Abstract
Generally, invasive treatment is contraindication for patients with severe thrombocytopenia, because it may increase risk of bleeding. However, many early hepatocellular carcinoma (HCC) patients with cirrhosis have platelet counts (PC) less than 50 × 109/L due to hypersplenism. These patients are often accompanied by hepatic insufficiency, which makes hepatectomy impossible, and local thermal ablation (LTA) has become a major treatment. The aim of our study is to investigate the correlation between severe thrombocytopenia and bleeding after LTA in HCC patients with cirrhosis, and evaluate risk factors of bleeding. 473 patients with cirrhosis who underwent LTA for HCC from 2016 to 2020 were enrolled, and 709 ablations were performed in total. Based on preoperative PC, cases were divided into three groups, namely, group A (PC > 50 × 109/L), group B (30 × 109/L < PC ≤ 50 × 109/L) and group C (PC ≤ 30 × 109/L). The incidence of bleeding after LTA was compared among the three groups. Logistic regression was used to explore the risk factors for bleeding after ablation. The overall incidence of bleeding complications was 4.4%, and no significant difference was observed between group A, B, and C (3.9% vs. 6.4% vs. 3.3%, P = 0.410). In multivariate analysis, tumor diameter (OR = 2.657 per 1 cm, P < 0.001), and multiple lesions (≥ 3) (OR = 3.723, P = 0.006) were found to be independent predictors of bleeding after LTA. In small HCC patients with cirrhosis and hypersplenism, the PC range 30–50 × 109/L will not increase the risk of bleeding after LTA. Tumor diameter and number of lesions are independent predictors for bleeding after LTA in HCC patients.
Similar content being viewed by others
Introduction
Primary liver cancer is one of the most common malignant tumors in the world. According to GLOBOCAN 20201, there were approximately 906,000 new cases and 830,000 deaths associated with primary liver cancer in 2020, making it the sixth most common cancer and the third leading cause of cancer death worldwide. Hepatocellular carcinoma (HCC) accounts for 75%-85% of primary liver cancer1,2. At present, radical treatments for HCC mainly include liver transplantation, hepatectomy and ablation3. However, liver transplantation is limited by the scarcity of donated organs, and few patients can receive the treatment3,4. Hepatectomy is indicated in patients with small HCC of Child–Pugh grade A5. However, 85%–90% of HCC patients in the world are accompanied by cirrhosis, which means that they have varying degrees of hepatic insufficiency6. In addition, due to multiple restrictions on tumor size, location and number, vascular and extrahepatic involvement, only less than 20% of HCC patients could perform hepatectomy7. In recent years, local thermal ablation has been widely used in the treatment of liver cancer with little effect on liver function and low complication incidence. In small HCC, thermal ablation provides similar overall survival (OS) and disease-free survival (DFS) to hepatectomy in randomized controlled trials (RCTs), making it a major radical treatment for HCC patients with cirrhosis8,9. These patients not only suffer from hepatic insufficiency, but also portal hypertension, which can cause splenomegaly, hypersplenism and finally thrombocytopenia. Previous literature reported that patients with cirrhosis accompanied by thrombocytopenia is in an extremely high proportion, approximately 64% to 78% 10,11,12. At present, most guidelines and expert consensus in China regard uncorrectable coagulation disorders and severe thrombocytopenia as contraindications for local thermal ablation13,14. Although there is no clear index threshold, most guidelines for liver cancer use platelet count 50 × 109/L as the low limit for invasive treatment2,15,16,17. In clinical practice, we have found a large number of HCC patients with platelet count < 50 × 109/L. To explore the safety of local thermal ablation for these patients, we designed this retrospective study, which traced back to the patients with cirrhosis who were admitted to our hospital for local thermal ablation for HCC from 2016 to 2020, to study the correlation between bleeding after ablation and thrombocytopenia, and to explore the risk factors of bleeding.
Patients and methods
Patients
Review the patients who were diagnosed with primary liver cancer and underwent local thermal ablation in our hospital from 2016 to 2020, the inclusion criteria of the patients were as follows: (1) Age > 18 years old; (2) The diagnosis of HCC is confirmed by pathology through needle biopsy or based on at least two types of contrast enhanced (at least 3-phase) scan (computed tomography (CT) or magnetic resonance imaging (MRI)) showing the typical characteristics of liver cancer in combination with serum tumor marker alpha fetoprotein (AFP) examination2; (3) Underwent percutaneous radiofrequency ablation or percutaneous microwave ablation treatment of HCC; (4) Child–Pugh grade A-B; (5) Complete routine blood test and coagulation function results within 3 days before percutaneous thermal ablation treatment; (6) Enhanced liver imaging shall be conducted within 2 weeks before percutaneous thermal ablation and within 1 month after operation. Exclusion criteria: (1) Metastatic hepatic carcinoma; (2) Has a history of trauma or other operations within a month before local thermal ablation treatment; (3) Gastrointestinal bleeding occurred within one month before local thermal ablation; (4) Patients with severe hematological diseases or dysfunction of heart, brain, kidney and other vital organs.The operations of this study were in accordance with the guidelines for the treatment of hepatocellular carcinoma. And this study was approved by the Ethics Committee of the General Hospital of the Chinese People’s Liberation Army.
Microwave ablation and radiofrequency ablation procedures
MWA was performed using the (KY-2000, Kangyou Medical, China) MWA generator with a transmitter frequency of 2450 MHz and the output power was set at 40–60 W. The therapeutic apparatus was equipped with a built-in water cooling cycle system, and the needle antenna was 1.9 mm (15G) in diameter. RFA was performed using a 470 kHz ± 10 multipole radiofrequency ablation generator (CelonLab Power; Celon, Berlin, Germany) with a maximum output power of 250 W. The radiofrequency electrode (CelonPro Surge; Celon) internal cold circulation bipolar needle (diameter 15.5G; 20 cm in length) contains two chambers capable of internal fluid circulation with exposed head lengths of 3 cm and 4 cm. For both MWA and RFA, peristaltic pumps were used to deliver distilled water at room temperature at a rate of 30 to 40 ml/min to prevent shaft overheating. Iron-constantan thermocouple was inserted into the tissue 5 mm from the radiating segment of the electrode and the temperature was measured under ultrasound guidance. Ascendus (Hitachi, China) color doppler ultrasonic diagnostic apparatus was used for ultrasonic guidance.
The patient took the supine position and underwent routine disinfection and towel laying. The microwave/radio frequency electrodes were inserted percutaneously under the guidance of real-time ultrasound after the puncture point was identified. After the antenna was properly placed, general anesthesia was applied and ablation therapy was performed according to the tumor location and volume until the lesion was completely covered by the strong echo in the ablation area, and the ablation area was extended to 5–10 mm from the outer edge of the lesion. After ablation of the tumor, the antenna was gradually retracted and microwave or radio frequency emission was continued until the antenna was pulled directly below the skin entrance site.
Data acquisition and complication assessment
The following items were collected from patients: baseline information, hematology up to 3 days prior to ablation (including hemoglobin, PC, prothrombin activity (PA), and liver function indices, etc.), and imaging up to 2 weeks prior to ablation. The patients were divided into three groups based on their PC before ablation: group A (PC > 50 × 109/L), group B (30 × 109/L < PC ≤ 50 × 109/L) and group C (PC ≤ 30 × 109/L). The above hematology testing and liver contrast-enhanced imaging were collected 24 h after ablation to assess complications.
Complications were graded according to Society of Interventional Radiology (SIR) classification criteria, including minor complications (Grade A-B) and major complications (Grade C-F). A: No therapy, no consequence; B: nominal therapy, no consequence; includes overnight admission for observation only; C: require therapy, minor hospitalization (< 48 h); D: require major therapy, unplanned increase in level of care, prolonged hospitalization (> 48 h); E: permanent adverse sequelae; F: death18,19. Bleeding after ablation was comprehensively judged according to the clinical symptoms, imaging features, and the decline of hemoglobin.
Efficacy evaluation
One month after ablation, patients were examined again with contrast-enhanced imaging to evaluate the local efficacy, which was divided into complete response (CR) and incomplete response (ICR). CR: After follow-up with contrast-enhanced imaging, no enhancement was observed during the arterial phase of tumor ablation lesions, suggesting that the tumor was completely necrotic; ICR: There was local enhancement in the intra-arterial phase of the lesion, suggesting residual tumor2.
Statistical analysis
SPSS 26.0 (IBM, Armonk, NY) were used for statistical analysis. One sample Kolmogorov-Smirnow test was used to test the normality of continuous variables. Continuous variables with normal distribution were reported as mean ± standard deviation (SD) and compared between groups using one-way analysis of variance (ANOVA). Continuous variables that were not conform to normal distribution were reported in the median and quartile ranges and compared between groups using the Kruskal–Wallis H test. Nominal and ordinal variables were reported as frequency (percentage) and differences between groups were compared using χ2 test and the Kruskal–Wallis H test, respectively.
A univariate binary logistic regression analysis was then performed for the demographic, hematology, and tumor factors evaluated to determine the risk factors of postoperative bleeding. Multivariate logistic regression model was constructed in a forward conditional method, and the results of logistic regression analysis were reported using 95% confidence intervals (CI) and odds ratios (OR). Double-tailed P < 0.05 was considered statistically significant throughout the study.
Results
Study population characteristics
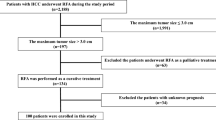
Altogether 515 patients, 751 local thermal ablations for liver cancer in the Fifth Medical Center, Chinese PLA General Hospital from January 2016 to October 2020 were screened in this study. Among them, 19 cases were excluded because of metastatic hepatic carcinoma, 5 cases were excluded because they underwent multiple local treatments within a month, 4 cases were excluded due to severe hematological diseases, and 14 cases because of missing baseline or outcome data (Fig. 1). A total of 473 patients were eventually included and 709 LTA were performed. There were 372 males and 101 females, with the average age of 57.6 years. Among the 709 local thermal ablation treatments, 624 were microwave ablation and 85 were radio frequency ablation. The baseline characteristics of the patients are presented in Table 1. The cases were divided into three groups based on PC, 539 cases in group A (PC > 50 × 109/L), 140 cases in group B (30 × 109/L < PC ≤ 50 × 109/L), and 30 cases in group C (PC < 30 × 109/L). The median PC in all cases was 96 × 109/L, and median PC for group A, B, C were 112 × 109/L, 42 × 109/L, and 28 × 109/L, respectively.
Among the three groups, leukocyte count, hemoglobin, PA, international normalized ratio (INR), albumin and cholinesterase of patients in group B and C were significantly lower than those in group A, and total bilirubin (TBIL) and aspartate aminotransferase (AST) were higher than those in group A with statistical significance (P < 0.001). The proportions of patients with Child–Pugh B in group B and C were significantly higher than that in group A (P < 0.001) and there were more patients with splenomegaly in group B and C than in group A (P < 0.001). The distributions of AFP, alanine aminotransferase (ALT), gamma-glutamyl transpeptdase (GGT), Eastern Cooperative Oncology Group (ECOG) performance status, ablation sessions, number of lesions, tumor location and tumor diameter, cirrhosis etiology and Barcelona Clinic Liver Cancer (BCLC) stage 3among the three groups were similar and the differences were not statistically significant (P > 0.05). Among the 709 treatments, 653 (92.1%) were CR, and 56 (7.9%) were ICR in the efficacy evaluation a month after thermal ablation.
Bleeding events after local thermal ablation in platelet count groups
Among all included patients, the incidence of bleeding after LTA was 4.4%. The incidence of bleeding was 3.9%, 6.4%, and 3.3% in group A, B, and C, respectively, with no significant differences between the three groups (P = 0.410). Similar results were obtained in subgroup analyses of RFA and MWA, where the bleeding rates were not significantly higher in patients with PC less than 50 × 109/L or 30 × 109/L than in patients with PC greater than 50 × 109/L (MWA: P = 0.774, RFA: P = 0.157) (Table 2).
A total of 31 bleeding events occurred in 709 ablations, of which 4 cases were major bleeding requiring massive treatment (blood transfusion or interventional surgery). Their preoperative PC were 40 × 109/L, 57 × 109/L, 69 × 109/L, and 103 × 109/L. Their treatments were as follows: 1 case presented with a large amount of encapsulated hemorrhagic effusion in the left thoracic cavity, and the hemoglobin decreased from 136 g/L to 67 g/L after ablation. Transcatheter arterial embolization (TAE) was performed and the bleeding was stopped after infusion of suspended red blood cells 2U supplemented with hemostasis drugs such as Carbazochrome Sodium Sulfonate and hemocoagulase from Agkistrodon halys. Another patient experienced thoracic hemorrhage after ablation. His hemoglobin decreased to 58 g/L from 109 g/L, and PC decreased to 28 × 109/L from 40 × 109/L. A thoracentesis catheter was placed for drainage, and 2U suspended red blood cells and 2U machine-collected platelets were infused. The patients were additionally treated with the above hemostatic drugs. The bleeding stopped after treatment, and a week later the hemoglobin and PC of the patient increased to 72 g/L and 50 × 109/L, respectively. A case of hemobilia complicated with upper gastrointestinal bleeding was improved after TAE intervention and hemostasis with intravenous drugs. In a patient with intraperitoneal hemorrhage, hemoglobin was decreased from 83 g/L to 56 g/L, and 6U of suspended red blood cells were successively infused. Meanwhile, hemostatic drugs were applied for treatment, and then the hemorrhage stopped. The efficacy of the above four patients with major bleeding was evaluated one month after ablation, and they all showed CR. The remaining 27 cases were treated with hemostatic drugs, and patients with PC < 50 × 109/L and PA < 50% after ablation were treated with platelet or plasma transfusion8. All patients were improved after active treatment.
Risk factors for bleeding after local thermal ablation
We analyzed the risk factors for bleeding after LTA (Table 3). First, univariate logistic analysis was performed, and tumor diameter (P < 0.001), number of lesions (P < 0.001) were found to be related to bleeding after ablation. Meanwhile, we observed no significant correlation between coagulation parameters: PA (P = 0.867), INR (P = 0.919), Child–Pugh grades(P = 0.198) and bleeding after ablation. Multivariate models were established and tumor diameter (OR = 2.657 per 1 cm, P < 0.001) and multiple lesions (≥ 3) (OR = 3.723, P = 0.006) were independent predictors for bleeding after local thermal ablation in HCC patients.
Other complications
No ablation-related deaths were observed in the overall cohort. Major complications occurred (Table 4) in 55 of 709 patients (7.8%). They included 21 cases (3.1%) of severe infection (liver abscess, biliary tract infection, abdominal cavity infection, thoracic cavity infection, and secondary blood infection), 11 cases (1.6%) of pleural effusion requiring drainage, 13 cases (1.8%) of abdominal effusion requiring drainage, 4 cases (0.6%) of hydropneumothorax, 2 cases (0.3%) of pneumothorax, 4 cases (0.6%) of major bleeding related to acupuncture pathway, 3 cases (0.4%) of gastrointestinal hemorrhage, and 4 cases (0.6%) of liver failure. There were 3 cases (0.4%) of acute kidney injury requiring continuous renal replacement therapy (CRRT), 5 cases (0.7%) of intestinal obstruction, 2 cases (0.3%) of hepatic encephalopathy, and 1 case (0.1%) of diaphragmatic injury. All major complications were observed to have improved after active treatment, with no long-term sequelae or mortality.
The incidences of major complications in group A, B, and C were 8.0%, 7.9%, and 3.3%, respectively, and no significant difference was observed (P = 0.651). In univariate analysis, we found that the occurrence of major complications was related to BMI (P = 0.049), hemoglobin (P = 0.026), albumin (P = 0.001), CHE (P = 0.003), Child–Pugh grade (P = 0.012) and tumor diameter (P < 0.001). After multivariate adjustment, lower BMI (OR = 0.887 per 1 kg/m2, P = 0.009), lower albumin (OR = 0.881per 1 g/L, P < 0.001), lager tumor diameter (OR = 1.768 per 1 cm, P < 0.001), and multiple ablation sessions (≥ 3 times) (OR = 3.570, P = 0.002) were found to be independent predictors for major complications of LTA.
In addition, the most common minor complication was post-ablation syndrome, and 350 cases (49.4%) of fever above 38 °C and 210 cases (29.6%) of hepatic pain requiring intervention were observed. In addition, asymptomatic ascites and pleural effusion were observed in 56 (7.9%) and 67 (9.4%) cases, respectively.
Discussion
Around the world, 85%-90% of HCC patients have varying degrees of liver cirrhosis, and about 30% of them have hypersplenism, which could eventually lead to thrombocytopenia6,20,21. Previous literature reported that patients with cirrhosis accompanied by thrombocytopenia in an extremely high proportion, PC < 150 × 109/L in the ratio of 64% to 78%, of which moderate thrombocytopenia (50 × 109/L ≤ PC ≤ 75 × 109/L) and severe thrombocytopenia (PC < 50 × 109/L) accounted for 13% to 1%[10,11]. In several studies in China, patients with severe thrombocytopenia (PC < 50 × 109/L) accounted for 22.1%-25.1% of cirrhosis patients22,23,24. In our study, of the 709 patients included, 170 (24.0%) had severe thrombocytopenia.
Hepatectomy is the main treatment for small HCC except liver transplantation. However, for patients with cirrhosis, the liver parenchyma is often stiff and fibrotic, which is less amenable to transection25. Additionally, portal hypertension, thrombocytopenia and impaired coagulation usually lead to a large amount of blood loss during the operation25. Therefore, cirrhotic patients with poor liver function are not candidates for hepatectomy. It has been shown that for patients with early-stage HCC, thermal ablation could provide similar or slightly inferior OS and DFS to hepatectomy, with low complication incidence and little effect on liver function8,9. Local thermal ablation has become the main treatment for HCC patients with cirrhosis who cannot tolerate surgery. Currently, most domestic guidelines and expert consensus include uncorrectable coagulation disorders and severe thrombocytopenia as contraindications to local thermal ablation therapy, but there is no clear conclusion on the issue of the correlation between the degree of thrombocytopenia and postoperative bleeding in patients. Although there is no clear indicator threshold, most hepatocellular carcinoma guidelines use a platelet count of < 50 × 109/L as the low limit for invasive therapy. Many patients with HCC combined with cirrhosis and severe thrombocytopenia are in urgent need of effective treatment2,26. In our study, we attempted local thermal ablation in these patients to investigate its safety.
In previous studies, the incidence of bleeding after percutaneous MWA or RFA of liver cancer ranged from 0.5% to 4.87%27,28,29,30,31. The bleeding rate in our study was 4.37%, with 31 bleeding complications occurring out of 709 ablation procedures. While PC 50 × 109/L was considered the lower limit of ablation in most previous studies, it accounted for 24.0% of patients in our study, without a significant increase in bleeding rates.
There is a lack of consistency in the results of current researches on the correlation between PC and bleeding after ablation of liver cancer. Among 996 patients with cirrhosis who underwent liver resection or surgical RFA for liver cancer, Ronca et al.32 found 89 cases of perioperative bleeding, and patients with PC < 50 × 109/L did not have a higher bleeding rate. Napolitano et al.27 observed only 10 bleeding events among 852 invasive procedures in 363 cirrhotic patients, and concluded that platelet count had no association with postprocedural bleeding. Thrombocytopenia might, however, increase the chance of bleeding after ablation, according to two other large studies. A retrospective study by Goto et al.29 evaluated 4133 RFA treatments of liver tumors and found that low PC was an important risk factor for hemorrhagic complications, and for every 10 × 103/mm3 increase in PC, the incidence of hemorrhage reduced by 11.9%, although PC < 50 × 109/L were excluded. Another study by Park et al.28 retrospectively analyzed 1,843 RFA procedures and found that in patients with thrombocytopenia (< 50 × 109/L), the incidence of hemorrhage after RFA was significantly increased despite pre-operative prophylactic platelet transfusion.
In our study, patients with PC 30–50 × 109/L did not receive special treatment and had no significant increase in postoperative bleeding risk. Local thermal ablation of liver cancer in these patients was safe. Patients with PC less than 30 × 109/L received treatments of raising platelets which included transfusion of 1 to 6 units of platelets, recombinant human thrombopoietin or platelet-elevating drugs. The median PC of these patients was 28 × 109/L, and after treatments mentioned above the corrected median PC was 36 × 109/L, ranged from 22 to 70 × 109/L. The bleeding rate of patients with PC < 30 × 109/L is not higher than that of patients with normal PC, and ablation of HCC in these patients is also relatively safe.
We found that bleeding after ablation of HCC in patients with cirrhosis had no significant correlation with either PC or common coagulation indicators (INR and PA). Current studies suggest that although platelet count and coagulation indicators of patients with liver disease suggest a bleeding tendency, the hemostatic system is in a rebalance state33,34. In primary hemostasis, although the number and function of platelet were decreased, correspondingly, the high level of von Willebrand factor (vWF) could restore platelet adhesion to the vessel wall. At the same time, the low plasma level of ADAMTS-13 could reduce the clearance of vWF multimers with high hemostatic activity, and contribute to the recovery of platelet function35. In secondary hemostasis, the concomitant reduction of procoagulant and anticoagulant factors, as well as the parallel changes in profibrinolytic and antifibrinolytic drivers ensure relatively normal hemostatic function. However, compared with the normal population, the new balance in patients with cirrhosis is unstable, and there is a potential risk of bleeding and thrombosis, vulnerable to some external factors, such as portal hypertension, infection, etc33.
In multivariate analysis, we found that tumor diameter and number of lesions were independent predictors for bleeding after ablation. The effect of tumor size on post-operative bleeding was similar to previous research9,29. In addition, bleeding after ablation is affected by many factors. It includes bleeding from multiple sites, in which intraperitoneal hemorrhage may be due to incomplete coagulative of a subcapsular tumor or tearing of liver surface by electrodes; The reason for hemothorax could be penetrating the intercostal artery when an intercostal approach was required for ablation; Hemobilia may result from simultaneous aspiration of the bile duct and a blood vessel28,29.
In 709 ablation procedures(Table 4) , 55 cases (7.8%) of major complications were observed, including severe infection, pleural effusion, ascites, pneumothorax, liver failure, and renal injury, etc. Major complications did not occured more frequently in patients with PC below 30 × 109/L or 50 × 109/L, suggesting that thrombocytopenia was not a contributing factor to major complications after ablation. However(Table 5) , lower BMI, lower albumin, larger tumor diameter, and multiple sessions of ablation were found to be independent predictors of major complications. Albumin can reflect the synthetic function of the liver. The decrease of albumin represents poor liver reserve function, which may lead to a lower tolerance to surgery and more prone to major complications. Two studies by Liang et al.36 and Park et al.28 both reported the effect of tumor diameter and the number of ablation sessions on major complications, which is similar to our report. Furthermore, experience of the surgeons, cooperation of the patients, and the quality of imaging equipment will also affect postoperative complications, and comprehensive evaluation before ablation should be conducted to reduce the occurrence of complications29.
There are several limitations in the present study. First, it was a retrospective study from a single center, and the symptoms and signs of patients after ablation could not be recorded in real time. Multicenter prospective studies are needed to validate our conclusions. Second, mechanisms of bleeding after ablation in HCC patients with cirrhosis should be investigated in search of biomarkers. Third, a prediction model for bleeding after ablation in HCC patients should be established, so that more patients can receive effective treatment.
Conclusion
In conclusion, there is no significant increase in bleeding risk after local thermal ablation for small HCC patients with cirrhosis and hypersplenism whose platelet count is 30–50 × 109/L. Tumor diameter and number of lesions are independent predictors for bleeding after LTA in HCC patients, which should be handled with caution. Furthermore, platelet count in the range of 30–50 × 109/L does not increase the incidence of major postoperative complications, and ablation is relatively safe in these patients.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- AFP:
-
Alpha fetoprotein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BCLC:
-
Barcelona Clinic Liver Cancer
- BMI:
-
Body mass index
- CHE:
-
Cholinesterase
- CI:
-
Confidence interval
- CR:
-
Complete response
- DFS:
-
Disease-free survival
- ECOG:
-
Eastern Cooperative Oncology Group
- GGT:
-
Gamma-glutamyl transpeptdase
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- ICR:
-
Incomplete response
- INR:
-
International normalized ratio
- LTA:
-
Local thermal ablation
- MWA:
-
Microwave ablation
- OR:
-
Odds ratio
- OS:
-
Overall survival
- PA:
-
Prothrombin activity
- PC:
-
Platelet count
- RCT:
-
Randomized controlled trials
- RFA:
-
Radiofrequency ablation
- TAE:
-
Transcatheter arterial embolization
- TBIL:
-
Total bilirubin
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 71, 209–249 (2021).
National Health Commission of the People’s Republic of China. Guidelines for Diagnosis and Treatment of Primary Liver Cancer. Edition). Journal of Multidisciplinary Cancer Management (Electronic Version). 2022(8), 16–53 (2022).
Apor, S.-K. et al. Outcomes after primary and repeat thermal ablation of hepatocellular carcinoma with or without liver transplantation. Eur Radiol. 32, 4168–4176 (2022).
Benson, A. B. et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 19, 541–565 (2021).
Chok, K. S. et al. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma. Br J Surg. 96, 81–87 (2009).
Villanueva, A. Hepatocellular Carcinoma. N Engl J Med. 380, 1450–1462 (2019).
Poulou, L. S. et al. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 7, 1054–1063 (2015).
Chen, M. S. et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 243, 321–328 (2006).
Feng, K. et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 57, 794–802 (2012).
Qamar, A. A. et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 7, 689–695 (2009).
Afdhal, N. et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 48, 1000–1007 (2008).
Bashour, F. N., Teran, J. C. & Mullen, K. D. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am J Gastroenterol. 95, 2936–2939 (2000).
Chinese Expert Consensus Statement Chinese Society of Liver Cancer, Chinese Society of Cinical Oncology, Liver Cancer Group, Chinese Society of Hepatology. Guidelines for radiofrequency ablation therapy of liver cancer. J Clini Hepatol. 2011;27:236–42.
Interventional Group, Radiology Branch of Chinese Medical Association. Expert consensus on standard therapeutic procedures of percutaneous radiofrequency ablation for liver tumor. Chin J Radiol. 2012;46:581–5.
Kaufman, R. M. et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 162, 205–213 (2015).
Solves, A. P. Platelet Transfusion: And Update on Challenges and Outcomes. J Blood Med. 11, 19–26 (2020).
Estcourt, L. J. et al. Guidelines for the use of platelet transfusions. Br J Haematol. 176, 365–394 (2017).
Sacks, D. et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 14, S199-202 (2003).
Ahmed, M. et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 273, 241–260 (2014).
Jie H, Wanqing C, Hongbing S, et al. China guideline for liver cancer screening (2022, Beijing). J Clin Hepatol. 2022;38:1739–58+954–967.
Chen, W. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 66, 115–132 (2016).
Ouyang R. A multi-center study of the correlation between thrombocytopenia and portal vein thrombosis in the short-term prognosis of patients with cirrhosis. 2021; https://doi.org/10.27003/d.cnki.gojyu.2021.001040.
Liu, B. et al. Relative factors analysis of hemorrhage after thermal ablation for liver cancer. Chin J Interv Imaging Ther. 13, 276–279 (2016).
Xiao P. Construction and validation of a nomogram prediction the risk of sever thromnocytopenia in patients with hepatitis B cirrhosis complicated with hypersplinism. 2021; https://doi.org/10.27234/d.cnki.gnhuu.2021.000613
Kabir, T. et al. Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: meta-analysis. Br J Surg. 109, 21–29 (2021).
2019 Expert Committee on Clinical Coordination of Traditional Chinese and Western Medicine for Liver Cancer. Interventional expert consensus on integrated traditional Chinese and Western medicine for the diagnosis and treatment of hepatocellular carcinoma (trial edition I). J Internent Radiol. 2021;30:1079–90.
Napolitano, G. et al. Bleeding after invasive procedures is rare and unpredicted by platelet counts in cirrhotic patients with thrombocytopenia. Eur J Intern Med. 38, 79–82 (2017).
Park, J. G. et al. Early complications after percutaneous radiofrequency ablation for hepatocellular carcinoma: an analysis of 1,843 ablations in 1,211 patients in a single centre: experience over 10 years. Clin Radiol. 72(692), e9–e15 (2017).
Goto, E. et al. Hemorrhagic complications of percutaneous radiofrequency ablation for liver tumors. J Clin Gastroenterol. 44, 374–380 (2010).
Tang, Y. et al. Reasons and preventions of bleeding after percutaneous microwave ablation for liver cancer. Chin J Bases Clin General Surg. 17, 1294–1298 (2010).
Camma, C. et al. Treatment of hepatocellular carcinoma in compensated cirrhosis with radio-frequency thermal ablation (RFTA): a prospective study. J Hepatol. 42, 535–540 (2005).
Ronca, V. et al. Impact of Platelet Count on Perioperative Bleeding in Patients With Cirrhosis Undergoing Surgical Treatments of Liver Cancer. Hepatol Commun. 6, 423–434 (2022).
Tripodi, A. & Mannucci, P. M. The coagulopathy of chronic liver disease. N Engl J Med. 365, 147–156 (2011).
Tripodi, A. Hemostasis abnormalities in cirrhosis. Curr Opin Hematol. 22, 406–412 (2015).
Feng, Y. et al. ADAMTS13: more than a regulator of thrombosis. Int J Hematol. 104, 534–539 (2016).
Liang, P. et al. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 251, 933–940 (2009).
Acknowledgements
We sincerely thank all the patients, their families, hospital staff, etc. who participated in this study.
Funding
This study was not funded.
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by TL.Z and NSQ. Methodology was contributed by NS.Q, F.Y and XM.Z. Data collection was performed by FY.Z. Statistical analysis was performed by QN.Y. The first draft of the manuscript was written by FY.Z, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study was a retrospective study approved by Ethics Committee of Chinese PLA General Hospital (K-Y-2023–1-1–1), and informed consent was waived.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, F., Zhang, T., Yang, Q. et al. Safety of local thermal ablation in hepatocellular carcinoma patients with cirrhosis and severe thrombocytopenia. Sci Rep 14, 28350 (2024). https://doi.org/10.1038/s41598-024-79416-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79416-w
Keywords
This article is cited by
-
Radiofrequency ablation is safe in patients with hepatocellular carcinoma and thrombocytopenia
Abdominal Radiology (2025)