its analgesic properties35,36,36,36,39,40. This activity is usually related to anti-inflammatory activities. Generally, people use kratom as a leaf powder or prepared as a tea for pain reliever, which contains lesser mitragynine than the methanolic extracts41. Nonetheless, no studies have been conducted on the anti-inflammatory effect of Kratom alkaloids or crude extracts derived from the Kratom leaf. In spite of the fact that, an in vivo study discovered that mitragynine exhibits an anti-inflammatory effect in chronic and acute inflammation models by inhibiting COX-2 mRNA translation and lowering PGs synthesis11,37,42. Additionally, the effects of kratom extract on 5-LOX enzyme activity during inflammation still remain unclear. In this study, the inhibitory activity of Kratom extracts, i.e., methanolic-crude and alkaloid extract, against COX-2 and 5-LOX enzymes was evaluated in vitro using RAW 264.7 macrophage cells induced with Lipopolysaccharides (LPS). Kratom extracts exhibit antioxidant and anti-inflammatory properties against pro-inflammatory mediators such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), suggesting their potential for synergistic modulation. The study of the dual inhibition of COX-2/5-LOX is expected to shed light on Kratom’s potential as a safer anti-inflammatory drug candidate.
Materials and methods
Samples preparation
Kratom leaves were collected from the local plantation areas in Kapuas Hulu, West Borneo, Indonesia. This plant was identified as Mitragyna speciosa Korth. by the Directorate of Scientific Collection Management, National Research and Innovation Agency (BRIN), Republic of Indonesia (B-3966/II.6.2/DI.05.07/10/2022) and deposited at Herbarium Bogoriense, BRIN. Kratom leaves were dried in the sun until the moisture of the biomass was less than 10%. After the leaves dried, a local industry used a good manufacturing process to powder them, separating the bone leaves and producing green kratom leaf powder. The study was conducted following the World Health Organization Quality Control Methods for Medicinal Plant Materials 1998 and approved by The Research Organization for Health, BRIN Republic of Indonesia. Kratom extracts were prepared according to our previous protocols41, resulting in two types of extract, i.e., methanolic-crude and alkaloid extract. Analysis of alkaloid compounds was performed using the chromatographic technique described in our previous report as well. The typical chromatograms are depicted in Fig. 1. Mitragynine was confirmed using liquid chromatographic-high resolution mass spectrometry (LC-HRMS) and proton nuclear magnetic resonance (1H NMR) spectroscopic analysis (Supplementary Information). LC-HRMS was conducted following the protocol described by other study43. The 1H NMR spectrum was recorded on a Bruker AVANCE III 500 spectrometer (Billerica, MA, USA). The sample was dissolved in deuterated methanol and scanned for 62 scans.
Antioxidant activities
Free radical scavenger activity
The ABTS (2,2ʹ-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid) scavenging activity was determined using a modified ABTS+ radical cation decolorization assay44. In brief, about 5 mL of 7 mM ABTS aqueous solution (Merck, USA) was reacted with 88 μL mM of 140 nM potassium persulfate solution (K2S2O8) (Sigma-Aldrich, USA). The mixture was incubated in a dark place at room temperature for 12–16 h to obtain ABTS+ solution. The prepared ABTS+ solution was then diluted with an analytical grade ethanol (Sigma-Aldrich, USA) to obtain an initial absorbance of 0.7 at 734 nm. Thereafter, an approximately 20 µL solution containing the extract or standard (Trolox, Sigma-Aldrich, USA) was added to 280 µL of the ABTS+-ethanolic solution. The mixture was transferred to a 96-well plate and incubated at 30 °C for 5 min in the dark. Afterward, the absorbance was measured at 734 nm using a microplate reader (Tecan Spectrophotometer, Tecan Group Ltd, Switzerland). The percentage inhibition of free radical scavenging activity was calculated using the following equation:
where AB = absorbance of the blank sample, and AE = absorbance of the extracts.
Ferric reducing antioxidant power (FRAP) assay
The FRAP method evaluates a sample’s capacity to convert Fe3+ ions in Fe3+-TPTZ complexes (ferric-2,4,6-tripyridyl-s-triazine) into Fe2+ ions44. The FRAP method was carried out with a slight modification. In each well of a 96-well plate, approximately 20 µL of extract or ascorbic acid standard solution was mixed with 280 µL of FRAP dye solution, i.e., a mixture of sodium acetate 300 mM, TPTZ solution and Fe solution in a ratio of 10:1:1. Subsequently, the solution was incubated at 37 °C for 10 min. The absorbance was measured at 593 nm using a microplate reader (Tecan Spectrophotometer, Tecan Group Ltd, Switzerland). Ascorbic acid (Sigma-Aldrich, USA) was plotted at several concentrations ranging from of 0–50 µg/mL to make a standard curve. All data were expressed as ascorbic acid equivalents (AAE) per gram of dry weight (d.w.) kratom leaf (mg AAE/g d.w.) using standard curve equation.
Total phenolic content (TPC)
TPC was determined using a modified Folin-Ciocalteu spectrophotometry method44. In each well of a 96-well plate, 25 µL of extract was mixed with 25 µL of Folin-Ciocalteu reagent (Sigma-Aldrich, USA) solution, which was diluted with water at a ratio of 1:3, and 200 µL of water. The mixture was then incubated at room temperature for 5 min. Following incubation, the reaction mixture was basified by adding 25 μL of 10% sodium carbonate and incubated for another 60 min in the dark. The absorbance was then measured at 765 nm using a spectrophotometric plate reader (Tecan Spectrophotometer; Tecan Group Ltd, Switzerland). Gallic acid (Sigma-Aldrich, USA) was plotted at several concentrations ranging from 0 to 200 μg/mL to create a standard curve. The TPC of the extracts was expressed as gallic acid equivalents (GAE) per gram (dried weight; d.w.) kratom leaf (mg GAE/g d.w.) using a standard curve equation.
Anti-inflammatory activities
Cell culture
A RAW 264.7 macrophage cell line was purchased from the European Collection of Authenticated Cell Culture (ECACC; 91062702). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Thermo Fisher Scientific, NY, USA) with 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin solution (Gibco, Thermo Fisher Scientific) at 37 °C in 5% CO2 incubator. The RAW 264.7 cells were sub cultured and plated until 70–80% confluency for assay.
Cell viability assay
The cell viability test was carried out using a modified MTT assay45 involving the conversion of the water-soluble yellow dye MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolilum bromide] (Sigma-Aldrich, USA). The RAW 264.7 macrophage cells were seeded, with 100 µL per well, onto 96-well microplates at 1 × 105 cells mL−1 and cultured for 24 h in a humidified 5% CO2 incubator at 37 °C to facilitate cell attachment (adherent cells). After that, the unattached cells (nonadherent cells) were removed carefully. The cells were subsequently treated with crude or alkaloid extracts in the final concentrations of 25, 50, 100, and 200 ppm (final media volume of 200 µL). The cells were then incubated in a humidified 5% CO2 incubator at 37 °C for 24 h. Thereafter, about 20 µL of MTT reagent (5 mg mL−1) was added to each well and incubated under 5% CO2 at 37 °C for 4 h in the dark to form formazan crystals. The formazan crystals were dissolved in 150 µL dimethyl sulfoxide (DMSO) (Sigma-Aldrich, USA), and the mixture was incubated in the dark for another 15 min at 30 °C. Cell viability was evaluated by measuring the absorbance at 570 nm using a microplate reader and determined using the formula below:
Determination of LPS and H2O2-induced intracellular ROS and NO production
Reactive Oxygen Species (ROS) levels were measured by assessing the fluorescent signal generated from oxidized 2ʹ,7ʹ-dichlorofluorescein diacetate (DCFH-DA), using a modified approach from a previous study46. The RAW 264.7 macrophage cells were seeded into 96-well plates at a density of 5 × 104 cells per well (100 μL per well). The cells were pre-treated with different concentrations of the sample solutions; i.e., crude (100, 50, and 25 ppm) and alkaloid extracts (25, 12.5, and 6.25 ppm); and the mixture was incubated for 1 h. The mixture was then treated with 1 μg mL−1 of LPS (Sigma Aldrich, USA) and directly incubated for another 24 h. After the LPS induction, the treated cells were washed with Phosphate Buffer Saline (PBS; Sigma Aldrich, USA), and 100 μL of 10 uM DCFH-DA (Sigma Aldrich, USA) was added. The mixture was incubated in the dark at 37 °C for 45 min. Thereafter, the supernatant was gently removed and washed twice using PBS. Subsequently, the cells were incubated with 100 uM H2O2 (Sigma Aldrich, USA) for 1 h. The fluorescence was determined using Cytation 5 instrument (Biotek Instruments, Agilent, USA) at excitation and emission wavelengths of 485 nm and 528 nm, respectively. The medium and LPS-induced medium served as negative and positive control, respectively.
The nitric oxide (NO) production assay was performed using a modified method from a previous study47. As described above, the treated cells were incubated with LPS for 24 h. Then, the medium from the LPS-induced treated cells was transferred to a centrifuge tube. The supernatant collected from LPS-treated cells was used to measure the levels of NO, TNF-α and IL-6. The supernatant was transferred 100 μL to another 96 well plate, followed by addition of 100 μL Gries reagent (Sigma Aldrich, USA). The mixture was incubated for 10 min and the NO production was measured at an absorbance of 540 nm.
Measurement of LPS-induced pro-inflammatory cytokines production (TNF-α and IL-6)
The supernatant obtained from the LPS induction (section determination of LPS and H2O2-induced Intracellular ROS and NO production) was used to assess TNF-α and IL-6 levels in the samples using a mouse TNF-α and IL-6 ELISA kit (Elabscience, China). The protein content was first calculated using the Bradford reagent. All procedures were carried out in accordance with the manual instructions for the ELISA kit to determine the TNF-α and IL-6 levels in samples.
Measurement of COX-2 and 5-LOX activities
The RAW 264.7 macrophage cells were cultured and treated with LPS (as described above in determination of LPS and H2O2-induced Intracellular ROS and NO production). The cells were harvested, washed using PBS, and then lysed using RIPA Buffer (Sigma-aldrich, USA) as the lysis buffer. The protein content in the cell lysates was measured using Bradford reagent, while COX-2 and 5-LOX activities were quantified using an ELISA Kit (Elabscience, China). All reagents, including washing buffer, substrate solution, stop solution, and standards, were prepared according to the manufacturer’s protocol.
Molecular docking simulation
The three-dimensional (3D) protein structures of 5-LOX and COX-2 were retrieved from the Protein Data Bank (PDB) using their respective PDB IDs 3V99 and 5IKR. Selection criteria for the crystal structures included considerations such as, resolution, presence of the protein–ligand complex, active site residues, and source organism. Marvinsketch was utilized to model the 3D structures of the ligands, identify the most stable conformation, and save them in mol2 format. Molecular docking analyses were performed using Molegro Virtual Docker 6.0, taking into account the cavities in the protein structure that could serve as active sites for complex formation through bonding with native ligands. The protein–ligand complexes were evaluated based on their binding free energies, represented as docking scores. Post-docking analysis was conducted using BIOVIA Discovery Studio 2021.
Statistical analysis
The statistical analysis was performed using OriginPro 2019 software (OriginLab, Japan), and all graphs were created using the same tool. Normality was confirmed statistically and justified using parametric analysis. The antioxidant assay values were analyzed using two-sample student t-tests, with p-values < 0.05 considered significant. The cell-based assays’ values were analyzed using one-way ANOVA followed by Tukey’s post hoc test, with p < 0.05 considered significant.
Results
Antioxidant activities of kratom extracts
It is known that ROS could be produced as a by-product of AA metabolism catalyzed by 5-LOX and COX-2 enzymes48. An oxidative defense system (antioxidant) in living organisms maintains the level of ROS, allowing ROS to perform their proper functions without negatively impacting the body. As a result, antioxidant-containing components can ensure an adequate amount of ROS to carry out signaling and physiological tasks without causing inflammation49,50,51. In light of this, the antioxidant activity of Kratom extracts was initially assessed to determine their efficiency in stabilizing ROS using several methods. Figure 2a shows that either crude or alkaloid extract exhibited significant activity, with 94% free radical scavenging activity based on the ABTS assay at a concentration of 1000 ppm. These two results indicate that both extracts contain active compounds with high antioxidant activity. Interestingly, the FRAP assay revealed that the crude extract was 20 times more effective at scavenging free radical ferric iron (Fe3+) than the alkaloid extract (Fig. 2b). TPC measurements supported this observation, revealing a high TPC content in the crude extract of Kratom leaf (7224 ± 46.4 mg GAE g−1) (Fig. 2c).
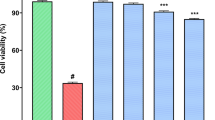
Cytotoxicity of kratom extracts on RAW 624.7 macrophage cells
Before assessing the anti-inflammatory activities of Kratom extracts in RAW 264.7 macrophage cells, we evaluated their cytotoxicity using an MTT assay. This assay was conducted to determine suitable concentrations of the extracts that do not kill the cells. As shown in Fig. 3, the crude extract was not cytotoxic on the RAW 264.7 cell line (91–100% cell viability) at concentrations of 25–200 ppm. In contrast, alkaloid extract exhibits concentration-dependent cytotoxicity. No toxicity was observed at 25 ppm (~ 100% cell viability), but at 50–100 ppm, cell viability decreased by 10–46%. Moreover, the alkaloid extracts completely killed RAW 264.7 macrophage cells at 200 ppm. Alkaloids are widely recognized as highly active natural products, and their biological activity has already been shown to be advantageous as therapeutic agents when used in sufficient quantities52. As a result, the anti- inflammatory efficacy of crude and alkaloid extracts was evaluated at 25–100 ppm and 6.25–25 ppm, respectively.
The cytotoxicity of Kratom extract in RAW 264.7 macrophage cells (a) and the cells images before (control media (b)) and after treated with the Kratom extracts (crude extract 50 ppm (c), alkaloid extract 50 ppm (d), alkaloid extract 25 ppm (e)). The percent viability data was processed statistically using One-way ANOVA followed by Tukey’s post hoc test; i.e., *p < 0.05 signifying significant difference compared to control media (without any treatment); #p < 0.05 indicating significant difference from its group (either within crude extract or within alkaloid extract).
Anti-inflammatory activity of kratom extracts
The effect on intracellular oxidative stress
In the previous discussion, Kratom extracts demonstrated significant antioxidant activity at a concentration of 1000 ppm. Given that ROS are produced during metabolic reactions at the cellular level, we evaluated the activity of Kratom extracts in suppressing ROS production in RAW 264.7 macrophage cells. The crude and alkaloid extract concentrations were chosen at 25–100 ppm and 6.25–25 ppm, respectively, to minimize their detrimental influence on cells as previously stated. Oxidative stress was induced by adding H2O2 into RAW 264.7 macrophage cells treated by LPS.
The addition of H2O2 exacerbates cellular oxidative stress as shown by an increase in ROS levels compared with control media (Fig. 4a). Exposing the cells to Kratom extracts significantly reduced ROS production in a concentration-dependent manner. When the cells were exposed to maximum concentrations of crude and alkaloid extract (100 ppm crude and 25 ppm alkaloid), ROS production was significantly reduced (50–64%). Fluorescence microscopic images revealed that the cells were destroyed after being exposed to the extracts (Fig. 4c,d). Interestingly, the results indicated that the alkaloid extract is four times more effective at decreasing ROS generation than the crude extract.
ROS (a) and NO (b) production assessment in LPS-induced RAW 264.7 macrophage cells after treatment with the crude and alkaloid extracts derived from Kratom leaf. The fluorescence images of ROS intensity in the LPS-induced cells without (c) and with the treatment using alkaloid extracts at 25 ppm (d). The ROS and NO production data were processed statistically using One-way ANOVA followed by Tukey’s post hoc test (*p < 0.05 indicating significant difference compared to control media (with treatment); #p < 0.05 indicating significant difference from its group (either within crude extract or within alkaloid extract)).
To further investigate this, the study was extended to evaluate the suppressive activity of Kratom extracts on the production of reactive nitrogen species (RNS), which are also known to induce excessive inflammation at the cellular level. NO is an RNS that participates in various physiological functions, including the pathogenesis of inflammation. Therefore, observing NO production during inflammatory stimulation may provide more insight into the intracellular antioxidative activity of the extracts. The intracellular NO production in RAW 264.7 macrophage cells treated with LPS was approximately 1.7-fold higher than the untreated cells (Fig. 4b). Similar to ROS, RNS was decreased when LPS-treated cells were exposed to Kratom extracts. Previous studies have found that plant extracts containing phyto-metabolites may compete with oxygen to capture nitrite free radicals, thereby limiting NO oxidation and displaying anti-inflammatory properties through NO inhibition53,54. Furthermore, the maximum NO attenuation was achieved at ~ 7–8 nM with 100 ppm and 25 ppm of crude and alkaloid extract, respectively. This result confirms that the alkaloid extract exhibits four times more activity than the crude extract in suppressing intracellular oxidative radicals. As a result, crude and alkaloid extracts derived from Kratom leaves have the ability to reduce cellular oxidative stress, hence lowering the risk of inflammation. This is supported by the observation that NO stimulates the expression of genes associated with inflammatory diseases, such as interleukins, 5-LOX, and COX 255,56.
The effect on TNF-α and IL-6 level
Increasing ROS/RNS production by inflammatory and host cells activates signal transduction cascades and alters the transcription factors such as NF-κB. These usually lead to the expression of inflammatory cytokines such as IL-6 and TNF-α, chemokines, growth factors, and pro-inflammatory enzymes such as COX-2 and 5-LOX, which attract more inflammatory cells to the site of inflammation and even produce more reactive species55,57. TNF-α and IL-6 are the prominent pro-inflammatory cytokines released in RAW 264.7 macrophage cells after infection or exposure to LPS58,59. These pro-inflammatory cytokines can exacerbate the inflammatory response and play an important role in the inflammatory process60. Furthermore, high levels of IL-6 and TNF-α have been linked to several inflammatory conditions. Therefore, measuring TNF-α and IL-6 can help determine the anti-inflammatory properties of bioactive components.
Following the LPS-induced inflammation, the treatment with alkaloid extracts inhibited TNF-α at concentrations greater than 12.5 ppm, whereas the crude extract inhibited TNF-α at concentrations greater than 50 ppm (Fig. 5a). However, the alkaloid extract effectively reduced IL-6 level below 25 ppm, whereas the crude extract inhibited IL-6 at all concentrations tested (Fig. 5b). Alkaloid extract derived from Kratom leaf inhibited IL-6 levels in an inverse concentration-dependent manner. Meanwhile, Kratom crude extract did not inhibit IL-6 levels in a concentration-dependent manner.
TNF-α levels (a) and IL-6 Levels (b) assessment in LPC-induced RAW 264.7 cells after being treated with the crude and alkaloid extracts derived from Kratom leaf. TNF-α levels and IL-6 Levels were processed statistically using One-way ANOVA with ***p < 0.001 indicating significant difference compared to control media (without any treatment); ##p < 0.01, ###p < 0.001 indicating significant difference from its group (either within crude extract or within alkaloid extract).
The effect on COX-2 and 5-LOX activities
To gain a better understanding of the crude and alkaloid extracts’ anti-inflammatory efficacy, their effect on COX-2 and 5-LOX activity was studied. Figure 6a shows that LPS-induced RAW 264.7 cells treated with alkaloid extract had an average COX-2 inhibition of 33.46% across all concentrations. The alkaloid extract demonstrated inhibition of 5-LOX activity in LPS-induced RAW 264.7 macrophage cells at lower concentration (6.25 ppm) in an inverse concentration-dependent manner (6.25 ppm = 5.5 ± 0.8 pmol/min/mg; 12.5 ppm = 14.2 ± 2.9 pmol/min/mg; p < 0.001 compared to media + LPS group) (Fig. 6b). Conversely, the crude extract treatment exhibited only a 35.06% reduction in COX-2 activity at the lowest concentration (25 ppm) and did not show any activity on inhibition of 5-LOX at any concentration.
The inhibition profile of COX-2 (a) and 5-LOX activity (b) in RAW 264.7 macrophage cells after exposed with crude and alkaloid extract derived from Kratom leaf. The COX-2 and 5-LOX activity were processed statistically using One-way ANOVA ***p < 0.001 indicating significant difference compared to control media (without any treatment); ##p < 0.01, ###p < 0.001 indicating significant difference from its group (either within crude extract or within alkaloid extract).
Discussion
Several studies have described the potential of methanolic kratom extract as an anti-inflammatory agent, with the majority being conducted in vivo using carrageenan-induced paw edema on rats61,62. The result showed that at the highest concentration (200 mg/kg), methanolic kratom extract inhibited the development of carrageenan-induce rat paw edema, implying that methanolic kratom extract has anti-inflammatory properties. Furthermore, Utar et al.11 discovered that mitragynine inhibited the expression of the COX-2 enzyme in RAW 264.7 cells-induced inflammation by lipopolysaccharide (LPS, hence reducing the formation of PGE2. However, the anti-inflammatory effects of the crude extract and alkaloid extract from Kratom leaves on in vitro inflammation model have yet to be discovered. The alkaloid extracts were obtained by treating the crude extract with acid–base solutions to separate alkaloid from other secondary metabolites. Regardless, both extracts contain higher mitragynine content than lyophilized kratom extract. We used an in vitro inflammation model to gain a better understanding of how both extracts work as anti-inflammatory agents. Our results showed that the crude and alkaloid extract of kratom has anti-inflammatory properties by reducing free radicals (ROS and RNS), pro-inflammatory cytokines (TNF-α and IL-6) and inflammatory-related enzymes (COX-2 and 5-LOX).
ROS are the intermediate products commonly produced during cellular metabolism. They are formed as by-products of cellular oxidative processes, including the metabolism of arachidonic acid by COX and LOX enzymes, and play a significant role as mediators in the modulation of inflammation48,63. These species mainly include the hydroxyl radical (⋅OH), peroxyl (RO2⋅), alkoxyl (RO⋅) superoxide anions (O2−), oxy singlet oxygen (1O2), and hydrogen peroxide (H2O2). At normal levels, ROS plays a critical role in cellular signaling pathways, such as cell metabolism, growth, differentiation, and death signaling64. At moderate levels, they are defense molecules that destroy inflammation agents such as exogenous pathogens48. However, ROS have a strong tendency to react with and damage cellular macromolecules such as proteins, lipids, and nucleic acids. This excessive oxidation can lead to increased inflammatory responses in the organs.
Naturally, the level of ROS is maintained by the components possessing antioxidant activity. The high antioxidant activity of crude and alkaloid extracts derived from Kratom leaf indicates that these extracts contain phytochemicals that are active in scavenging ROS. A significant amount of phenolics observed in the crude extract should contribute to the high antioxidant activity of Kratom’s crude extract (Fig. 1c). Phenolic compounds are reported to be one of the effective nutrients in the prevention of oxidative stress65,66. In this study, the TPC of the crude extract was greater compared to that of the alkaloid extract, which is in line with previous findings67. This is because the cascade process during alkaloid extraction involves acid–base reaction and the use of non-polar organic solvents, which separate non-polar alkaloids and polar compounds (non-alkaloids) such as phenolics. Phenolics are a group of polar compounds and, thus, are not extracted when non-polar organic solvents are used during the extraction process to obtain the alkaloid extract.
Furthermore, the antioxidant activity of kratom’s crude and alkaloid extracts can be attributed to the presence of alkaloid compounds such as mitragynine and its derivatives (Fig. 1). In this study, the alkaloid extract exhibited significant ABTS antioxidant activity and was found to contain a high content of mitragynine (45.9 ± 0.9%). The presence of mitragynine was confirmed through LC–MS/MS analysis (Fig. S1) and by the proton signals of mitragynine obtained in the 1H NMR spectra (Fig. S2). Kratom alkaloids such as 7-hydroxy mitragynine, paynantheine, speciogynine, and speciociliatine have been reported to show antioxidant activity68 It should be noted that the lower FRAP value of the alkaloid extract compared to the crude extract does not necessarily indicate a lack of activity in the alkaloid extract. It might be a result of the fact that alkaloids, being weak bases, could potentially alter the redox reaction during the FRAP measurement. The FRAP assay requires an acidic condition (pH 3.6) to get the optimal redox reaction65. In contrast, the ABTS assay is carried out under neutral pH conditions, wherein the chromophore reaction should not be significantly affected by the weak basicity of the alkaloid components. In general, kratom extracts exhibit high antioxidant activity (Fig. 2), which should be beneficial for scavenging ROS/RNS in LPS-H2O2-stimulated RAW 264.7 (Fig. 4) and for acting as anti-inflammatory agents by inhibiting inflammatory-related enzymes (Fig. 6).
The ability of crude and alkaloid extracts to substantially decrease ROS in LPS-H2O2-stimulated RAW 264.7 cells without killing the cells should be associated with their antioxidant activity to scavenge free radicals (Figs. 2a, 4a). This is supported by the observation that these kratom extracts could also reduce NO levels in the LPS-treated RAW 264.7 macrophage cells (Fig. 4b). It should be noted that LPS stimulation can activate NF-κB, which upregulates the inducible nitric oxide synthase (iNOS) enzyme to result in the enhancement of NO production in RAW 264.7 macrophage cells49. This is corroborated by previous research that found plant extracts with a high concentration of phytochemical metabolites may compete with oxygen to capture nitrite free radicals, limiting nitric oxide oxidation and thus displaying anti-inflammatory properties through NO inhibition53,54.
RAW 264.7 macrophages cells stimulated by LPS, will release various pro-inflammatory cytokines; e.g., TNF-α, interleukin-1 beta (IL-1β), and interleukin 6 (IL-6)58,59. TNF-α has the ability to induce apoptosis and trigger the release of other inflammatory cytokines such as IL-1, IL-6, and IL-10. Additionally, it stimulates T cells as well as other inflammatory cells. The alkaloid extract exhibited four times more potent (~ 50% reduction) than the crude extract did (Fig. 5a). Furthermore, when the alkaloid extract concentration exceeded 6.25 ppm, TNF-α levels decreased. Nonetheless, the alkaloid extract exhibited significant suppression of IL-6 at the inverse concentration, whereas IL-6 levels were not reduced at 25 ppm of alkaloid extract exposure. In contrast, a crude extract exhibited IL-6 inhibition at concentrations ranging from 25 to 100 ppm but only exhibited TNF-α inhibition at 100 ppm. We hypothesized that each of the phytochemicals contained in the extracts possessed various activities on these pro-inflammatory cytokines. In particular, the alkaloid components manifest a different mechanism of action, prompting further analysis to ascertain the individual magnitude of their effects.
The AA pathway plays a key role in numerous inflammatory diseases, where its metabolism involves COXs and LOXs enzymes that lead to the production of a variety of bioactive mediators such as prostanoids and LTs69. COX-2 catalyzes the conversion of arachidonate to inflammatory PGs, a process pivotal in inflammation. COX-2 inhibitors are among the most widely used medications due to their anti-inflammatory, antipyretic, and analgesic properties8. On the other hand, it has been reported that 5-LOX plays the most important role in the production of LTs, which also act as inflammatory mediators in respiratory, dermatological, and gastrointestinal disorders18.
The alkaloid extract demonstrated consistent inhibition of COX-2 across all tested concentrations (6.25–25 ppm), while crude extract concentrations higher than 25 ppm did not reduce COX-2 activity (Fig. 6a). The alkaloid extract is mainly composed of mitragynine and its derivatives. Mitragynine has been found to inhibit the expression of COX-2 in a concentration-dependent manner11. Meanwhile, multiple components in the crude extract, besides alkaloids, might work synergistically or antagonistically to induce inflammation to some extent as the crude extract concentration increases. Similar to the interaction between artemisinin and casticin, which is antagonistic at a ratio of 1:3 (v/v), a synergistic interaction has been described for combination ratios ranging from 1:10 to 1:1000 (artemisinin to casticin, v/v)70.
Interestingly, the alkaloid extract exhibited the 5-LOX enzyme inhibition in a concentration-dependent manner, with 86% and 33% inhibition at 6.25 and 12.5 ppm, respectively (Fig. 6b). Such activities were not observed when the crude extract was tested. Given that the alkaloid extract contained more mitragynine and its derivatives than the crude extract, these compounds could account for this activity. This hypothesis is supported by molecular docking results, which showed that mitragynine and some of its derivatives, like paynantheine and speciophylline, exhibited good binding affinity with COX-2 and 5-LOX proteins (Table S1). Moreover, mitragynine showed higher binding affinity compared to several opioids, including morphine and methadone.
COX-2 possesses three regions of main active sites; i.e., the hydrophobic pocket (Tyr385, Trp 387, Phe518, Ala201, Tyr 248, Leu352), the hydrophilic region (Arg120, Glu524, Tyr 355), the side pocket (His90, Arg513, Val523); while the binding pocket of 5-LOX is primarily Phe169, Phe610, Ala410, Ala672, Gln363, Gln413 and Ile40671. In this study, mitragynine demostrated multiple interactions with several main active sites of COX-2; i.e., Arg120, Tyr355, Phe518, Leu 352, Trp 387, Val 523; and with 5-LOX; i.e., Phe610, Ala410, Ile406, Gln413 (Fig. S3a,b). In comparison, celecoxib, a selective COX-2 inhibitor72, formed multiple bonds with COX-2 active sites than with 5-LOX proteins (Fig. S3c,d). Morphine showed more limited interactions with these two enzymes (Fig. S3e,f), resulting in lower binding affinity compared to mitragynine, as indicated docking scores (Table S1). These findings suggest that an alkaloid extract derived from Kratom leaf effectively inhibits both COX-2 and 5-LOX enzymes, demonstrating dual anti-inflammatory activity. Inhibiting the 5-LOX enzyme concurrently may help reduce the negative effects of COX-2 inhibition since COX-2 inhibition can enhance the availability of the AA-LOXs pathway, which is primarily linked to COX inhibitor side effects8. In contrast, the crude extract, similar to most NSAIDs, only inhibits COX-2 enzymes. However, it should be noted that our molecular docking study indicated variations in the binding affinity of each alkaloid compound, and thus, further investigations are warranted to determine the level of inhibitory activity of each component against COX-2 and 5-LOX enzymes. Overall, the results of this study shed the light on the possible application of alkaloid kratoms as an analgesic to reduce pain, most likely due to their dual anti-inflammatory properties. Further studies are needed to determine whether the combination of alkaloid or single alkaloid compound in kratom extracts mainly responsible to gives them their anti-inflammatory or analgesic trait.
Conclusion
The alkaloid extract derived from kratom leaf, containing ~ 46% mitragynine, was found to exhibit significant antioxidant activity, effectively scavenging ROS (< 50%) and NO (34%) in vitro using RAW 264.7 macrophage cells induced with LPS. Its free radical scavenging activity correlated with the extract concentration, where a significant reduction of ROS (< 50%) and NO (34%) levels is observed at 25 ppm, without exhibiting toxicity to the cells. At a sufficient concentration (~ 12.5 ppm), the alkaloid extract also demonstrates activity in reducing the proinflammatory cytokines TNF-α and IL-6, with approximately 4-folds more activity than the crude extract. An interesting feature of the alkaloid extract is its dual anti-inflammatory activity, inhibiting both COX-2 and 5-LOX to some extent. This contrasts with the crude extract, which shows inhibition of COX-2 only. These results support previous reports that describe mitragynine, one of the alkaloids derived from kratom leaf, as being active in inhibiting COX-2. The additional information on the inhibition activity of the kratom alkaloid extract towards 5-LOX enzyme opens a new insight into the potential of the alkaloid compounds in Kratom to be developed as an alternative NSAID with fewer and safer side effects.
Data availability
Data is provided within the manuscript or Supplementary Information files.
References
Cock, I. E. Terminalia ferdinandiana Exell. extracts reduce pro-inflammatory cytokine and PGE2 secretion, decrease COX-2 expression and down-regulate cytosolic NF-κB levels. Inflammopharmacology 32(3), 1839–1853. https://doi.org/10.1007/S10787-024-01462-7 (2024).
Giménez-Bastida, J. A., González-Sarrías, A., Laparra-Llopis, J. M., Schneider, C. & Espín, J. C. Targeting mammalian 5-lipoxygenase by dietary phenolics as an anti-inflammatory mechanism: A systematic review. Int. J. Mol. Sci. 22(15), 937. https://doi.org/10.3390/IJMS22157937 (2021).
Wolfarth, B., Speed, C., Raymuev, K., Vanden Bossche, L. & Migliore, A. Managing pain and inflammation associated with musculoskeletal disease: Time for a change?. Curr. Med. Res. Opin. 38(10), 1695–1701. https://doi.org/10.1080/03007995.2022.2108618 (2022).
Tai, F. W. D. & McAlindon, M. E. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin. Med. 21(2), 131. https://doi.org/10.7861/CLINMED.2021-0039 (2021).
Sriuttha, P., Sirichanchuen, B. & Permsuwan, U. Hepatotoxicity of nonsteroidal anti-inflammatory drugs: A systematic review of randomized controlled trials. Int. J. Hepatol. 2018, 1. https://doi.org/10.1155/2018/5253623 (2018).
Frishman, W. H. Effects of nonsteroidal anti-inflammatory drug therapy on blood pressure and peripheral edema. Am. J. Cardiol. 89, 18–25. https://doi.org/10.1016/S0002-9149(02)02233-6 (2002).
Schafer, A. I. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J. Clin. Pharmacol. 35(3), 209–219. https://doi.org/10.1002/J.1552-4604.1995.TB04050.X (1995).
Ahmadi, M., Bekeschus, S., Weltmann, K. D., von Woedtke, T. & Wende, K. Non-steroidal anti-inflammatory drugs: Recent advances in the use of synthetic COX-2 inhibitors. RSC Med. Chem. 13(5), 1. https://doi.org/10.1039/D1MD00280E (2022).
Prasher, P., Mudila, H., Sharma, M. & Khati, B. Developmental perspectives of the drugs targeting enzyme-instigated inflammation: A mini review. Med. Chem. Res. 28(4), 417–449. https://doi.org/10.1007/s00044-019-02315-7 (2019).
Tsatsanis, C., Androulidaki, A., Venihaki, M. & Margioris, A. N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 38(10), 1654–1661. https://doi.org/10.1016/J.BIOCEL.2006.03.021 (2006).
Utar, Z., Majid, M. I. A., Adenan, M. I., Jamil, M. F. A. & Lan, T. M. Mitragynine inhibits the COX-2 mRNA expression and prostaglandin E2 production induced by lipopolysaccharide in RAW264.7 macrophage cells. J. Ethnopharmacol. 136(1), 75–82. https://doi.org/10.1016/J.JEP.2011.04.011 (2011).
Hyde, C. A. C. & Missailidis, S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int. Immunopharmacol. 9(6), 701–715. https://doi.org/10.1016/j.intimp.2009.02.003 (2009).
Mukhopadhyay, N., Shukla, A., Makhal, P. N. & Kaki, V. R. Natural product-driven dual COX-LOX inhibitors: Overview of recent studies on the development of novel anti-inflammatory agents. Heliyon 9(3), 569. https://doi.org/10.1016/j.heliyon.2023.e14569 (2023).
Wisastra, R. & Dekker, F. J. Inflammation, cancer and oxidative lipoxygenase activity are intimately linked. Cancers 6(3), 1500. https://doi.org/10.3390/CANCERS6031500 (2014).
Kretzer, C. et al. Shifting the biosynthesis of leukotrienes toward specialized pro-resolving mediators by the 5-lipoxygenase-activating protein (FLAP) antagonist BRP-201. J. Inflamm. Res. 15, 911. https://doi.org/10.2147/JIR.S345510 (2022).
Fiorucci, S., Meli, R., Bucci, M. & Cirino, G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy?. Biochem. Pharmacol. 62, 1433 (2001).
Gilroy, D. W., Tomlinson, A. & Willoughby, D. A. Differential effects of inhibitors of cyclooxygenase cyclooxygenase 1/and cyclooxygenase 2 in acute inflammation. Eur. J. Pharmacol. 355, 1 (1998).
Charlier, C. & Michaux, C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur. J. Med. Chem. 38(7–8), 645–659. https://doi.org/10.1016/S0223-5234(03)00115-6 (2003).
Inagaki, M. et al. Novel antiarthritic agents with 1,2-isothiazolidine-1-,1-dioxide (γ-sultam) skeleton: Cytokine suppressive dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase. J. Med. Chem. 43(10), 2040–2048. https://doi.org/10.1021/jm9906015 (2000).
Bitto, A. et al. Effects of COX1-2/5-LOX blockade in Alzheimer transgenic 3xTg-AD mice. Inflamm. Res. 66(5), 389–398. https://doi.org/10.1007/S00011-017-1022-X (2017).
Gouda, N. A., Alshammari, S. O., Abourehab, M. A. S., Alshammari, Q. A. & Elkamhawy, A. Therapeutic potential of natural products in inflammation: Underlying molecular mechanisms, clinical outcomes, technological advances, and future perspectives. Inflammopharmacology 31(6), 2857–2883. https://doi.org/10.1007/S10787-023-01366-Y (2023).
Salmerón-Manzano, E., Garrido-Cardenas, J. A. & Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 17(10), 3376. https://doi.org/10.3390/IJERPH17103376 (2020).
Dal Cero, M., Saller, R., Leonti, M. & Weckerle, C. S. Trends of medicinal plant use over the last 2000 years in Central Europe. Plants 12(1), 135. https://doi.org/10.3390/PLANTS12010135/S1 (2023).
Atanasov, A. G. et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 33(8), 1582–1614. https://doi.org/10.1016/J.BIOTECHADV.2015.08.001 (2015).
Klayman, D. L. et al. Isolation of artemisinin (qinghaosu) from Artemisia annua growing in the United States. J. Nat. Prod. 47(4), 715–717. https://doi.org/10.1021/NP50034A027 (1984).
Luo, J. et al. Masoprocol (nordihydroguaiaretic acid): A new antihyperglycemic agent isolated from the creosote bush (Larrea tridentata). Eur. J. Pharmacol. 346(1), 77–79. https://doi.org/10.1016/S0014-2999(98)00139-3 (1998).
Hohmann, J., Evanics, F., Berta, L. & Bartók, T. Diterpenoids from Euphorbia peplus. Planta Med. 66(3), 291–294. https://doi.org/10.1055/S-2000-8568/ID/4/BIB (2000).
Parthasarathy, S. et al. A simple HPLC–DAD method for the detection and quantification of psychotropic mitragynine in Mitragyna speciosa (ketum) and its products for the application in forensic investigation. Forensic Sci. Int. 226(1–3), 183–187. https://doi.org/10.1016/J.FORSCIINT.2013.01.014 (2013).
Garcia-Romeu, A., Cox, D. J., Smith, K. E., Dunn, K. E. & Griffiths, R. R. Kratom (Mitragyna speciosa): User demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 208, 107849. https://doi.org/10.1016/J.DRUGALCDEP.2020.107849 (2020).
Hassan, Z. et al. From Kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neurosci. Biobehav. Rev. 37(2), 138–151. https://doi.org/10.1016/J.NEUBIOREV.2012.11.012 (2013).
Singh, D., Narayanan, S. & Vicknasingam, B. Traditional and non-traditional uses of Mitragynine (Kratom): A survey of the literature. Brain Res. Bull. 126(Pt 1), 41–46. https://doi.org/10.1016/J.BRAINRESBULL.2016.05.004 (2016).
Vicknasingam, B. et al. Focus: Plant-based medicine and pharmacology: Kratom and pain tolerance: A randomized, placebo-controlled, double-blind study. Yale J. Biol. Med. 93(2), 229 (2020).
Veltri, C. & Grundmann, O. Current perspectives on the impact of Kratom use. Subst Abuse Rehabil. 10, 23–31.https://doi.org/10.2147/SAR.S164261 (2019)
Avery, B.A. et al. Comparative Pharmacokinetics of Mitragynine after Oral Administration of Mitragyna speciosa (Kratom) Leaf Extracts in Rats. Planta Med. 85(4), 340–346. https://doi.org/10.1055/a-0770-3683 (2019)
Kimura, M., Obata, H. & Saito, S. Peripheral nerve injury reduces analgesic effects of systemic morphine via spinal 5-hydroxytryptamine 3 receptors. Anesthesiology 121(2), 362–371. https://doi.org/10.1097/ALN.0000000000000324 (2014).
Kruegel, A. C. et al. 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent. Sci. 5(6), 992–1001. https://doi.org/10.1021/ACSCENTSCI.9B00141/ASSET/IMAGES/LARGE/OC-2019-00141P_0007.JPEG (2019).
Mat, N. H., Bakar, S. N. S., Murugaiyah, V., Chawarski, M. C. & Hassan, Z. Analgesic effects of main indole alkaloid of kratom, mitragynine in acute pain animal model. Behav. Brain Res. 439, 114251. https://doi.org/10.1016/J.BBR.2022.114251 (2023).
Takayama, H. Chemistry and pharmacology of analgesic indole alkaloids from the Rubiaceous plant, Mitragyna speciosa. Chem. Pharm. Bull. 52(8), 916–928. https://doi.org/10.1248/CPB.52.916 (2004).
Váradi, A. et al. Mitragynine/corynantheidine pseudoindoxyls as opioid analgesics with Mu agonism and delta antagonism, which do not recruit β-Arrestin-2. J. Med. Chem. 59(18), 8381. https://doi.org/10.1021/ACS.JMEDCHEM.6B00748 (2016).
Watanabe, K., Yano, S., Horie, S. & Yamamoto, L. T. Inhibitory effect of mitragynine, an alkaloid with analgesic effect from Thai medicinal plant Mitragyna speciosa, on electrically stimulated contraction of isolated guinea-pig ileum through the opioid receptor. Life Sci. 60(12), 933–942. https://doi.org/10.1016/S0024-3205(97)00023-4 (1997).
Bayu, A. et al. An in vitro examination of whether kratom extracts enhance the cytotoxicity of low-dose doxorubicin against A549 human lung cancer cells. Molecules 29(6), 404. https://doi.org/10.3390/molecules29061404 (2024).
Wilson, L. L. et al. Kratom alkaloids, natural and semi-synthetic, show less physical dependence and ameliorate opioid withdrawal. Cell. Mol. Neurobiol. 41(5), 1131–1143. https://doi.org/10.1007/S10571-020-01034-7 (2021).
Windarsih, A. et al. Untargeted metabolomics and proteomics approach using liquid chromatography-Orbitrap high resolution mass spectrometry to detect pork adulteration in Pangasius hypopthalmus meat. Food Chem. 386, 132856. https://doi.org/10.1016/J.FOODCHEM.2022.132856 (2022).
Tang, J., Dunshea, F. R. & Suleria, H. A. R. LC-ESI-QTOF/ms characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 9(1), 7. https://doi.org/10.3390/FOODS9010007 (2020).
Kumar, P., Nagarajan, A. & Uchil, P. D. Analysis of cell viability by the MTT assay. Cold Spring Harbor Protoc. 2018(6), 469–471. https://doi.org/10.1101/PDB.PROT095505 (2018).
Kongkatitham, V. et al. Anti-oxidant and anti-inflammatory effects of new bibenzyl derivatives from Dendrobium parishii in hydrogen peroxide and lipopolysaccharide treated RAW264.7 cells. Phytochem. Lett. 24, 31–38. https://doi.org/10.1016/J.PHYTOL.2018.01.006 (2018).
Divate, R. D. & Chung, Y. C. In vitro and in vivo assessment of anti-inflammatory and immunomodulatory activities of Xylaria nigripes mycelium. J. Funct. Foods 35, 81–89. https://doi.org/10.1016/J.JFF.2017.05.027 (2017).
Liu, J. et al. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 16(1), 7. https://doi.org/10.1186/S13045-023-01512-7 (2023).
Jones, E., Adcock, I. M., Ahmed, B. Y. & Punchard, N. A. Modulation of LPS stimulated NF-kappaB mediated nitric oxide production by PKCε and JAK2 in RAW macrophages. J. Inflamm. 4(1), 1–9. https://doi.org/10.1186/1476-9255-4-23/FIGURES/8 (2007).
Neha, K., Haider, M. R., Pathak, A. & Yar, M. S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 178, 687–704. https://doi.org/10.1016/J.EJMECH.2019.06.010 (2019).
Snezhkina, A. V. et al. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid. Med. Cell. Longevity 2019, 804. https://doi.org/10.1155/2019/6175804 (2019).
Dos Nunes, C. R. et al. Plants as sources of anti-inflammatory agents. Molecules 25(16), 726. https://doi.org/10.3390/molecules25163726 (2020).
Gomathi, D., Ravikumar, G., Kalaiselvi, M., Vidya, B. & Uma, C. In vitro free radical scavenging activity of ethanolic extract of the whole plant of Evolvulus alsinoides (L.). Chin. J. Integr. Med. 21(6), 453–458. https://doi.org/10.1007/S11655-014-1763-0/METRICS (2015).
Ramya, R. et al. Secondary metabolite credentials and in vitro free radical scavenging activity of Alpinia calcarata. JACME 5(2), 33–37. https://doi.org/10.1016/J.JACME.2015.02.005 (2015).
Cumpstey, A. & Feelisch, M. (2018). Free Radicals in Inflammation.
Laroux, F. S. et al. Role of nitric oxide in inflammation. Acta Physiol. Scand. 173, 113–118 (2001).
Lei, Y. et al. Redox regulation of inflammation: Old elements, a new story. Med. Res. Rev. 35(2), 306–340. https://doi.org/10.1002/med.21330 (2015).
Won, A. N. et al. HO-1 induction by Selaginella tamariscina extract inhibits inflammatory response in lipopolysaccharide-stimulated RAW 264.7 macrophages. Evid. Based Complement. Altern. Med. 2018, 923. https://doi.org/10.1155/2018/7816923 (2018).
Zhang, W. et al. Inhibition of HDAC6 attenuates LPS-induced inflammation in macrophages by regulating oxidative stress and suppressing the TLR4-MAPK/NF-κB pathways. Biomed. Pharmacother. 117, 109166. https://doi.org/10.1016/J.BIOPHA.2019.109166 (2019).
Facchin, B. M. et al. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 71(7–8), 741–758. https://doi.org/10.1007/S00011-022-01584-0 (2022).
Salim, H. M. et al. Anti-inflammatory effects and potential mechanisms of Mitragyna speciosa methanol extract on λ-karagenan-induced inflammation model. Bali Med. J. 11(3), 1172–1175. https://doi.org/10.15562/BMJ.V11I3.3535 (2022).
Shaik Mossadeq, W. M. et al. Anti-inflammatory and antinociceptive effects of Mitragyna speciosa Korth methanolic extract. Med. Princ. Pract. 18(5), 378–384. https://doi.org/10.1159/000226292 (2009).
Cho, K. J., Seo, J. M. & Kim, J. H. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol. Cells 32(1), 1–6. https://doi.org/10.1007/S10059-011-1021-7 (2011).
Zou, Z., Chang, H., Li, H. & Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 22(11), 1321–1335. https://doi.org/10.1007/s10495-017-1424-9 (2017).
Huang, D., Boxin, O. U. & Prior, R. L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53(6), 1841–1856. https://doi.org/10.1021/jf030723c (2005).
Parthasarathy, S. et al. Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from Mitragyna speciosa (rubiaceae family) leaves. Molecules 14(10), 3964–3974. https://doi.org/10.3390/molecules14103964 (2009).
Zhang, P. et al. Antidiabetic and antioxidant activities of Mitragyna speciosa (kratom) leaf extract in type 2 diabetic rats. Biomed. Pharmacother. 162, 114689–114689. https://doi.org/10.1016/J.BIOPHA.2023.114689 (2023).
Elahian, F., Zahedian, S., Safaei, M., Pahlevani-Gazi, E. & Abbas Mirzaei, S. Unlike morphine, long-term exposure to analgesic mitragynine, 7-hydroxymitragynine, paynantheine, and speciociliatine alkaloids does not contribute to antinociceptive tolerance of μ-opioid receptors. Res. Sq. 1, 1–15. https://doi.org/10.2203/rs.3.rs-39727/v1 (2020).
Wang, B. et al. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther. 6(1):94. https://doi.org/10.1038/s41392-020-00443-w (2021).
Suberu, J. O. et al. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract—Possible synergistic and resistance mechanisms. PLoS ONE 8(11), e80790. https://doi.org/10.1371/JOURNAL.PONE.0080790 (2013).
Goldenberg, M. M. Celecoxib, a selective cyclooxygenase-2 inhibitor for the treatment of rheumatoid arthritis and osteoarthritis. Clin Ther. 21(9):1497-513; discussion 1427-8. https://doi.org/10.1016/s0149-2918(00)80005-3
Bar, F. M. A., Sameti, M., Foudah, A. I., Haque, A. & Elsbaey, M. In vitro and in silico inhibition of COX-2 and 5-LOX by beta-carboline alkaloids from the seeds of Peganum harmala L., South African Journal of Botany. 147, 926–936. https://doi.org/10.1016/j.sajb.2022.03.044 (2022).
Acknowledgements
The authors gratefully acknowledge the financial support and administrative assistance provided by the Research Organization for Health, National Research and Innovation Agency (BRIN), Republic of Indonesia. The authors would like to acknowledge Jonathan Ardhianto Panggabean and Firmansyah Karim for their assistance with the extraction and chromatographic analysis
Funding
This research was supported by the Research Organization for Health, National Research and Innovation Agency (BRIN), Republic of Indonesia through “Rumah Program Biofarmaseutikal and Biosimilar” 2024, Project Number NOMOR 2/III.9/HK/2024.
Author information
Authors and Affiliations
Contributions
All authors played significant roles in the research and manuscript preparation processes. S.I.R. contributed to the conceptualization, methodology, data analysis, investigation, writing—original draft, review, and editing. D.W.I. contributed to the conceptualization, methodology, data analysis, writing—original draft, review and editing, visualization. F.N.N. contributed to the investigation, writing—original draft, review, and editing. M.H. contributed to the investigation and editing. P.A. contributed to the data analysis, review, and editing. A.R. contributed to the data analysis, review, and editing. F. contributed to in silico study. E.S. contributed to the data analysis and resources. N.L.P.I.D. contributed to the writing—review and editing, funding acquisition. A.B. contributed to the data analysis, investigation, review, and editing. M.Y.P. contributed to the supervision, writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rahmawati, S.I., Indriani, D.W., Ningsih, F.N. et al. Dual anti-inflammatory activities of COX-2/5-LOX driven by kratom alkaloid extracts in lipopolysaccharide-induced RAW 264.7 cells. Sci Rep 14, 28993 (2024). https://doi.org/10.1038/s41598-024-79229-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79229-x
Keywords
This article is cited by
-
Serotonin release mediates analgesia via opioidergic system and withdrawal symptoms in chronic kratom extract-treated mice
BMC Complementary Medicine and Therapies (2025)
-
Neuroinflammatory crosstalk in migraine: consolidated activity of rizatriptan and meloxicam in suppressing CGRP-induced nociception and COX-mediated inflammation
Inflammopharmacology (2025)